
Special Article – Cerebral Palsy
Phys Med Rehabil Int. 2016; 3(1): 1075.
Hyperselective Neurectomy in the Treatment of the Spastic Upper Limb
Leclercq C* and Gras M
Institut de la Main, Paris, France
*Corresponding author: Leclercq C, Institut de la Main, 6 Square Jouvenet, 75016, Paris, France
Received: December 28, 2015; Accepted: January 27, 2016; Published: January 30, 2016
Abstract
Cerebral palsy is a general term that includes all the sequelae of infantile encephalopathies occurring during the perinatal period or during infancy, responsible for a permanent disorder of the development of movement and posture, resulting in a diminution of function. It manifests progressively during growth, but once established, follows a non-progressive course. Surgery is one element of the rehabilitative care, which includes primarily physiotherapy and splinting, occupational therapy, and pharmacological treatment as needed. Different surgical techniques are utilized to address the different components of the deformities, namely spasticity itself, muscle contracture, joint contractures, and paralysis.
Partial neurectomy of motor nerves has been shown to reduce spasticity in the target muscles. It is effective only on the spastic component of the deformity, underlining the need for a preliminary thorough clinical examination. Hyperselective neurectomy, which consists in following each motor ramus until its entry point into the target muscle, aims at improving selectivity, widespread partial denervation, and durability of the results.
Keywords: Cerebral palsy; Spastic upper limb; Hyperselective neurectomy
Introduction
Cerebral palsy (CP) is a general term that includes all the sequelae of infantile encephalopathies occurring during the perinatal period, or during infancy, responsible for a permanent disorder of the development of movement and posture, resulting in a diminution of function. It can also be associated with disturbances of sensation, perception, cognition and communication, as well as behavior. It manifests progressively during growth, but once established, follows a non progressive course, which makes it amenable to surgical treatment in selected cases.
The essential treatment for spasticity is physical management, including a wide range of physical and occupational therapy techniques [1]. Pharmacological agents may be used as an adjunct, whether orally, intra-thecal or locally. In selective cases, surgery may be indicated following proper conservative treatment.
The goals of surgical treatment can vary greatly, depending on the extent of the functional impairment. Whenever possible, it is to improve function. In some cases, however, it will be limited to improving nursing and comfort, reducing pain, or correcting a severe deformity.
Different surgical techniques are utilized to address the different components of the deformities, namely spasticity itself, muscle contracture, joint contractures, and paralysis, underlining the need for a preliminary thorough clinical examination.
Partial neurectomy described by Stoffel [2] in 1913, and developed by Brunelli [3] in 1983, has been shown to reduce spasticity in the operated muscles.It is effective only on the spastic component of the deformity. Satisfactory results of this technique have been reported in the literature, mainly for the lower limb [4]. In the upper limb, the results are more difficult to interpret because of the lack of quantitative tools, and there is a general perception that recurrence is frequent. In light of our recent anatomic studies [5-7], new guidelines for a “hyperselective” neurectomy (HSN) have been described and we have conducted a prospective study in order to re-evaluate the results of this treatment.
Clinical Examination
The clinical picture may vary greatly from one individual patient to the other, depending on the amount and location of the initial brain insult.
Clinical examination is a critical part of the assessment. Its goal is fourfold: evaluate spasticity, detect possible muscle contracture and joint deformity, evaluate motor and sensory impairment in the upper limb, and evaluate existing function, and functional needs of the upper limb.
This examination is best performed as a team, including all the specialists involved in the child’s care (physiatrist, physical and occupational therapist, pediatrician, surgeon…). This should ideally be done in a warm, quiet, and friendly environment, ensuring that the child is comfortable and confident, both because the child’s cooperation is essential for sensory and motor evaluation, and because spasticity may increase considerably if he/she is frightened or recalcitrant.
Some CP children also have limited concentration capacities, and cannot cooperate throughout the entire examination. Therefore it is not wise to decide on surgery after a single session, and assessment should be repeated before any decision making.
The data are recorded on standardized charts, which will allow intra- and inter-comparisons of the results of surgical treatment. Video recording of each patient before and after every step of treatment is extremely helpful for decision-making, and for evaluation of surgical outcome.
