
Review Article
Austin J Plant Biol. 2018; 4(1): 1019.
How Auxin May Contribute to the Regulation of Plant Defense Responses against Herbivory
Pérez-Alonso MM* and Pollmann S
Centro de Biotecnología y Genómica de Plantas (UPM - INIA), Spain, Universidad Politécnica de Madrid (UPM), Spain
*Corresponding author: Pérez-Alonso MM, Centro de Biotecnología y Genómica de Plantas, Universidad Politécnica de Madrid (UPM)-Instituto Nacional de Investigación y Tecnología Agraria y Alimentación (INIA), Pozuelo de Alarcón, Spain
Received: April 16, 2018; Accepted: August 31, 2018; Published: September 07, 2018
Abstract
Numerous studies have indicated that investing in defense usually comes at the expense of plant growth. A phenomenon known as the “growth-defense tradeoff”. Nevertheless, recent research puts emphasize on the fact that the degree of cooperation and crosstalk between phytohormones is of key importance in driving plant responses to their ever-changing environment. In this context, the growth hormone indole-3-acetic acid, the most common auxin in the plant kingdom, makes no exception. Several lines of evidence indicate that the relationship between auxin and jasmonates is of particular relevance for the control of plant defenses responses. In this review we discuss multiple levels at which auxin homeostasis can intimately regulate plant defense against biotic foes, paying special attention to responses towards phytophagous predators.
Keywords: Phytohormones; Phytophagous pathogens; Direct/indirect defense; Indole-3-acetic acid; Jasmonates
Abbreviations
RNAi: RNA interference
Auxin: An Overview of the Growth-Defense Dilemma
Excluding seed dispersion, the entire life cycle of higher plants proceeds anchored to a fixed place. Due to this fact, the rapid integration of environmental signals is of key importance to develop effective mechanisms to cope with changes including the defense against abiotic and biotic stresses [1,2]. Plant hormones or phytohormones, are small chemical messengers derived from secondary metabolism that take center stage in the integration and translation of environmental demands into physical plant responses [3,4]. The major hormones produced by plants include the “classical” five, i.e. auxins, gibberellins (GA), cytokinins (CK), abscisic acid (ABA), ethylene (ET), and the relatively new compounds salicylic acid (SA), jasmonates, brassinosteroids (BR) and strigolactones [5- 7]. Regarding their function, auxins, BR, CK, GA and strigolactones are known to play decisive roles in the orchestration of growth and development [8]. In contrast, ABA, SA, jasmonates and ET are considered to play crucial roles in mediating plant defense responses against pathogens and abiotic stresses, such as drought, light, salinity, or high temperatures [6,9]. Historically, it has been assumed that these two functional blocks act antagonistically and, therefore, growth and defense-related hormones work in separate modules. Supporting this hypothesis, jasmonic acid (JA) and its metabolites, collectively known as jasmonates (JAs), are plant growth inhibitors, mainly involved in counteracting stress against herbivore and pathogen attack [10-12]. In vivo-analyses demonstrated that induction of endogenous JAs in Arabidopsis is sufficient to impair primary root growth and leaf expansion [13]. In leaves, JAs exert their growth inhibitory effect through the suppression of mitosis, by arresting the cell cycle in G1 prior to the S transition [14]. In contrast to the growth inhibitory effect triggered by JAs, indole-3-acetic acid (IAA) constitutes the most important plant growth hormone [15-18]. In agreement with the antagonistic relationship between growth and defense, Mutka and co-workers [19] demonstrated that elevation of the endogenous content of IAA, driven by the constitutive overproduction of the Auxin biosynthetic gene YUC1, increased the susceptibility of the Arabidopsis mutant against the plant pathogen Pseudomonas syringae. Moreover, it has been observed that inactivation of IAAmediated processes, i.e. cell expansion and plant cell wall relaxation, activates Nicotiana attenuata defense in response to the herbivore Manduca sexta oral secretion elicitation [10]. Nonetheless, despite all this insight suggesting a negative impact of auxin in plant defense, a number of recent studies have called this assumption into question. For example, it has been observed that several herbivory insects are capable of manipulating IAA biosynthesis to induce abnormal tissue formation, such as galls [20]. In view of these evidences, we specifically try to summarize and discuss recent findings concerning the contribution of auxin in the regulation of plant defense against phytophagous predators in this mini-review. Furthermore, we try to highlight new molecular targets for plant bioengineering that may facilitate increased crop resistance against herbivore foes. The intricacies of IAA biosynthesis and signaling will not be considered here, and readers interested in this aspect should consult the recent reviews on this topic [15,16,21,22].
