
Research Article
Austin J Psychiatry Behav Sci. 2023; 9(1): 1092.
Environmental Enrichments Prevent Rats from Maternal Deprivation-Induced Depression through Regulating Hippocampal VEGF/Flk-Signaling
Mei Bai1,2*; Xin Zhang1#; Huanhuan Yan2#; Lu Yang1; Yang Yang1; Huijie Du1; Zhe Yan1
¹The First People’s Hospital of Guiyang, Guiyang, Guizhou 550001, China
²Guizhou Medical University, Guizhou, China
*Corresponding author: Mei Bai The First People’s Hospital of Guiyang, Guiyang, Guizhou 550001, China. Tel: 86-0851-88575658 Email: keaikoala @126.com.
#These authors have equally contributed to this article.
Received: May 13, 2023 Accepted: June 13, 2023 Published: June 20, 2023
Abstract
Background: Adverse events in early life can induce depression in adolescents. Environmental Enrichment (EE) has anti-depression effects in adulthood animals and humans. However, whether EE has effects on adolescent depression and the underlying mechanisms has not been documented.
Aim: To explore the effects of EE on Maternal Deprivation (MD)-induced adolescent depression and associated molecular and cellular mechanisms.
Methods: The animal model of adolescent depression was established with MD in newborn rats. Depressive-like behaviors were evaluated by sucrose preference test, forced swimming test, and invasion test. VEGF and Flk-1 mRNA and protein expression were measured by Real-time PCR and Western blot, respectively. Hippocampal cell proliferation was assessed by Bromodeoxyuridine (BrdU) incorporation and immunohistochemical staining of BrdU and neuronal nuclear protein (NeuN).
Results: MD significantly lowered sucrose preference rate, shortened the attack latency in invasion test, and increased the time of motionless while shortened the climbing time in the forced swimming test in rats compared to the control and control-EE rats. MD significantly decreased VEGF and Flk-1 mRNA and protein expression, and the number of positive BrdU and NeuN staining cells in the hippocampus of rats compared to the control and control-EE rats. EE decreased depressive-like behaviors, normalized VEGF and Flk-1 mRNA and protein expression, as well as increased positive BrdU and NeuN staining in the MD rats.
Conclusion: Maternal deprivation induced depressive-like behaviors in adolescent rats through negatively regulating VEGF and Flk-1 signaling and reducing hippocampal neurogenesis. Environmental enrichment can reduce the depressive-like behaviors through positive regulation of VEGF/Flk-1 signaling and subsequent neuron regeneration in the hippocampus.
Keywords: Early life stress; Adolescent depression; Maternal deprivation; Environmental enrichment; VEGF; Flk-1; Hippocampus; Neuronal regeneration
Introduction
Adolescent depression is a common and serious mental illness in adolescence, with a prevalence of about 4-8% [1]. It is reported that the suicide rate in adolescents with depression is higher than that in adulthood [2], while it is increasing in recent years [3]. However, most of antidepressants are not effective on adolescent depression. In contrast, antidepressants may increase the risk of suicide in adolescent patients [4]. Psychological interventions have become the first-line treatment of adolescent depression because of its small side effects. However, the involved neurobiological mechanisms of psychological interventions are still unclear.
Numerous studies have verified the impacts of Environmental Enrichment (EE) (a non-pharmaceutical intervention) on the neuron system of adulthood animals and humans [5-7]. Studies in SD rats have found that EE has anti-anxiety and anti-depression effects through regulating the inflammatory state in the rat brain [8]. Moreover, EE interventions can improve individual cognitive function and attenuate the establishment of fear condition [7,9]. It is also thought that EE can promote the morphology, structures, and functions of the neural circuits in the brain, and subsequently improve the cognitive functions, and behavioral responses [4,5,10]. The EE-caused changes in the brain include the increased neonatal neoplasia and synapses, neurotrophic factors, and volume and thickness of the cerebral cortex [7,11,12].