Examination of the upper limb is completed by a general examination in order to seek associated neurological disorders, and potential contraindications to surgery.
Evaluation of Spasticity
Spasticity is infrequent around the shoulder in CP children. At the elbow, it usually involves predominantly the biceps and brachialis muscles, but also the brachioradialis to a lesser degree. The triceps, classically spared, can actually be quite spastic.
Wrist flexors and forearm pronators are most frequently involved, leading sometimes to an extremely hyper flexed (100°) and hyperpronated (150°) position.
Spasticity is difficult to assess in the fingers because of the wrist deformity, and of the many muscle groups involved. It usually involves the finger flexors, and to a various degree, the interossei muscles.
In the first web space and the thumb, spasticity involves not only the thumb adductor, but also frequently the first dorsal interosseous and the flexor pollicis brevis. Associated spasticity of the flexor pollicis longus leads to the classical “thumb in palm” deformity.
Spastic involvement is recorded on a standard chart for each muscle or muscle group. Its quantification is quite difficult [4]. The Ashworth scale has been developed to assess the efficacy of antispastic treatment in patients with multiple sclerosis. It is descriptive, and despite subsequent modification, remains subject to personal interpretation, with suboptimal inter observer reliability [8]. There is evidence that the Tardieu scale [9] is currently the most reliable tool for evaluating spasticity [10-14].
Muscle Contracture
Muscle contracture may involve spastic muscles. Unlike spasticity, it is permanent and cannot be overcome, although shortening the involved articular segment can alleviate it. Contracture of the finger flexors is assessed by the Volkmann’s angle, which is the degree of wrist flexion required to obtain full passive finger extension. Contracture of the intrinsic muscles of the fingers is assessed by the Finochietto test.
Clinical distinction between contracture and spasticity may be difficult to establish. However it is critical for the decision making. In such cases nerve blocks or botulinum toxin are very helpful: spasticity yields completely whereas contracture persists [15-16].
Joint Deformity
Passive motion of the involved joints may be difficult to assess, not so much because of spasticity but because of muscle contractures. Motor blocks are not very helpful here, as they do not alleviate muscle contracture. Sometimes it is not until surgical release of the muscle contracture (especially elbow flexors and wrist flexors) that the actual range of passive motion can be evaluated. Joint contracture is rare in young children, but may develop as a result of lack of physical therapy, or of severe intractable spasticity.
Joint instability is more frequent in the fingers, due to increased passive extension, especially at the thumb MP joint, and at the finger PIP joints (leading to a swan neck deformity).
Motor Impairment
Motor examination of the upper limb is not easy in small children. Rather than individual muscles, it is easier to evaluate muscle groups contributing to the same function. Observation at play, and video recording are very helpful at this stage, avoiding lengthy repetitions of tasks.
The palsy (or ‘pseudo palsy’) usually predominates in the distal part of the upper limb, and involves the extensor and supinator muscles, namely the wrist and finger extensors, thumb extensors, and supinator muscle. Motor examination of these muscles may be difficult when the antagonist flexors and pronators are severely spastic. Actually, rather than really paralysed, these muscles may be present, but made ineffective by the spastic antagonists. Botulinum toxin has proved very helpful in assessing these muscles: when injected in the spastic antagonist muscles, it reduces dramatically their tone for several months, making it possible to evaluate and exercise the ‘paralysed’ muscles, which may end up demonstrating, in a number of cases, a satisfactory voluntary control.
The flexor, adductor, and pronator muscles, mostly spastic, usually retain some voluntary control. However, their examination is made difficult when severe deformities are present. For example, extreme flexion deformity of the wrist prevents evaluation of the strength of the finger flexors, which are rendered ineffective because of the mechanical shortening of their muscle-tendon unit.
We have not found electromyographic (EMG) studies to be helpful in quantifying the motor function of either the pseudoparalytic or the spastic muscles. Three dimensional movement analysis is complex in the upper limb, and not routinely utilized [17- 18].
Involuntary movements, whether spontaneous (chorea, athetosis) or voluntary (dystonia) are recorded: they may be a contra-indication to surgery.
Sensory Impairment
Sensory examination is practically impossible before the age of four to five. Light touch and two-point discrimination are generally intact, whereas complex sensations (fine sensibility, proprioception, stereognosis) are more readily affected.