Direct Plant Defense Activates Auxin Biosynthesis and Signalling
To understand plant defense responses against phytophagous pathogens we should establish two different levels of action, known as direct and indirect defense. Direct defenses involve all the appliances used by the host plant to counteract a specific attack from predators [23-27]. A good example of this is the mechanical protection of the plant surface by the development of spines, thorns and trichomes [28,29]. Focusing on leaf trichomes, studies performed in several plant species have consistently demonstrated that the formation of these structural barriers is controlled by the combined action of JA, GA, and CK [30-33]. Interestingly, Auxin Response Factors (ARFs), which are key-components of auxin signaling [34,35], have been described to be required for this process. Fahlgren et al. [36] reported that an increased accumulation of ARF3 transcripts promote the formation of abaxial tracheas in Arabidopsis thaliana. Likewise, in tomato (Solanum lycopersicum) is has been observed that SlARF3 down-regulation by applying a RNAi approach significantly reduced the trichome density in leaves [37]. On the other hand, plants also limit phytophagous attacks by increasing leaf rigidity and stem strength through the lignification of their cell walls. In this regard, wounding and JA have been associated with lignin biosynthesis through the transcriptional regulation of a series of woundinduced genes, as for example the CAFFEIC ACID-O-METHYL TRANSFERASE (COMT) [38], which encodes an enzyme implicated in the synthesis of G-lignin [39]. Alternatively, quite some time ago, Vanholme et al. [40] reported that auxin stimulation induced lignin formation in the secondary xylem of Coelus blumei. In Arabidopsis, the constitutive overexpression of YUC8 and YUC9, two key enzymes in IAA biosynthesis, has been reported to translate into enhanced lignification [41]. Further underlining the role of increased IAA levels, the tobacco auxin overproducer mutant 35S-iaaM/iaaH has been described to show increased peroxidase gene transcription levels [42]. Peroxidases are known to be involved in the polymerization of lignin monomers [39]. Thus, it appears tempting to speculate that JA-IAA crosstalk can mediate lignin biosynthesis in response to challenges by biotic foes. The positive interplay between mechanical defense and auxin becomes also evident during the process of herbivore recognition. Perception of a predator challenge mainly occurs after egg deposition or feeding. For pea (Pisum sativum) it is known that ovoposition stimulates cell division and neoplasm formation at the egg location, with the intention of impeding the entry of larvae [43]. In agreement with this, the positive effect of auxin in cell division has been well documented [15,44]. Moreover, experiments with the gall-inducing caterpillar Gnorimochema gallaesolidaginis showed a significant increment of IAA contents in galls. Thus, although further elucidation is required, it can be hypothesized that egg perception can activate auxin biosynthesis in the host plant. On the other hand, phytophagous predator herbivory commonly entails disruption of plant tissue integrity. It is well characterized that after wounding or oral elicitation the production of the bioactive JA, jasmonoyl-Lisoleucine (JA-Ile), is stimulated [45]. Subsequently, synthetized JAIle is perceived by the F-box protein CORONATINE INSENSITIVE 1 (COI1) [46]. As shown in (Figure 1A), the perception of JA-ILE enables the SCFCOI1 complex to bind and ubiquitinate specific repressor proteins, the so-called Jasmonate ZIM Domain (JAZ) family of transcription repressors. The JAZ repressors are, in turn, labeled for degradation by the 26S proteasome, thereby relieving MYC transcription factors from repression and triggering the expression or different subsets of JA-responsive genes [47-49]. Analogously, auxin perception shares a conserved signal transduction mechanism that also uses the 26S-proteasome apparatus (Figure 1B). Importantly, it has been