Previous studies have found that negative experiences in early life have adverse effects on the individual's brain functions and behaviors [13,14], in which negative events occurring before the age of 12 are mainly related to depression in adolescence [15]. Depression in adolescence is thought to be associated with the reduction in hippocampal volume [16] and synaptic density [17]. Vascular Endothelial Growth Factor (VEGF) is a highly conserved vasoactive growth factor with two types of receptors, including Fetal Kiver Kinase-1 (Flk-1), which is mainly found in the matured neurons, neural progenitor cells, and endothelial cells in the hippocampus of rodents [18,19]. Previous studies have demonstrated that VEGF-Flk-1 signaling pathway plays an important role in the process of chronic stress-induced depression phenotype and interventions of various antidepressants on the depression in adult individuals. For example, some studies have found that chronic stress can induce depression-like behaviors through down-regulating of the VEGF expression in the hippocampus of adult rats. Moreover, the increase of VEGF can relieve the inhibition of chronic stressors on neuronal regeneration in the hippocampus [20]. Intraventricular injection of VEGF can promote neuronal regeneration in the subcutaneous area of the hippocampus in adult rats [21,22], while injection of adeno-associated virus expressing VEGF into the hippocampus can stimulate neuronal regeneration. In contrast, reducing VEGF levels can inhibit neuronal regeneration in the hippocampus [23]. These above studies suggest that the activation of VEGF-Flk-1 signaling pathway in the hippocampus plays an important role in the emergence and improvement of adult depression phenotypes, but its role in the occurrence and development of adolescent depression needs further evidence.
In this study, the negative events in early life were established in rats by Maternal Deprivation (MD) and depression-like behaviors as well as VEGF/Flk-1 expression in the hippocampus were measured in adolescent rats. Recent studies suggest that stress induced loss of hippocampal neurons which may contribute to the pathophysiology of depression. This study therefore investigated the effect of EE on hippocampal neurogenesis in the adolescent rat, using the thymidine analog bromodeoxyuridine (BrdU) as a marker for dividing cells and neuronal nuclear protein (NeuN) as a specific neuronal marker.
Materials and Methods
Animals
The animal use protocol listed below has been reviewed and approved by the Animal Experimental Ethical Inspection Form of Guizhou Medical University. Sixty newborn male Sprague-Dawley rats (Slac Laboratory Animal Inc., Shanghai, China) were assigned into Maternal Deprivation (MD) group (n=30) and control group (n=30). In MD group, pups were separated from their mothers and placed in a single cell for 6 hours each day from 9:00 am to 3:00 pm and from PND 1 to PND 21. After 6 hours, pups were returned to their respective cages with their mothers. Rats in control group were housed with their mothers until PND21. On day-22, rats in the MD group were divided into depression group to receive routine housing from day-22 today-49 and depression-EE group to house under environmental enrichment (EE) from day-22 today-49. The rats in the control group were also divided into control group to receive routine hosing and control-EE group to house under EE from day-22 today-49. Briefly, in the control-EE and depression-EE group, every 10 rats were housed in a large cage (60×50×70cm). The large cage was consisted of two floors connected by inclined ladders. The cages contained wood filings, runners, small houses, pipes and a variety of plastic toys, etc. The houses, runners and pipes remained unchanged, and the rest of the toys are changed once a week for 4 weeks. At 8th week, behavior tests were performed. All experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and Chinese legislation on the use and care of laboratory animals.
Sucrose Preference Test
The sucrose preference test spanned a total of 4 days and rats were housed individually (Lin et al., 2005). On the first day, rats were given free access to 2 bottles of 2% sucrose solution. On the day 2, 3, and 4, one pre-weighed bottle of sucrose solution was replaced with pre-weighed water. The positions of the water and sucrose bottles were switched. The volume of liquid consumed from both bottles was recorded. The sucrose preference rate was calculated to assess the degree of anhedonia in rats using the following formula: sucrose preference rate=sucrose consumption (g)/[water consumption (g) +sucrose consumption (g)]×100%.
Forced Swimming Test
The experiment was divided into pre-experiment (15 minutes) and formal experiment (5 minutes) (Weaver et al., 2005). On the first day, the rats were placed separately in a transparent tempered glass cylinder (a diameter of 20cm and a height of 40cm) with a water depth of 30cm. The water temperature was 25±1&°C. After 15 minutes of pre-experiment, the rats were removed, dried at 32&°C, and returned to the cage. On the second day, the rats were again placed in the forced swimming cylinder, and the rats were observed to be stationary for 5 minutes (the rats were passively floating, only the tail and four paws were slightly swayed to maintain body balance and keep the head out of the water), swimming time (coordinated treading, paddling time) and struggling time (time of drowning, climbing and turbulence) as indicators. The water in the cylinders was emptied and refilled after each experiment to avoid affecting the next test rat.