Pain may be present, but is difficult to evaluate, especially in children. It may be linked to severe contractures, or to a deformed joint, or, occasionally at the wrist to a Kienböck’s disease secondary to a severe flexion deformity [19].
Functional Assessment
The International Classification of Functioning, Disability and Health (ICF) provides a standard language and framework for assessing function and disability [20]. According to the ICF evaluation of activity distinguishes capacity and performance. Capacity is the ability to execute a task at the highest possible level of functioning. Performance is the spontaneous use of the hand during activities or play. They differentiate what the patients can do versus what they actually do, for a better understanding of their ability [21].
Indeed, in a number of cases the CP child neglects the upper extremity in spite of some potential function. In these cases, the child may persist in ignoring it even when functional ability can be improved through surgery.
Validated tools for assessing capacity include some multitask tests (MUUL, SHUUE, Jebsen Taylor) and unitask tests (Box and Block, Nine Hole Peg). Performance can be accessed through questionnaires (AbilhandKids, CHEQ) and tests (AHA, VOAA).
General Preoperative Assessment
The aim of this general examination is to evaluate the real benefit the child could gain from surgery, taking into account other neurological impairments, the patient’s age, intellectual status, motivation and environment.
Surgery is not indicated for abnormal movements or dystonia. Contra-indications may also include lack of compliance, emotional disorders, or unrealistic expectations. Cognitive problems are not per se contra-indications, but must be carefully evaluated prior to surgery.
Medical Treatment: The Role of Botulinum Toxin
Besides systemic medications such as benzodiazepines, dantrolene sodium, baclofen, or tizanidine, and intrathecal baclofen which are used for treating generalized spasticity, some agents are effective locally [22]. Nowadays, rather than lidocaïne, alcohol or phenol [15], the efficacy of botulinum toxin (BT) has gained wide recognition. Injected in the body of the involved muscle(s), it produces a reduction of spasticity lasting up to several months. It is now routinely used in the cerebral palsied upper limb with measurable and reproducible effects [23-24]. Charts are available which indicate the effective dose for each age group and for each individual muscle. The role of associated physiotherapy and splinting has been emphasized [25].
BT may be repeated as required, sometimes yielding a permanent improvement if the antagonist muscles improve their strength, thus balancing the spasticity more effectively. But if spasticity recurs after each injection, a more definitive procedure can subsequently be performed. BT plays, in such cases, an educational role in simulating the effect of surgery [26-27].
BT is also useful as a preoperative test, in order to decide which muscles require a permanent surgical reduction of spasticity. This is particularly informative in groups of muscles with the same function, in order to understand which of them should be treated (ex: elbow flexors).
Finally BT may be used pre- or immediately postoperatively to attenuate the spastic tone of the antagonists muscles when performing a tendon transfer, in order to protect and facilitate education of the transferred muscle.
Surgical Treatment: The Role of Neurectomy
Three groups of surgical procedures may be indicated in the spastic upper limb, in isolation or together: those which aim at reducing spasticity, those which aim at reducing muscle and/or joint contracture, and those which aim at reinforcing paralysed muscles.
Spasticity may be addressed surgically by selective neurectomy, performed at the neuro-muscular level, or by neurosurgical procedures (rhizotomy). This paper deals with the surgical reduction of spasticity by selective neurectomy.
Neurotomy
Neurotomy (complete section of a nerve trunk) may be indicated in non-functional upper limbs with severe spasticity, in order to facilitate hygiene and nursing, and to improve cosmesis.
It has a single indication in the upper limb, in non-functional hands with severe spasticity of the intrinsic muscles, where a neurotomy of the motor branch of the ulnar nerve at the wrist will reduce the deformities, and improve hygiene and cosmesis. It creates a flaccid palsy of the involved muscles, but is ineffective on established muscle contractures.
Selective Neurectomy
Selective or “partial” neurectomy involving only part of the nerve fascicles had been suggested as early as 1913 in an attempt to retain some function [2]. This technique has gained some popularity after Brunelli published a series of clinical cases in 1983 [3], coining the term “hyponeurotization”. The procedure is performed at the entry point of the nerve into the muscle, where it usually divides into several fine fascicles. Under magnifying loops, part of the fascicles is resected. Brunelli initially advocated removing 50% of the fascicles, but after experiencing some recurrence of the spasticity, he recommended resection of a larger amount of fibres. He also recommended planning a second partial neurectomy 6 months later in cases of recurrence.
