
Special Article – Gait Rehabilitation
Phys Med Rehabil Int. 2015; 2(10): 1072.
Changes in Post-Stroke Gait Biomechanics Induced by One Session of Gait Training
Kesar TM¹*, Reisman DS², Higginson JS³, Awad LN4 and Binder-Macleod SA5
¹Division of Physical Therapy, Department of Rehabilitation Medicine, Emory University, Atlanta, Georgia.USA
²Department of Physical Therapy, University of Delaware, Newark, Delaware, USA
²Department of Mechanical Engineering, University of Delaware, Newark, Delaware, USA
4Harvard John A Paulson School of Engineering and Applied Sciences and Wyss Institute For Biologically Inspired Engineering, Harvard University, Cambridge, MA, USA
5Department of Physical Therapy, University of Delaware, Newark, Delaware, USA
*Corresponding author: Kesar Trisha M, Division of Physical Therapy, Department of Rehabilitation Medicine, Emory University, 1441 Clifton Rd NE, Atlanta, Georgia, USA
Received: November 28, 2015; Accepted: December 23, 2015; Published: December 28, 2015
Abstract
The objective of this study was to determine whether one session of targeted locomotor training can induce measurable improvements in the post-stroke gait impairments. Thirteen individuals with chronic post-stroke hemiparesis participated in one locomotor training session combining fast treadmill training and functional electrical stimulation (FES) of ankle dorsi- and plantar-flexor muscles. Three dimensional gait analysis was performed to assess withinsession changes (after versus before training) in gait biomechanics at the subject’s self-selected speed without FES. Our results showed that one session of locomotor training resulted in significant improvements in peak anterior ground reaction force (AGRF) and AGRF integral for the paretic leg. Additionally, individual subject data showed that a majority of study participants demonstrated improvements in the primary outcome variables following the training session. This study demonstrates, for the first time, that a single session of intense, targeted post-stroke locomotor retraining can induce significant improvements in post-stroke gait biomechanics. We posit that the within-session changes induced by a single exposure to gait training can be used to predict whether an individual is responsive to a particular gait intervention, and aid with the development of individualized gait retraining strategies. Future studies are needed to determine whether these single-session improvements in biomechanics are accompanied by short-term changes in corticospinal excitability, and whether single-session responses can serve as predictors for the longer-term effects of the intervention with other targeted gait interventions.
Keywords: Gait rehabilitation; Stroke; Biomechanics; Propulsion; Functional electrical stimulation
Abbreviations
FES: Functional Electrical Stimulation; AGRF: Anterior Ground Reaction Force; SS Speed: Self-Selected Speed
Introduction
Common impairments in post-stroke gait kinematics include reduced hip, knee, and ankle flexion during swing phase. To alleviate reduced dorsiflexion during swing phase or ‘foot drop’, functional electrical stimulation (FES) delivered to ankle dorsi-flexor muscles is commonly used as an intervention [1-3]. Previous studies from our laboratory have shown that in contrast to the traditional FES approach of stimulating the ankle dorsiflexors during swing phase, delivering FES to both dorsi- and plantar-flexor muscles provides greater biomechanical advantages and may address both swing phase and stance phase gait deficits post-stroke [4,5]. Decreased push-off force generation by the paretic limb during terminal stance is a poststroke impairment that has recently received attention in the literature due to its relationships with hemiparetic severity and walking speed [6,7]. ‘FastFES’, a novel gait rehabilitation intervention involving the combination of fast treadmill training with functional electrical stimulation of ankle plantar-and dorsi-flexor muscles, is a novel and effective post-stroke locomotor training intervention [5,8-10]. The FastFES intervention targets slowed walking speed, decreased paretic push-off and decreased knee and ankle flexion during swing. Twelve weeks (36 sessions) of FastFES gait training have been shown to produce significant improvements in walking function, activity, and participation post-stroke [8,9,10,11].