Aboriginal/Invasion Test
This experiment is to reflect the irritability and aggression of depressed individuals. Each experimental rat was fed in a single cage for 24 hours (free diet) before the experiment. At the beginning of the experiment, the invading rats (another 4-week-old male SD rat not participating in other steps of the experiment) was placed in the cage of experimental rats (on the end of the diagonal with the experimental rat), and then began recording the time that the experimental rat took to attack the invading rat within six minutes (called the attack latency). The shorter attack latency means higher irritability in the experimental rats.
Real-Time Reverse Transcription Quantitative PCR
After behavioral tests, eight rats from each group were euthanized, and the hippocampus was immediately dissected (left for QPCR while right for Western blot). Total RNA was isolated using TRIzol reagent (Life Technologies). Reverse transcription was performed using Bestar qPCR RT Kit (DBI Bioscience). Real-time quantitative PCR was performed using Bestar® SybrGreen qPCR master Mix (DBI Bioscience). Relative quantification of gene expression was conducted using the Stratagene Mx3000P Real time PCR system (). VEGF gene was amplified using forward primer: 5’-GTCCTGTGTGCCCCTAATG-3’ and reverse primer: 5’GGCTTTGGTGAGGTTTGAT-3’. Flk-1 was amplified using forward primer: 5’- ACGGGGCAAGAGAAATGAAT-3’ and reverse primer: 5’- ACAGATGAGATGCTCCAAGGTC -3’. GAPDH was amplified using forward primer: 5’- CCTCGTCTCATAGACAAGATGGT -3’ and reverse primer: 5’-GGGTAGAGTCATACTGGAACATG-3’ as an internal control. Data analysis was performed using the comparative 2-ΔΔCt method.
Western Blot
The rabbit polyclonal anti-VEGF (1:500 dilution), anti-Flk-1 (1:1000 dilution), and anti-GAPDH (1:10000 dilution) antibody was purchased from abcam (San Diego, CA). The horseradish peroxidase-conjugated anti-rabbit IgG was purchased from Sigma-Aldrich (St. Louis, MO). Total protein was extracted, and Western blot was conducted as previously described (Zhang et al., 2006). Twenty micrograms of total protein were loaded onto a 10% sodium dodecyl sulfate–polyacrylamide gel. The same blot was reprobed for GAPDH to serve as a loading control. The intensity of each band was quantified with Bio-Rad Quantity One software (Bio-Rad, Hercules, CA).
Immunohistochemical Staining of BrdU and NeuN Protein Expression
After behavioral tests, seven rats from each group were intraperitoneally injected with BrdU (5-Bromo-2’-Deoxyuridine: 50 mg/kg/day) for three consecutive days. 24 hours after the last injection, the whole brain tissue was immediately dissected and fixed with 4% paraformaldehyde after euthanasia. The embedded whole brain was sectioned serially at 40-μM using a freezing microtome from rostro-caudal coordinates covering the whole hippocampal formation (-2.12 to -6.3 mm relative to bregma). After dehydrated and antigen retrieve, slices were incubated with primary Anti-BrdU and Anti-NeuN antibody (Invitrogen: Cat. No. MA3-071 and PA5-37407) for overnight at 4&°C, and then HRP-labeled secondary antibody for 30 min at room temperature. After washing, the slices were stained using the chromogenic substrate DAB (3,3'-Diaminobenzidine). The number of positive stained cells and the number of total cells were analyzed using Image-Pro Plus 6.0 analysis software. The positive rate was calculated: Positive rate (%) = positive cell number / total cell number *100.
Statistics
Data were analyzed using SPSS (SPSS Inc., Chicago, IL, USA) and reported as means±SD. Differences between experimental groups were determined by ANOVA and Student's t-test with subsequent Bonferroni correction. A P<0.05 was considered significant.
Results
The Effects of Environmental Enrichment on weights and behaviors in MD rats
The weight in the Depression group was significantly lower than that in the Control and Control-EE group from day 7 to day 49. The weight in the Depression-EE group was significantly lower than that in the Control and/or Control-EE group from day 7 to day 28 without significant difference from Depression group, but it was significantly higher than Depression group from day 35 to day 49 (Table 1).