Anatomical Study
We performed several anatomical studies to establish “cartography” of the motor branches of the musculo-cutaneous, median, ulnar, and radial nerves in the forearm, as well as the terminal motor branch of the ulnar nerve in the hand. In total 96 cadaveric upper limbs were dissected [5-7].
In 16 cadaver dissections, the musculo-cutaneous nerve gave off 1 to 5 trunks for the biceps, which reached the muscle from 18 to 64% of the arm length; then it gave off 1 to 3 branches for the brachialis, which reached the muscle from 35 till 75% of the arm length [5]. Therefore the skin incision for selective neurectomy of the musculocutaneous nerve should run from 18 to 75% of the arm length.
Motor branches of the median nerve in the forearm had the most complex and variable distribution [7]. In 20 cadaver dissections, the median nerve gave off 1 or 2 trunks for the pronator teres (PT), starting at 3% of the forearm length, 1 trunk only for the flexor carpi radialis (FCR), which in almost all cases arose as a common trunk with other branches from 21 to 41% of the forearm length. For the flexor digitorum superficialis muscles (FDS), there were 2 to 4 trunks, which reached the muscle from 20 to 77% of the forearm length. For the flexor digitorum profundi (FDP), the anterior interosseous nerve (AIN) gave off 1 to 5 branches between 29 and 72% of the antebrachial segment. For both the FDS and the FDP, part of the terminal branches were located under the muscle bellies, requiring detaching these muscles for access to the nerve-muscle junction. The flexor digitorum profundus was most frequently innervated by 1 branch of the AIN, which reached the muscle between 44 and 63% of the antebrachial segment
The ulnar nerve, in a further dissection of 20 upper limbs, gave off 1 to 4 trunks for the flexor carpi ulnaris, which originated from 20 mm above the medial epicondyle till 38 mm below, and reached the muscle between 0 and 50% of the forearm length [6]. The majority of these branches originated from the posterior aspect of the ulnar nerve, stressing the importance of elevating the nerve trunk, in order to identify all of them. The FDP received in most cases a single branch from the ulnar nerve, originating on average 50 mm from the medial epicondyle, and no motor branch was found after the ulnar artery joined the nerve. Therefore skin incision for NHS of the flexor carpi ulnaris should extend from just above the medial epicondyle until 50% of the forearm length.
Combined approach of the median and ulnar nerve for HSN of the wrist flexors may be performed trough a single C-shape incision starting above the elbow, curving towards the medial epicondyle, and then turning anteriorly (Figure 1).
Figure 1: Combined approach of the median and ulnar nerve for HSN of the
wrist flexors. (NM: median nerve; NC: ulnar nerve, O: medial epicondyle).
Similar studies have been done for the radial nerve and the terminal motor branch of the ulnar nerve in the palm (20 cadaver dissection each, unpublished data). The radial nerve gave off one to three trunks for the brachioradialis muscle, and the branches penetrated the muscle from 66% of the arm segment until 10% of the forearm segment (distal to the lateral epicondyle). The motor branches of the ulnar nerve for the adductor pollicis (AP) and the 1st dorsal interosseous was easily identified distal to the point on penetration of the ulnar nerve between the two heads of the adductor pollicis. There was more than one terminal branch for the oblique head of the AP in 95% cases, and for the transverse head in 62%. And there was more than one terminal branch for the 1st dorsal interosseous muscle in all cases, making HSN possible in most cases.
Surgical Technique
Depending on the involved nerve, the surgery can be performed under local anaesthesia, i.e. axillary block, or general anaesthesia (for proximal nerves). Peroperative nerve stimulation is most helpful, and therefore curare is contraindicated during induction.
The skin incision follows the guidelines from anatomical dissections. Combined approaches of the median and the ulnar nerve may be performed through a single incision for hyperselective neurectomy of the wrist flexors. Once the main trunk has been exposed, its motor branches are identified. Common branches for several muscles are frequent, thus the need for nerve stimulation in order to select the target motor branches. There are usually several rami for each involved muscle, and all of them must be identified for a complete result. Each ramus is dissected until the neuro-muscular junction (Figure 2). Then with micro-surgical instruments and magnifying loops [28], the required amount of fascicles is resected from each ramus (usually two-thirds, depending on the amount of spasticity and the anticipated result). Some authors coagulate the proximal stump in order to prevent nerve regrowth [29].