observed that a point mutation of a SCF subunit of Arabidopsis resulted not only in reduced auxin response, but also in a diminished expression of several specifically JA-induced genes, indicating a reduction in JA sensitivity [50]. This evolutionary connection emphasized the value of the synergistic interaction between the two plant hormones, JA and IAA, for the fine-tuning of plant stress responses. As aforementioned, elicitation by oral secretion or wounding play prominent roles in herbivore attack perception. In relation to this, the polymer of N-acetyl-β-D-glucosamine, commonly termed chitin, is a structural component of insect and spider mite exoskeletons, as well as fungal cell walls and nematode egg [51-54]. However, it has never been reported in plant cell walls. Chitin is a recognized elicitor of plant defense responses [53,55,56], being an important activator of JA-signaling [57]. Recently, Lopez-Moya et al. [58] demonstrated that chitosan, a deacetylated form of chitin, which is widely used in agriculture, stimulates the production of auxin through the modulation of two auxin biosynthetic genes, YUC2 and AMI1, in A. thaliana. Remarkably, chitosan also induces the expression of MYC2. In addition, Hentrich et al. [59] further reported the induction of two IAA biosynthetic genes, YUC8 and YUC9, after the application of several oxylipins. In their work the authors also described the activation of YUC9 after mechanical wounding. This observation has recently been confirmed [60], showing the activation of YUC8 after Plutella xylostella larvae attack in A. thaliana. YUC8 induction was accompanied by an elevation of IAA levels in the place of damage, but also in distal parts, such as roots. Therefore, further elucidation of the molecular mechanism underlying auxin transport upon herbivory attack will help to shed light onto the role of auxin in tolerance against pathogens. In the same way, the exposition of N. attenuata plants to M. sexta herbivory leads to a significant increase of IAA levels in leaves and roots of attacked plants [61]. In a later study, Machado et al. [62] reported that the M. sexta-mediated increase of IAA in N. attenuata correlates with a rapid induction of several YUC-like genes. Additionally, the authors demonstrated that the simultaneous application of IAA and methyl jasmonte (MeJA) induces the production of anthocyanin in N. attenuate, which is believed to acts as a chemical repellent [63]. This observed induction was further reproduced under real or simulated M. sexta attack using A. thaliana.
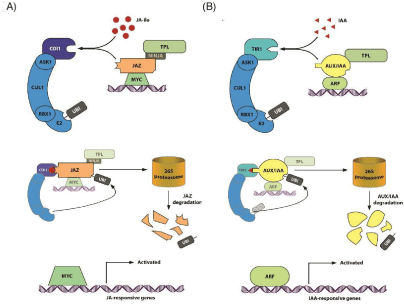
Figure 1: Comparison between JA and IAA signaling in Arabidopsis A) At high levels, the bioactive jasmonate JA-Ile is perceived by the F-box receptor
CORONATINE INSENSITIVE1 (COI1). The SCFCOI1-E3 ligase multiprotein complex, including COI1, ARABIDOPSIS S-PHASE KINASE PROTEIN1 (ASK1),
CULLIN1 (CUL1), and RING-BOX1 (RBX1), mediates the ubiquitination and degradation of the JASMONATE ZIM DOMAIN (JAZ) transcriptional repressors
through the 26S proteasome, thereby releasing the repressor COMPLEX-NINJA-TOPLESS and the MYC family of transcriptional factors. This culminates with
the activation of the JA-responsive genes. B) TRANSPORT INHIBITOR RESPONSE1 (TIR1) and AUXIN SIGNALING F-BOX1-4 (AFB), are F-box proteins that
function as auxin receptors. TIR1 is known to form part of the SCF protein complex, which comprises the ASK1, CUL1, and RBX1. In presence of biological levels
of IAA, the bioactive hormone is bound to the bottom of a cavity located at the surface of the TIR1 receptor. After binding, the affinity of TIR1 for the AUX/INDOLE-
3-ACETIC ACID (Aux/IAA) transcriptional repressors increases. Subsequently, the SCFTIR1-E3 ligase mediates the ubiquitination of the bound Aux/IAA protein
and their degradation via the 26S proteasome. The ubiquitin mediated proteolysis of Aux/IAA results in the liberation of the co-repressor TOPLESS and the Auxin
Responsive Factor (ARFs), thereby, permitting ARFs to control the transcription of auxin responsive genes.