While improvements in post-stroke gait are commonly observed after several weeks of training with FastFES and other gait retraining interventions, when and how these improvements in gait first evolve is unclear. Experiments employing a single session of exposure to unique, challenging, and well-controlled locomotor paradigm, such as walking with a weight added to one leg [12], stepping on a rotating disc [13], and split-belt treadmill walking [14] demonstrate that human locomotion adapts rapidly in the short-term. However, longlasting therapeutic effects of these locomotor adaptation paradigms have not been well studied. In contrast, FastFES has been shown to produce marked long-term therapeutic benefits on post-stroke gait, but it is not known whether it can induce measurable improvements in post-stroke gait impairments within a single session.
The changes induced in gait following each session of gait training may be important because they form the ‘building blocks’ that accumulate to produce the long-term therapeutic effects of an intervention. We posit that within-session changes in gait performance, if present, may provide a valuable ‘probe’ or evaluation tool to assess an individual’s short-term responsiveness to an intervention and aid with future development of customized gait retraining strategies. For example, if a single session of training is found to induce changes in gait biomechanics, before implementing a long-term gait rehabilitation program comprising 18 to 24 sessions over the course of 6 to 12 weeks, perhaps biomechanical data obtained during a single session of training can be utilized to determine whether the individual shows any measurable improvements in gait biomechanics following a single session of training. Furthermore, establishing that within-session changes in gait biomechanics are induced by clinical training protocols such as FastFES would lay foundations for future studies investigating the neurophysiological and biomechanical mechanisms underlying the effects of gait rehabilitation. The objective of this study, therefore, was to determine whether one FastFES gait training session (comprising 30-minutes of stepping practice with and without FES) can induce improvements in post-stroke gait biomechanics, as demonstrated by carry-over of improvements in gait during walking without FES.
Materials and Methods
Subjects
Thirteen individuals with chronic post-stroke hemiparesis (age 42-75 years, 2 females, and time post-stroke 5-90 months) were recruited for the study (Table 1). Study inclusion criteria included >6 months post-stroke and the ability to walk for 4-minutes without an orthosis. Individuals were excluded if they had neurological diseases other than stroke, neglect, hemianopia, orthopedic problems affecting walking, or inability to communicate with investigators. Participants provided informed consent and study procedures were approved by the institutional review board.
Subject
Gender
Age (years)
Side of paresis
Stroke Onset (months)
SS Gait Speed (m/s)
Fast (Training) Speed (m/s)
Fugl-Meyer LE Score
TUG Time (s)
6-min walk distance (meters)
1
M
70.5
L
44.0
0.9
0.95-1.05
21
15.7
242.7
2
M
75
R
87.0
0.5
0.66-0.76
14
18.8
262.8
8
M
60
L
32.0
0.38
0.42
12
30.3
146.6
3
M
47
R
15.5
0.4
0.93-1.05
15
27.1
177.9
4
F
71
L
15.9
0.3
0.6-0.73
22
35.1
116.0
5
F
60.5
R
10.3
0.32
0.36-0.49
11
28.9
108.1
6
M
68
L
35.6
0.79
1.01
31
10.1
316.3
7
M
68
L
11.0
0.65
0.65
21
17.0
316.3
10
M
57.5
L
6.6
0.87
0.5-0.61
28
11.4
322.5
11
M
63.5
R
90.3
0.92
1.02-1.05
23
9.6
424.9
9
M
54.5
L
5.8
1.03
1.19
27
10.9
335.1
12
M
42
L
9.5
0.94
0.87
22
12.0
395.6
13
M
60
R
14.5
0.83
1.0-1.05
18
15.7
370.6
Mean
61.3
29.1
0.7
0.8
20.4
18.7
284.9
Stdev
9.6
29.0
0.3
0.3
6.2
8.7
99.6
Table 1: Subject demographics and clinical characteristics.