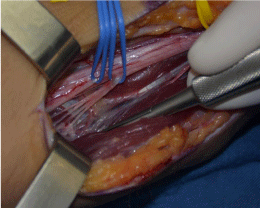
Figure 2: Microdissection of the 4 motor rami to the flexor carpi radialis atr the
neuro-muscular junction, immediately before they enter the muscle.
Post-operative care consists in a simple dressing for two weeks. Then gentle exercises of the involved muscles and joints are initiated. A temporary paresis of the target muscles is frequent during the first few weeks. Not unfrequently, this technique is associated with other procedures (tendon lengthening, tendon transfers…), and the postoperative regimen will vary according to the requirements of these other procedures.
Indications
Selective neurectomy is indicated when one wishes to reduce spasticity permanently. Resection of the motor fibres usually includes 2/3 of the fascicles, knowing that it will reduce the strength of the muscle in the same proportion as spasticity.
It has no effect on muscle or joint contractures; muscle contractures should be addressed by muscle or tendon lengthening, and joint contractures by arthrolysis, bone shortening or osteotomies. Neither is it effective on true paralysis of the antagonist muscles, which must be addressed by reconstructive procedures (tendon transfers). But, as it reduces the tone of the spastic agonist muscles, it may allow a previously weak antagonist to become effective by restoring the balance.
More general contra-indications of surgery in spasticity of the upper limb also apply to this technique: dystonia or predominant abnormal movements. Cognitive problems are not necessarily a contra-indication to this technique, as long as the patient can cope with a surgical procedure.
The postoperative regimen includes a dressing for 12 to 15 days, then return to the usual physiotherapy routine. The neurectomized muscles as well as the antagonist muscles are exercised ideally three times a week.
Results
Our earlier experience with this technique in the previous years had been globally satisfactory, although at times unpredictable. Following our recent anatomic studies, we have adjusted our skin incisions in order to include for certain all branches of the motor nerves and perform a truly hyperselective neurectomy. We report our prospective results following this modification. Between November 2013 and October 2015, 20 patients including 11 adults and 9 children have undergone 22 HSN. There were 14 males and 6 females; ages ranged from 12 to 59y.o. (average 33y.o). The cause of spasticity was cerebral palsy in nine, stroke in eight, head injury in two, and brain tumour in one patient. Patient selection was based on repeated clinical examination, and the results of pre-operative botulinum toxin injections. All patients had previous medical treatment for the spasticity: medications, physical and occupational therapy, orthoses as needed, and botulinum toxin injections (1 to 12).
Pre and postoperative assessment was based on spontaneous limb position, active and passive motion, and muscle strength (MRC scale). Spasticity was evaluated with the modified Ashworth and the Tardieu scales. Functional results were evaluated with the House score and an ADL questionnaire. Patient satisfaction was rated according to the VAS scale.
HSN involved 63 muscles (11 biceps, 11 brachialis, 4 brachioradialis, 10 FCR, 8 FCU, 5 PL, 7 pronator teres, 2 subscapularis, 2 teres minor, 2 supraspinatus and 2 infraspinatus, and 1 pectoralis major), amounting to 2.9 neurectomies per surgery. Associated procedures were performed simultaneously in 11 cases, including 24 muscle lengthening, one first web opening and one interosseous membrane release.
There were two postoperative complications: one hematoma in a patient treated by Coumadin, and a complete failure of the procedure (HSN of the infra-spinatus) related to a technical problem.
After a mean follow-up of 10 months (1.5-20), spontaneous position, which was recorded for the elbows, improved on average from 97° to 40°, active motion of the involved joints improved by 19, 4 degrees and passive motion by 15 degrees. Spasticity decreased from 1.9 to 0.4 on the Ashworth scale, and from 45.6 to 8,5 on the Tardieu scale (V1-V3). Loss of strength was minimal, from 4.1 to 3.8. The House functional score increased from 2 to 3.1. Patient satisfaction averaged 6.9 /10. In the six cases with a follow up longer than 12 months (average 17.5 months), results have remained stable over time.