A Role of Auxin in Indirect Defense
Indirect defense is based on the capability of attacked plants to emit volatile organic compounds (VOCs), such as terpenoids, glucosinolates, fatty acid derivatives and ET, to attract natural enemies of herbivores [23-25]. A broad number of genes relates to VOC production are induced by JAs [26]. However, earlier studies indicated that IAA is capable of stimulating ET production through the activation of specific ACC-synthase genes, which encode enzymes involved in a rate- limiting step in this process [64-66]. In Arabidopsis, it has been demonstrated that the overproduction of auxin mediated by YUC8 and YUC9 likely results in increased ET production, as these lines are less sensitive to the ET biosynthesis inhibitor 2-aminoisobutyric acid [41]. Thus, it may be concluded that a hormonal cascade employing JA, IAA, and ET contributes to plant defense responses. The main role of VOC emission is to prepare (prime) distal tissues or neighboring plants for a possible imminent attack. Such a VOC-mediated induction of the defense machinery can provide substantial advantages to responding plants, providing time for the production a battery of secondary metabolites to deter potential aggressors. Interestingly, the chemical communication between plants seemingly involves the canonical auxin perception pathway, too. Sweeney et al. [67] reported that mechanical wounding stimulates both root growth and the activation of auxin signaling, as analyzed by using the DR5: GUS reporter line, in neighboring unwounded A. thaliana plants.
Conclusion
Taking all these observation into account, we conclude that although elicitation of the JA-mediated response is the primary event, reconfiguration and adjustment, respectively, of IAA biosynthesis and signaling is likely to contribute to direct/indirect plant resistance against herbivory (Figure 2). However, many questions still remain elusive and require more detailed investigations in the future.
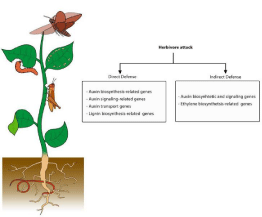
Figure 2: Summary of IAA-related targets involved in tolerance against herbivore foes. The figure contrasts the different auxin-related modules activated in direct
and indirect plant responses.
Acknowledgement
The work was supported in by grants from the Spanish Ministry of Economy, Industry and Competitiveness (MINECO) (BFU2014- 55575-R, PCIN-2016-037 to S.P.).
References
- Depuydt S, Hardtke CS. Hormone signalling crosstalk in plant growth regulation. Curr Biol. 2011; 21: R365-R373.
- Hoffmann M, Hentrich M, Pollmann S. Auxin-Oxylipin Crosstalk: Relationship of Antagonists. J Integr Plant Biol. 2011; 53: 429-445.
- Santner A, Estelle M. Recent advances and emerging trends in plant hormone signalling. Nature. 2009; 459: 1071-1078.
- Kazan K. Auxin and the integration of environmental signals into plant root development. Ann Bot. 2013; 112: 1655-1665.
- Kende H, Zeevaart J. The Five “Classical” Plant Hormones. Plant Cell. 1997; 9: 1197-1210.
- Bari R, Jones JDG. Role of plant hormones in plant defence responses. Plant Mol Biol. 2009; 69: 473-488.
- Verma V, Ravindran P, Kumar PP. Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 2016; 16: 86.
- Pozo MJ, López-Ráez JA, Azcón-Aguilar C, García-Garrido JM. Phytohormones as integrators of environmental signals in the regulation of mycorrhizal symbioses. New Phytol. 2015; 205: 1431-1436.
- De Bruyne L, Höfte M, De Vleesschauwer D. Connecting growth and defense: The emerging roles of brassinosteroids and gibberellins in plant innate immunity. Mol Plant. 2014; 7: 943-959.
- Onkokesung N, Gális I, Von Dahl CC, Matsuoka K, Saluz HP, Baldwin IT. Jasmonic Acid and Ethylene Modulate Local Responses to Wounding and Simulated Herbivory in Nicotiana Attenuata Leaves. Plant Physiol. 2010; 153: 785-798.