After completion of initial screening and informed consent, subjects participated in a clinical testing session comprising measurement of: (i) over ground self-selected walking speed using the 6-meter walk test (SS speed used for pre-test and post-test measurements during each session), (ii) overground endurance using the 6-minute walk test, (iii) fastest speed subjects could maintain on a treadmill for 4-minutes (Fast speed used during gait training), (iv) lower extremity portion of the Fugl-Meyer, and (v) the timed up and go test. Following completion of clinical testing, all participants completed one FastFES training session. Three dimensional gait analyses were performed to measure gait biomechanics before (Pretest) and after (Post-test) the gait training session (Figure 1). These tests were performed during walking without FES and at the same speed (each participant’s self-selected gait speed determined at the start of the training session).
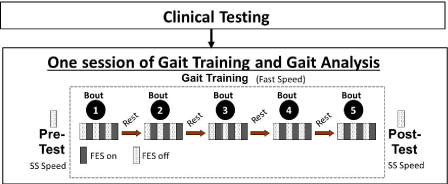
Figure 1: Overview of study methodology.
Methods for gait analysis
An 8-camera motion analysis system (Motion Analysis, Santa Rosa, CA) was used to measure positions of retro-reflective markers attached to the pelvis and bilateral thigh, shank, and foot segments [15]. Participants walked on a split-belt treadmill instrumented with force platforms embedded within each belt (Bertec, Columbus, OH). Marker and ground reaction force (GRF) data were sampled at 100- and 1000-Hz respectively. During gait analysis, participants walked without FES or an orthoses. An overhead harness without bodyweight support provided safety.
Methods for locomotor training
The FastFES training comprised a total of 30 minutes of walking practice (five 6-minute bouts with seated rest breaks between bouts), as described previously [4,16] (See Figure 1 for additional details). Each 6-minute bout comprised treadmill walking at the fastest speed participants could maintain with alternating 1-minute periods of walking with and without FES (Figure 1). The fast training speed was determined at the start of the session and if the subjects were able, the fast training speed was progressed during the training session as well (i.e. training speed may be increased from the 1st to the 5th training bout). During walking with FES, FES was delivered via surface electrodes to ankle dorsi-flexor muscles during paretic swing phase and to ankle plantar-flexor muscles during paretic terminal stance phase, as described previously [4,9,16].
Dependent variables and statistical analyses
Gait events were determined using a 20-Newton vertical GRF force threshold. GRF data were normalized to body weight. The gait phase between the point where the antero-posterior GRF crossed zero (i.e. transitioned from posterior to anterior) and the end of stance phase was identified as the portion of the gait cycle when participants demonstrated anteriorly-directed GRFs. The peak paretic anterior GRF (peak AGRF) and paretic AGRF integral (i.e., area underthe AGRF curve) were the primary dependent variables measuring paretic propulsion. Secondary outcome variables included peak knee flexion angle during swing phase and ankle dorsi/plantar-flexion angle at initial contact. These 4 variables were selected to assess the specific impairments targeted by the intervention.
For each dependent variable, a paired t-test was performed to determine if there was a difference in the dependent variable at pre pretest versus post-test. Improvements in gait biomechanics induced by the single session of gait training would be present if the comparisons demonstrated greater peak AGRF, greater AGRF integral, greater peak swing phase knee flexion angle, and reduced foot-drop (ankle angles closer to 0º plantar/dorsi-flexion) at Post-test versus Pre-test. The Kolmogorov-Smirnov Test was used to confirm that the data were normally distributed.
Results
Complete data were obtained on all 13 subjects (Table 1) for peak AGRF, paretic AGRF integral, and ankle angle at initial contact. Data from the first exposure to FastFES gait training session were utilized for the current study. However, for one participant, due to gaps and issues with marker data obtained during from the first training session, data from a second training session were included in the analysis.