Discussion
Favourable results have been reported after partial neurectomies in the upper limb [29-39].
However there is little consensus on the technique itself, its indications, and even the results. We performed a literature search [40] in order to analyse the different techniques of partial neurectomy in the upper limbs of spastic patients and their results. We were able to retrieve 14 articles which met our inclusion criteria, amounting to 425 cases of partial neurectomy.
The terminology was confusing in 5 articles, where the term partial neurectomy (or neurotomy) described resection of the whole of the motor branch with conservation of the sensory branch (1 musculo-cutaneous nerve, 4 terminal motor branches of the ulnar nerve in the hand).
The technique varied: some authors, following Brunelli’s original description, follow the motor branches until they enter the muscle, and perform the partial neurectomy at this level [35,37]. Others, in order to simplify the procedure, have chosen to perform a partial neurectomy at the level of the main nerve trunk, without approaching the target muscle(s). The motor fascicles are identified proximally using a stimulator and partially resected [4,34,38-39]. While faster and more limited in exposure, this technique is less accurate, with possible errors in target muscles, possible insufficient reduction of spasticity by missing some branches, and potential injury to sensory fibres [4].
Some authors coagulate the proximal stump to avoid nerve regrowth, or put a metal clip [29], but others don’t, fearing to damage the remaining fibers.
And even in those who follow Brunelli’s original technique, it often appears through a critical analysis of their material (operative description and photographs) that incisions were often quite small, compared to the branching pattern of the motor nerves, and that some motor branches may have been missed [40]. From our cadaveric studies [5-7], it appears that skin incisions should be longer than generally reported in the literature. As for the FDS and FDP, our findings suggest that HSN is not feasible without harming the muscles, in contradiction with Brunelli’s experience [3].
The amount of fibres resected varies in this literature review from 33 to 80%. Some authors decide on the amount of neurectomy depending on the Ashworth score [35] whereas others rely on the response to peroperative stimulation after resection of 50% fibres [37]. One must keep in mind in mind that excessive resection might cause paralysis.
Brunelli initially advocated removing 50% of the fascicles, but after experiencing some recurrence of the spasticity, he recommended resection of a larger amount of fibres. He explained this recurrence by what he termed the “adoption” phenomenon, describing the adoption of orphan muscle fibers by the remaining nerve branches, equivalent to the“sprouting” phenomenon described by others [41- 42], rather than a nerve regrowth from the transected stumps. He also recommended to plan a second neurectomy 6 months later in cases of recurrence, which he states he performed in 90% cases [3]. However stable results without the need for a “second look”have been reported by several authors, [29,37]. Maarrawi reports 5% recurrence at 1 year [35]. Our single case of recurrence was probably due to a technical error.
Finally, results were difficult to analyze in terms of function, because out of 14 series, 9 involved mainly nonfunctional hands (65 to 100% of cases), and the neurectomy was often performed in case of failure of all other treatments.
After extensive cadaver studies, we have been able to develop guidelines for a hyperselective neurectomy of the major motor nerves of the upper limb. A prospective study of 22 HSN has shown significant reduction of spasticity quantified by current tools, with a minimal non-significant loss of strength. Except for one technical failure, the goal of surgery, whether functional, nursing or cosmesis, has been attained in all cases.
Conclusion
Surgery is only one element of the rehabilitative care of CP patients, which includes primarily physiotherapy and splinting, occupational therapy, and pharmacological treatment as needed. A careful clinical examination and local chemodenervation agents (BT) are required in order to select the proper candidates for partial neurectomy.
Although spasticity is difficult to evaluate, our results of the technique of hyperselective neurectomy in spasticity of the upper limb have been promising, showing effective reduction of spasticity and improved motion, with minimal loss of strength, and some functional gain. These results have remained stable over time in the middle term.A longer follow-up will be necessary in order to evaluate the long term outcomes of this procedure.
References
- Koman LA, Smith BP, Shilt JS. Cerebral palsy. Lancet. 2004; 363: 1619-1631.
- Stoffel A. Treatment of spastic contractures. AmJ Ort Surg. 1913; 210: 611.