- Antico CJ, Colon C, Banks T, Ramonell KM. Insights into the role of jasmonic acid-mediated defenses against necrotrophic and biotrophic fungal pathogens. Front Biol. 2012; 7: 48-56.
- Studham ME, Macintosh GC. Phytohormone signaling pathway analysis method for comparing hormone responses in plant-pest interactions. BMC Res. 2012; 5: 392.
- Zhang Y, Turner JG. Wound-Induced Endogenous Jasmonates Stunt Plant Growth by Inhibiting Mitosis. PLoS One. 2008; 3: e3699.
- Noir S, Bömer M, Takahashi N, Ishida T, Tsui TL, Balbi V, et al. Jasmonate Controls Leaf Growth by Repressing Cell Proliferation and the Onset of Endo Reduplication While Maintaining a Potential Stand-By Mode. Plant Physiol. 2013; 161: 1930-1951.
- Woodward AW, Bartel B. Auxin: Regulation, Action, and Interaction. Ann Bot. 2005; 95: 707-735.
- Abel S, Theologis A. Odyssey of auxin. Cold Spring Harb. Perspect Biol. 2010; 2: 1-13.
- Zhao Y. Auxin biosynthesis and its role in plant development. Annu Rev Plant Biol. 2010; 61: 49-64.
- Mano Y, Nemoto K. The pathway of auxin biosynthesis in plants. J Exp Bot. 2012; 63: 2853-2872.
- Mutka AM, Fawley S, Tsao T, Kunkel BN, Louis S. Auxin promotes susceptibility to Pseudomonas syringae via a mechanism independent of suppression of salicylic acid-mediated defenses. Plant J. 2013; 746-754.
- Suzuki H, Yokokura J, Ito T, Arai R, Yokoyama C, et al. Biosynthetic pathway of the phytohormone auxin in insects and screening of its inhibitors. Insect Biochem Mol Biol. 2014; 53: 66-72.
- Lavy M, Estelle M. Mechanism of auxin signaling. Development. 2016; 143: 3226-3229.
- Zhao Y. Essential roles of local auxin biosynthesis in plant development and in adaptation to environmental changes. Annu Rev Plant Biol. 2018; 69: 12.1- 12.19.
- Muroi A, Ramadan A, Nishihara M, Yamamoto M, Ozawa R, Junji T. The Composite Effect of Transgenic Plant Volatiles for Acquired Immunity to Herbivory Caused by Inter-Plant Communications. PLoS One. 2011; 6: 1-8.
- Erb M, Meldau S, Howe GA. Role of phytohormones in insect-specific plant interaction. Trends Plant Sci. 2012; 17: 250-259.
- Mithöfer A, Boland W. Plant defense against herbivores: chemical aspects. Annu Rev Plant Biol. 2012; 63: 431-450.
- War AR, Paulraj MG, Ahmad T, Buhroo AA, Hussain B, Ignacimuthu S, et al. Mechanisms of plant defense against insect herbivores. Plant Signal Behav. 2012; 7: 1306-1320.
- Schuman MC, Baldwin IT. The Layers of Plant Responses to Insect Herbivores. 2015; 373-394.
- Wu J, Baldwin IT. Herbivory-induced signalling in plants: perception and action. Plant Cell Environ. 2009; 1161-1174.
- Hanley ME, Lamont BB, Fairbanks MM, Rafferty CM. Plant structural traits and their role in anti-herbivore defence. Perspect Plant Ecol Syst. 2007; 8: 157-158.
- Traw MB, Bergelson J. Interactive Effects of Jasmonic Acid, Salicylic Acid, and Gibberellin on Induction of Trichomes in Arabidopsis. Plant Physiol. 2003; 133: 1367-1375.
- Boughton AJ, Hoover K, Felton GW. Rapid communication methyl jasmonate application induces increased densities of glandular trichomes on tomato, Lycopersicon esculentum. J Chem Ecol. 2005; 31: 2211-2216.
- Maes l, Goossens A. Hormone-mediated promotion of trichome initiation in plants is conserved but utilizes species- and trichome-specific regulatory mechanisms. Plan Signal, Behav. 2010; 5: 205-207.