The paired t-tests revealed significantly greater values of peak AGRF (p=0.014) and AGRF integral (p=0.004) during the post-test versus the pre-test (Figure 2). The mean difference between Posttest and Pre-test (mean difference±standard error of difference) was 1.11±0.39% body weight for peak AGRF and 0.33±.08 % body weight. seconds for AGRF integral. The paired t-test for peak swing phase knee flexion was not significant but showed a trend (p=0.12) for greater values at post-test versus pre-test (Figure 2; mean difference between Post- and Pre-test = 2.3±1.4º). The paired t-test detected no significant difference between pre- and post-test values for ankle angle at initial contact (p=0.95; mean difference between Post- and Pre-test = 0.07±1.3º) (Figure 2). Additionally, analysis of the values of the 4 dependent variables at Pre- and Post-test for individual subject data corresponding to these group statistics showed that for the primary outcome variables, i.e. peak AGRF and AGRF integral, a majority of the subjects (11 of 13) showed improvements following the training session (depicted by bold lines in Figure 3). For the secondary outcome variable of ankle angle at initial contact, there was greater inter-individual variability in the magnitude of within-session changes, and while a majority of subjects still showed within-session improvements (shown by bold lines in Figure 3), 5 of the participants showed no change or a worsening of the variables following training (shown by shaded lines in Figure 3).
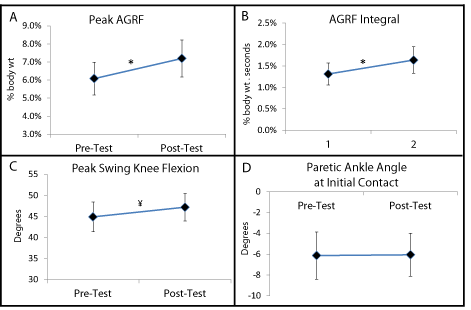
Figure 2: Group means and standard errors for peak AGRF (N=13), paretic AGRF integral (N=13), swing phase peak knee flexion (N=13, positive values indicate
knee flexion), and ankle angle at initial contact (N=13, positive values indicate dorsiflexion) obtained before (pre-test) and after (post-test) after one session of
FastFES gait training. The individual subject data corresponding to these group means are shown in Figure 3.*Significant effect of time (post-test versus pre-test)
(p<0.05). ¥ Trend for statistical significance (p=0.1).
Discussion
This study demonstrates, for the first time, that a single session of intense gait training targeting paretic push-off force generation, an important post-stroke gait impairment shown to be associated with hemiparetic severity and over ground gait speed, can induce significant improvements in push-off of the paretic leg of individuals post-stroke. Significantly greater push-off forces (paretic peak AGRF and AGRF integral), and a trend for greater swing phase knee flexion angles,were demonstrated by the paretic limb after the FastFES gait training session, indicating that FastFES training maybe sufficiently intensive and robust to induce positive effects on gait impairments within one session. It is important to note that although previous studies have shown improvements in gait biomechanics and walking function after several weeks of FastFES gait training [8,9,11], or immediate improvements in gait biomechanics during short bouts of walking with versus without FES [4,16], improvements in gait deficits (measured during walking without FES) following a single training session have not been previously documented in the literature.
During FastFES, the combination of fast treadmill training with plantar-flexor FES is designed to train stroke survivors to generate greater push-off forces with their paretic ankle muscles [4,5]. The within-session improvements in push-off forces observed in our study suggest that the FastFES training strategy successfully modulated push-off force generation after a single session. Some what consistent with previous biomechanical simulations [17] and experimental studies [4] suggesting that push-off force generation contributes to swing phase knee flexion, in the current study, a trend for improvements in knee flexion were observed after the training session. In addition, the consistency of the direction of the withinsession change in paretic push-off forcesand knee flexion across subjects suggests that these within-session improvements were robust (see individual subject data in Figure 3). The lack of within-session change in ankle kinematics may be attributed to dorsi-flexor muscle fatigue, and is an interesting finding that merits further investigation, especially because foot drop is an important and prevalent poststroke impairment [1]. Furthermore, it is important to note that these within-session changes were observed as subjects walked at a speed (self-selected speed) different from the speed they trained at (fast speed), and during walking without FES, suggesting short term carry-over or retention of modified gait biomechanics patterns learned during training.