- Brunelli G, Brunelli F . Partial selective denervation in spastic palsies (hyponeurotization). Microsurgery. 1983; 4: 221-224.
- Decq P, Shin M, Carrillo-Ruiz J. Surgery in the Peripheral Nerves for Lower Limb Spasticity. Oper Tech Neurosurg. 2005; 7: 136-140.
- Cambon-Binder A, Leclercq C. Anatomical study of the musculocutaneous nerve branching pattern: application for selective neurectomy in the treatment of elbow flexors spasticity. Surg Radiol Anat. 2015; 37: 341-348.
- Paulos R, Leclercq C. Motor branches of the ulnar nerve to the forearm: an anatomical study and guidelines for selective neurectomy. Surg Radiol Anat. 2015; 37: 1043-1048.
- Parot C, Leclercq C. Anatomical study of the motor branches of the median nerve to the forearm and guidelines for selective neurectomy. Surg Radiol Anat. 2015; .
- Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther. 1987; 67: 206-207.
- TARDIEU G, SHENTOUB S, DELARUE R. Research on a technic for measurement of spasticity. Rev Neurol (Paris). 1954; 91: 143-144.
- Boyd RN, Barwood SA, Ballieu C, Graham HK. Validity of a clinical measure of spasticity in children with cerebral palsy in a double blinded randomised controlled clinical trial. Dev Med Child Neurol. 1998; 40: 7.
- Mackey AH, Walt SE, Lobb G, Stott NS. Intraobserver reliability of the modified Tardieu scale in the upper limb of children with hemiplegia. Dev Med Child Neurol. 2004; 46: 267-272.
- Yam WK, Leung MS. Interrater reliability of Modified Ashworth Scale and Modified Tardieu Scale in children with spastic cerebral palsy. J Child Neurol. 2006; 21: 1031-1035.
- Patrick E, Ada L. The Tardieu Scale differentiates contracture from spasticity whereas the Ashworth Scale is confounded by it. Clin Rehabil. 2006; 20: 173-182.
- Gracies JM, Burke K, Clegg NJ, Browne R, Rushing C, Fehlings D, et al. Reliability of the Tardieu Scale for assessing spasticity in children with cerebral palsy. Arch Phys Med Rehabil. 2010; 91: 421-428.
- Braun RM, Hoffer MM, Mooney V, McKeever J, Roper B. Phenol nerve block in the treatment of acquired spastic hemiplegia in the upper limb. J Bone Joint Surg Am. 1973; 55: 580-585.
- Roper B. Evaluation of spasticity. Hand. 1975; 7: 11-14.
- Fitoussi F, Diop A, Maurel N, Laassel el M, Penneçot GF. Kinematic analysis of the upper limb: a useful tool in children with cerebral palsy. J Pediatr Orthop B. 2006; 15: 247-256.
- Jaspers E, Desloovere K, Bruyninckx H, Molenaers G, Klingels K, Feys H. Review of quantitative measurements of upper limb movements in hemiplegic cerebral palsy. Gait Posture. 2009; 30: 395-404.
- Leclercq C, Xarchas C. Kienböck's disease in cerebral palsy. J Hand Surg Br. 1998; 23: 746-748.
- World Health Organization (WHO). International Classification of Functioning, Disability and Health. Geneva: World Health Organization. 2001.
- Klingels K, Jaspers E, Van de Winckel A, De Cock P, Molenaers G, Feys H. A systematic review of arm activity measures for children with hemiplegic cerebral palsy. Clin Rehabil. 2010; 24: 887-900.
- Chung CY, Chen CL, Wong AM. Pharmacotherapy of spasticity in children with cerebral palsy. J Formos Med Assoc. 2011; 110: 215-222.
- Koman LA, Mooney JF 3rd, Smith BP, Goodman A, Mulvaney T. Management of spasticity in cerebral palsy with botulinum-A toxin: report of a preliminary, randomized, double-blind trial. J Pediatr Orthop. 1994; 14: 299-303.
- Fehlings D, Rang M, Glazier J, Steele C. An evaluation of botulinum-A toxin injections to improve upper extremity function in children with hemiplegic cerebral palsy. J Pediatr. 2000; 137: 331-337.