Group /Time (days)
Stress Stimulation
Enriched Environment Stimulation
1
7
14
21
28
35
42
49
Control (n=15)
6.05±1.04
15.96±1.31
29.70±3.02
50.74±5.32
82.61±7.39
121.73±10.32
172.17±7.69
220.02±14.02
Control-EE (n=15)
6.26±1.12
15.21±1.11
30.90±2.02
51.04±5.95
83.58±7.21
127.82±15.01
179.38±13.45
236.79±17.58
6.10±0.99
13.42±1.31ab
26.12±2.78ab
43.25±5.18ab
73.95±6.48ab
104.59±8.31ab
158.22±11.52ab
202.71±17.90ab
Depression-EE (n=16)
6.16±0.71
13.72±1.23ab
26.67±2.22ab
43.98±5.26ab
76.33±6.92b
116.82±11.62c
171.046±11.93c
221.08±17.53c
+a, mean P<0.05, compared with Control group; b, mean P<0.05, compared with Control-EE group; c, mean P<0.05, compared with Depression group.
Table 1: Weights.
The sucrose preference rate was significantly lower in the MD group than the Control and Control-EE group. The sucrose preference rate in the MD-EE group was significantly higher than that of the MD group, but it was still significantly lower than the Control group (Figure 1A). EE treatment had no effect on the control rats. These results suggested that EE reduced anhedonia behavior in MD rats.
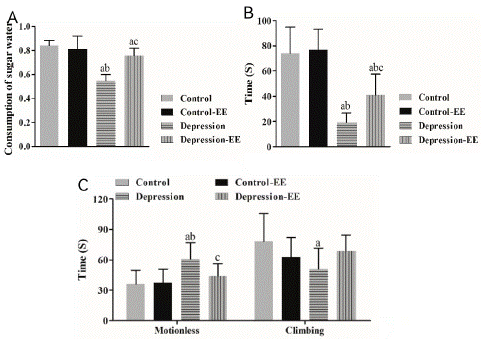
Figure 1: Behavioral tests. A) The sucrose preference rate. MD: maternal deprivation; EE: Environmental enrichment. a P<0.05 vs. Control group; b P<0.05 vs. Control-EE group; c P<0.05 vs. MD group. B) Invasion test. a P<0.05 vs. Control group; b P<0.05 vs. Control-EE group; c P<0.05 vs. MD group. C) Forced swimming test. a P<0.05 vs. Control group; b P<0.05 vs. Control-EE group; c P<0.05 vs. MD group.
Invasion test showed that the attack latency in the MD group was significantly shorter than that in the Control and Control-EE group. The attack latency in the MD-EE group was significantly longer than that in the MD group, but it was still significantly different from the Control group (Figure 1B). EE treatment had no effect on the control rats. These results suggested that EE decreased irritability in MD rats.
Forced swimming test showed that the time of motionless was significantly longer in the MD rats than control rats, whereas the climbing time in the MD rats was significantly shorter than Control rats. The time of motionless in the MD-EE rats was significantly decreased compared to the control rats, but no significant difference in the climbing time was observed between the MD-EE and other groups (Figure 1C). These results suggested that EE reduced behavioral despair in MD rats.
Environmental Enrichment Affected VEGF and Flk-1 Expression in MD Rats
Real-time PCR showed that VEGF (Figure 2A) and Flk-1 (Figure 2B) mRNA expression in the hippocampus was significantly decreased in the MD group compared to the Control and Control-EE group. VEGF and Flk-1 mRNA expression in the MD-EE group was significantly higher than that of the MD group. Environmental enrichment treatment had no effect on the control rats in VEGF and Flk-1 mRNA expression.

Figure 2: VEGF and Flk-1 expression in the hippocampus. A) Relative VEGF mRNA expression measured by real-time PCR. B) Relative Flk-1 mRNA expression measured by real-time PCR. C) Western blot of VEGF protein expression. D) Western blot of Flk-1 protein expression. a P<0.05 vs. Control group; b P<0.05 vs. Control-EE group; c P<0.05 vs. MD group.
Western blot showed that VEGF (Figure 2C) and Flk-1 (Figure 2D) protein expression was significantly decreased in the hippocampus of MD rats compared to the Control and Control-EE group. VEGF and Flk-1 protein expression in the MD-EE group was significantly higher than that of the MD group. EE treatment had no significant effect on VEGF protein expression, but decreased Flk-1 protein expression.
Environmental Enrichment Affected Hippocampal Neurogenesis
Immunohistochemical staining showed that positive BrdU and NeuN staining cells were significantly decreased in the hippocampus of MD rats compared to the Control and Control-EE group. Positive BrdU and NeuN staining cells in the MD-EE group were significantly higher than that of the MD group. EE treatment had significant effect on BrdU and NeuN protein expression in the hippocampus of MD rats (Figure 3).