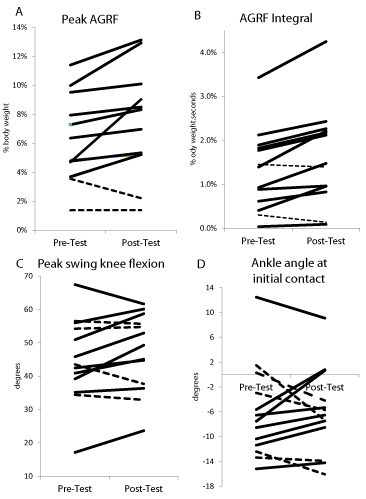
Figure 3: Individual subject data (corresponding to the averaged or group data shown in Figure 2) showing (A) peak AGRF, (B) paretic AGRF integral, (C) swing
phase peak knee flexion, and (D) ankle angle at initial contact (positive values indicate dorsiflexion) obtained before (pre-test) and after (post-test) after one
session of FastFES gait training. The study subjects who showed an increase (or improvement) in the gait variable are depicted with bold lines, and subjects who
showed no change or decrement (worsening) of the gait variable are depicted with the shaded lines. These graphs demonstrate the inter-subject variability in the
single-session responses to gait training, and also show that for the primary gait deficit targeted during training (paretic propulsion (A and B)), a majority of subjects
showed improvements following the training session.
In the current study, unlike previous studies reporting the immediate or long-term (over many weeks) therapeutic effects of the gait training, we focused on changes in gait induced after a single session of gait training. However, the study is limited by a small sample size comprising a majority of male stroke survivors. Additionally, although we demonstrate that a single training session induces improvements in post-stroke biomechanical impairments targeted during training, the longevity or retention of these singlesession changes was not evaluated, and would be an interesting focus of future investigations.
To our knowledge, no previous study has shown that improvements in post-stroke gait biomechanics can occur after a single session of clinical gait rehabilitation. We posit that withinsession biomechanical changes, such as those demonstrated here during a single session of FastFES gait training, can be used as a quick, cost-effective probe or test to assess whether an individual is responsive to a particular treatment paradigm. Future studies are needed to determine whether other gait training interventions produce similar within-session improvements, to investigate the neural correlates underlying these within-session improvements in gait impairments, to develop strategies to maximize the magnitude of these within-session improvements, and to determine whether single-session responses to a rehabilitation treatment can serve as predictors of longer-term effects of the intervention.
Conclusion
This study demonstrated that changes in post-stroke gait biomechanics may occur following a single gait rehabilitation treatment session. Our results showed that a single session of intense, post-stroke locomotor training (FastFES) that targets paretic propulsion induced improvements in paretic push-off (peak AGRF and AGRF integral). Additionally, we demonstrated that withinsession responses to gait training may vary across subjects and across different gait deficits (e.g. primary variable of peak AGRF and AGRF integral in our current study versus secondary variables of peak swing phase knee angle and ankle angle at initial contact). We postulate that an in-depth understanding of such within- and across-session time courses of change during gait retraining can help to maximize the effects of each session and each week of rehabilitation. Our findings provide support for the need for studying the effects of a single treatment session on gait as a ‘probe’ or ‘test’ for an individual’s response to a rehabilitation intervention [18].
Acknowledgements
The authors would like to thank Erin Helm, BS, Tamara Wright, PT and Margie Roos, PT, PhD, NCS for assistance with data collection and clinical testing. This work was funded by American Heart Association Scientist Development Grant (13SDG13320000) and NIH K01 HD079584 awarded to Dr. Kesar, and an NIH R01 NR0786 awarded to Dr. Binder-Macleod. None of the authors have any conflict of interest related to this manuscript.
References
- Kesar TM, Perumal R, Jancosko A, Reisman DS, Rudolph KS, Higginson JS et al. Novel patterns of functional electrical stimulation have an immediate effect on dorsiflexor muscle function during gait for people poststroke. Phys Ther. 2010; 90: 55-66.