- Autti-Rämö I, Larsen A, Taimo A, von Wendt L. Management of the upper limb with botulinum toxin type A in children with spastic type cerebral palsy and acquired brain injury: clinical implications. Eur J Neurol. 2001; 8 Suppl 5: 136-144.
- Buffenoir K, Decq P, Lefaucheur JP. Interest of peripheral anesthetic blocks as a diagnosis and prognosis tool in patient with spastic equines foot: a clinical and electrophysiological study of the effect of block of nerve branches to the triceps surae muscle. Clin Neurophysiol. 2005; 116: 1596–1600.
- Hodgkinson I, Sindou M. Decision-Making for Treatment of Disabling Spasticity in Children. Oper Tech Neurosurg. 2005; 7: 120-123.
- Leclercq C. Prévention et traitement des troubles orthopédiques. Chirurgie du membre supérieur de l’enfant IMC. Motricitécérébrale. 2013; 34: 97–102.
- Reddy S, Puligopu AK, Purohit AK. Results of selective motor fasciculotomy in spastic upper limbs due to cerebral palsy (a review of 30 children and adults). Indian J CerebPalsy. 2015; 1: 21-27.
- Cahuzac M, Claverie P, Nichil J. L’enfant infirme moteur d’originecérébrale. Paris: Masson. 1980.
- Garland DE, Thompson R, Waters RL. Musculocutaneous neurectomy for spastic elbow flexion in non-functional upper extremities in adults. J Bone Joint Surg Am. 1980; 62: 108-112.
- Msaddi AK, Mazroue AR, Shahwan S, al Amri N, Dubayan N, Livingston D, et al. Microsurgical selective peripheral neurotomy in the treatment of spasticity in cerebral-palsy children. Stereotact Funct Neurosurg. 1997; 69: 251-258.
- Decq P, Filipetti P, Feve A, Djindjian M, Saraoui A, Kéravel Y. Peripheral selective neurotomy of the brachial plexus collateral branches for treatment of the spastic shoulder: anatomical study and clinical results in five patients. J Neurosurg. 1997; 86: 648-653.
- Purohit AK, Raju BS, Kumar KS, Mallikarjun KD. Selective musculocutaneous fasciculotomy for spastic elbow in cerebral palsy: a preliminary study. Acta Neurochir (Wien). 1998; 140: 473-478.
- Maarrawi J, Mertens P, Luaute J, Vial C, Chardonnet N, Cosson M, et al. Long-term functional results of selective peripheral neurotomy for the treatment of spastic upper limb: prospective study in 31 patients. J Neurosurg. 2006; 104: 215-225.
- Sindou MP, Simon F, Mertens P, Decq P. Selective peripheral neurotomy (SPN) for spasticity in childhood. Childs Nerv Syst. 2007; 23: 957-970.
- Buffenoir K, Rigoard P, Ferrand-Sorbets S, Lapierre F. Étude rétrospective du résultat à long terme des neurotomies sélectives périphériques dans le traitement du membre supérieur spastique. (Retrospective study of the long-term results of selective peripheral neurotomy for the treatment of spastic upper limb). Neurochirurgie. 2009; 55: S150–S160.
- Puligopu AK, Purohit AK. Outcome of selective motor fasciculotomy in the treatment of upper limb spasticity. J Pediatr Neurosci. 2011; 6: S118-125.
- Sitthinamsuwan B, Chanvanitkulchai K, Phonwijit L, Nunta-Aree S, Kumthornthip W, Ploypetch T. Surgical outcomes of microsurgical selective peripheral neurotomy for intractable limb spasticity. Stereotact Funct Neurosurg. 2013; 91: 248-257.
- Gras M, Cambon-Binder A, Paulos R, Parot C, Leclercq C. Neurectomy in upper limb spasticity: Review of the literature in light of recent anatomical studies. Eur J Hand Surg. 2016; in press.
- Dengler R, Konstanzer A, Hesse S, Schubert M, Wolf W. Collateral nerve sprouting and twitch forces of single motor units in conditions with partial denervation in man. Neurosci Lett. 1989; 97: 118-122.
- Einsiedel LJ, Luff AR. Activity and motor unit size in partially denervated rat medial gastrocnemius. J Appl Physiol (1985). 1994; 76: 2663-2671.