- Pattanaik S, Patra B, Singh SK, Yuan. An overview of the gene regulatory network controlling trichome development in the model plant, Arabidopsis. Front Plant Sci. 2014; 5: 1-8.
- Teale WD, Paponov IA, Palme K. Auxin in action: signalling, transport and the control of plant growth and development. Nat Rev Mol Cell Biol. 2006; 7: 847-859.
- Santner A, Calderon-Villalobos LI, Estelle M. Plant hormones are versatile chemical regulators of plant growth. Nat Chem Biol. 2009; 5: 301-307.
- Fahlgren N, Montgomery TA, Howell MD, Allen E, Dvorak SK, Alexander AL, et al. Regulation of AUXIN RESPONSE FACTOR3 by TAS3 ta-siRNA affects develpmental timing and patterning in Arabidopsis. Curr Biol. 2006; 16: 939- 944.
- Zhang X, Yan F, Tang Y, Yuan Y, Deng W, LI Z. Auxin response gene SlARF3 plays multiple roles in tomato development and is involved in the formation of epidermal cell trichomes. PCP. 2015; 56: 2110-2124.
- Vélez-Bermúdez IC, Salazar-Henao JE, Fornalé S, López-Vidriero I, Franco- Zorrilla JM, Grotewold E, et al. A MYB/ZML complex regulates woundinduced lignin genes in maize. Plant Cell. 2015; 27: 3245-3259.
- Vanholme R, Demedts B, Morreel K, Ralph J, Boerjan W. Lignin biosynthesis and structure. Plant Physiol. 2010; 153: 895-905.
- Aloni R, Tollier MT, Monties B. The Role of Auxin and Gibberellin in Controlling Lignin Formation in Primary Phloem Fibers and in Xylem of Coleus Blumei Stems. Plant Physiol. 1990; 94: 1743-1747.
- Hentrich M, Sánchez-Parra B, Pérez Alonso MM, Carrasco Loba V, Carrillo l, Vicente-Carbajosa J, et al. YUCCA8 and YUCCA9 overexpression reveals a link between auxin signaling and lignification through the induction of ethylene biosynthesis. Plant Signal Behav. 2013; 8: 7-10.
- Huang WN, Liu HK, Zhang HH, Chen Z, Guo YD, Kang YF. Ethylene-induced changes in lignifications and cell wall-degrading enzymes in the roots of mungbean (Vigna radiata) sprouts. Plant Physiol Biochem. 2013; 73: 412- 419.
- Doss RP, Oliver JE, Proebsting WM, Potter SW, Kuy, Clement RT, et al. Bruchins: insect-derived plant regulators that stimulate neoplasm formation. Porc Nat Acad Sci USA. 2000. 23; 97: 6218-6223.
- Perrot-Rechenmann C. Cellular Responses to Auxin: division versus expansion. Cold Spring Harb Perspect. Biol. 2010; 2: a001446.
- Huot B, Yao J, Montgomery B, He SY. Growth-Defense Tradeoffs in Plants: A Balancing Act to Optimize Fitness Mol. Plant. 2014; 7: 1267-1287.
- Chini A, Fonseca S, Fernández G, Adie B, Chico JM, Lorenzo O, et al. The JAZ family of repressors is the missing link in jasmonate signalling. Nature. 2007; 448: 666-671.
- Kazan K, Manners JM. Jasmonate signaling: Toward an Integrated View. Plant Physiol. 2008; 146: 1459-1468.
- Chini A, Boter M, Solano R. Plant oxylipins: COI1/JAZs/MYC2 as the core jasmonic acid-signalling module. FEBS J. 2009; 276: 4682-4692.
- Fernandez-Calvo P, Chini A, Fernandez-Barbero G, Chico JM, Gimenez- Ibanez S, Geerinck J, et al. The Arabidopsis bHLH Transcription Factors MYC3 and MYC4 Are Targets of JAZ Repressors and Act Additively with MYC2 in the Activation of Jasmonate Responses. Plant Cell. 2011; 23: 701- 715.
- Ren C, Pan J, Peng W, Genschik P, Hobbie L, Hellmann H, et al. Point mutations in Arabidopsis Cullin1 reveal its essential role in jasmonate response. Plant J. 2005; 42: 514-524.