- Stein RB, Chong S, Everaert DG, Rolf R, Thompson AK, Whittaker M, et al. A multicenter trial of a footdrop stimulator controlled by a tilt sensor. Neurorehabil Neural Repair. 2006; 20: 371-379.
- LIBERSON WT, HOLMQUEST HJ, SCOT D, DOW M. Functional electrotherapy: stimulation of the peroneal nerve synchronized with the swing phase of the gait of hemiplegic patients. Arch Phys Med Rehabil. 1961; 42: 101-105.
- Kesar TM, Perumal R, Reisman DS, Jancosko A, Rudolph KS, Higginson JS, et al. Functional electrical stimulation of ankle plantarflexor and dorsiflexor muscles: effects on poststroke gait. Stroke. 2009; 40: 3821-3827.
- Kesar TM, Reisman DS, Perumal R, Jancosko AM, Higginson JS, Rudolph KS, et al. Combined effects of fast treadmill walking and functional electrical stimulation on post-stroke gait. Gait Posture. 2011; 33: 309-313.
- Bowden MG, Balasubramanian CK, Neptune RR, Kautz SA. Anterior-posterior ground reaction forces as a measure of paretic leg contribution in hemiparetic walking. Stroke. 2006; 37: 872-876.
- Balasubramanian CK, Bowden MG, Neptune RR, Kautz SA. Relationship between step length asymmetry and walking performance in subjects with chronic hemiparesis. Arch Phys Med Rehabil. 2007; 88: 43-49.
- Awad LN, Binder-Macleod SA, Pohlig RT, Reisman DS. Paretic Propulsion and Trailing Limb Angle Are Key Determinants of Long-Distance Walking Function After Stroke. Neurorehabil Neural Repair. 2015; 2 9: 499-508.
- Reisman D, Kesar T, Perumal R, Roos M, Rudolph K, Higginson J, et al. Time Course of Functional and Biomechanical Improvements During a Gait Training Intervention in Persons With Chronic Stroke. Journal of Neurologic Physical Therapy. 2013; 37: 159-165.
- Awad LN, Reisman DS, Pohlig RT, Binder-Macleod SA. Reducing The Cost of Transport and Increasing Walking Distance After Stroke: A Randomized Controlled Trial on Fast Locomotor Training Combined With Functional Electrical Stimulation. Neurorehabil Neural Repair. 2015.
- Awad LN, Reisman DS, Kesar TM, Binder-Macleod SA. Targeting paretic propulsion to improve poststroke walking function: a preliminary study. Arch Phys Med Rehabil. 2014; 95: 840-848.
- Noble JW, Prentice SD. Adaptation to unilateral change in lower limb mechanical properties during human walking. Exp Brain Res. 2006; 169: 482-495.
- Weber KD, Fletcher WA, Gordon CR, Melvill Jones G, Block EW. Motor learning in the "podokinetic" system and its role in spatial orientation during locomotion. Exp Brain Res. 1998; 120: 377-385.
- Reisman DS, Bastian AJ, Morton SM. Neurophysiologic and rehabilitation insights from the split-belt and other locomotor adaptation paradigms. Phys Ther. 2010; 90: 187-195.
- Kesar TM, Binder-Macleod SA, Hicks GE, Reisman DS. Minimal detectable change for gait variables collected during treadmill walking in individuals post-stroke. Gait Posture. 2011; 33: 314-317.
- Perumal R, Kesar TM, Wexler AS, Binder-Macleod SA, editors. Novel FES System to Stimulate Both Dorsi– and Plantar–Flexor Muscles During Stroke Gait. 12th Annual Conference of the International FES Society; 2007; Philadelphia, PA, USA.
- Neptune RR, Kautz SA, Zajac FE. Contributions of the individual ankle plantar flexors to support, forward progression and swing initiation during walking. J Biomech. 2001; 34: 1387-1398.
- Harris-Love ML, Morton SM, Perez MA, Cohen LG. Mechanisms of short-term training-induced reaching improvement in severely hemiparetic stroke patients: a TMS study. Neurorehabilitation and neural repair. 2011; 25: 398-411.