- Punja ZK, Zhang YY. Plant Chitinases and Their Roles in Resistance to Fungal Diseases. J Nematol. 1993; 25: 526-540.
- Merzendorfer H, Zimoch L. Chitin metabolism in insects: structure, function and regulation of chitin synthases and chitinases. J Exp Biol. 2003; 206: 4393-4412.
- Son GH, Wan J, Kim HJ, Nguyen XC, Chung WS, Hong JC, et al. Ethylene- Responsive Element-Binding Factor 5, ERF5, is Involved in chitin-induced innate immunity response. Mol Plant Microbe Interact. 2012; 25: 48-60.
- Van Leeuwen T, Demaeght P, Osborne EJ, Dermauw W, Gohlke S, Nauen R, et al. Population bulk segregant mapping uncovers resistance mutations and the mode of action of a chitin synthesis inhibitor in arthropods. Proc Natl Acad Sci USA. 2012; 109: 4407-4412.
- Miya A, Albert P, Shinya T, Desaki Y, Ichimura K, Shirasu K, et al. CERK1, a LysM receptor kinase, is essential for chitin elicitor signaling in Arabidopsis. Proc Natl Acad Sci USA. 2007; 104: 19613-19618.
- Iizasa E, Mitsutomi M, Nagano Y. Direct binding of a plant LysM receptorlike kinase, LysM RLK1/CERK1, to chitin in vitro. J Biol Chem. 2010; 285: 2996-3004.
- Turner JG, Ellis C, Devoto A. The jasmonate signal pathway. The Plant Cell. 2002; S153-S164.
- Lopez-Moya F, Escudero N, Zavala-Gonzalez EA, Esteve-Bruna D, Blázquez MA, Alabadí D, et al. Induction of auxin biosynthesis and WOX5 repression mediate changes in root development in Arabidopsis exposed to chitosa. Sci Rep. 2017; 7: 16813.
- Hentrich M, Böttcher C, Düchting P, Cheng Y, Zhao Y, Berkowitz O, et al. The jasmonic acid signaling pathway is linked to auxin homeostasis through the modulation of YUCCA8 and YUCCA9 gene expression. Plant J. 2013; 74: 626-637.
- Yan S, Jiao C, Mclamore ES, Wang N, Yao H, Shen Y. Insect Herbivory of Leaves Affects the Auxin Flux Along Root Apices in Arabisopsis thaliana. J Plant Growth Regul. 2017; 36: 846-854.
- Machado RA, Ferrieri AP, Robert CA, Kallenbach M, Baldwin IT, Erb M. Leafherbivore attack reduces carbon reserves and regrowth from the roots via jasmonate and auxin signaling. New Phytol. 2013; 200: 1234-1246.
- Machado RAR, Robert CAM, Arce CCM, Ferrieri AP, Xu S, Jimenez-Aleman GH, et al. Auxin is rapidly induced by herbivore attack and regulates a subset of systemic, jasmonate-dependent defenses. Plant Physiol. 2016; 172: 521- 532.
- Lev-Yadun S, Gould KS. Role of Anthocyanins in Plant Defense. Anthocyanins. 2008; 2: 21-48.
- Jones JF, Kende H. Auxin-induced ethylene biosynthesis in subapical stem sections of etiolated seedlings of Pisum sativum L. Planta. 1979; 146: 649- 656.
- Yang SF, Hoffman NE. Ethylene Biosynthesis and its Regulation in Higher Plants. Annu Rev Plant Physiol. 1984; 35: 155-189.
- Abel S, Nguyen MD, Chow W, Theologis A. ASC4, a primary indoleacetic acid-responsive gene encoding1-aminocyclopropane-1-carboxylate synthase in Arabidopsis thaliana: Structural characterization, expression in Escherichia coli, and expression characteristics in response to auxin. J Biol Chem. 1995; 270: 19093-19099.
- Sweeney C, Lakshmanan V, Bais HP. Interplant Aboveground Signaling Prompts Upregulation of Auxin Promoter and Malate Transporter as Part of Defensive Response in the Neighboring Plants. Front Plant Sci. 2017; 8: 1-10.