Figure 3: Immunohistochemical staining. BrdU (A,B) and NeuN (C,D) protein expression in the hippocampus of rats were measured using immunohistochemistry.
Discussion
In this study, an animal model of adolescent depression was established with maternal deprivation for 21 days with starting in newborn rats. The lower rate in sucrose preference test, the shorter attack latency in aboriginal/invasion test, and the longer time of motionless / shorter climbing time in forced swimming test in MD rats reflect a status a like depression in human beings. Environmental Enrichment (EE) can partially or entirely reduce these depression-like behaviors in MD rats. Both the VEGF and Flk-1 mRNA and protein expression were significantly decreased in the hippocampus of the MD rats, but their expressions can be recovered by the EE treatment. In addition, EE can improve the proliferation of hippocampal neurons which were decreased in the MD rats. Thus, this study suggests that 1) adolescent depression can be induced by negative experiences in the early life; 2) EE is effective in treating adolescent depression through regulating VEGF/Flk-1signaling; and 3) subsequently increasing the hippocampal neurogenesis.
Although the protocols of environmental enrichment vary between different laboratories, the most common procedure in rats should provide three basic components: novelty, social contact, and exercise [24]. In this study, rats were housed in a large cage with two floors to provide a social contact; the ladder, wood filings, runners, small houses, pipes, and a variety of plastic toys changed once a week to provide a novelty and exercise. Our EE protocol showed no effects on depression-like behaviors in native rats. In contrast, EE given after MD can partially or entirely reduce depression-like behaviors in MD rats. These findings suggest that the antidepressant effects of EE are different in native rats and the rats pre-exposed to the stress, but the mechanisms need further studies. Our EE protocol can be used as a treatment for adolescent depression.
A previous study demonstrated that EE can reduce learned helpless behavior in 14 weeks old, but not 28 weeks old congenital helpless rats. Also, EE had no effect on anhedonic-like behavior [25]. Mileva and Bielajew studied the impact of immediate environment on depression- and anxiety-like behaviors in Wistar Kyoto (validated animal model of depression) and native Wistar rats receiving standard, enriched, and isolated housing. In contrast, they found that enriched housing significantly increased sucrose preference (anhedonic behavior) in both strains of rats compared with isolated housing, but there were no significant effects on the measures of open arm duration in the EPM (anxiety-like behaviors) and immobility in the FST (depressive-like behaviors) in two strains, suggesting that enrichment appears to reduce anhedonia [26]. Seong et al study revealed that EE can decrease hopelessness and anxiety in a depressive rat model established by chronic stress [27]. Gong et al study demonstrated that EE can reduce adolescent anxiety- and depression-like behaviors in rats that received infant spared nerve injury through normalizing the inflammation balance in the brain [28]. Moreover, Doreste-Mendez et al study showed no rescue on the majority of the anxiety and depressive-like behaviors in adulthood of rats received maternal separation for 21 days [29]. In this study, our EE protocol affected anhedonic- and depressive-like behaviors, as well as irritability of depressed individuals in MD rats. Thus, our and others findings suggest that 1) the differential environmental factors may mediate the different depression-related symptoms; 2) environmental enrichment is complex and different protocols for enriching rats may produce conflicting results, and 3) it is important to establish a universal standard of environmental enrichment for the treatment of depression.
Previous studies have demonstrated that EE can increase neonatal neoplasia and synapses, as well as the volume and thickness of the cerebral cortex [11,12]. Inconsistent with previous studies, our EE protocol had no effects on the number of hippocampal neurons in native rats, but significantly increased the proliferation of neurons in the hippocampus of MD rats. Previous studies have revealed that VEGF-Flk-1 signaling is involved in the chronic stress-induced depression and the interventions of various antidepressants on the depression [20-22] through regulating the neuronal regeneration in the hippocampus [23]. Our study demonstrated that maternal deprivation starting in newborn rats induced adolescent depression through downregulation of VEGF-Flk-1 expression in the hippocampus, however, our EE protocol increased VEGF-Flk-1 expression in the hippocampus of MD rats. The changes of VEGF-Flk-1 expression paralleled to the changes in the BrdU and NeuN expression in the hippocampal neurons. The incorporation of BrdU and subsequent examination of BrdU protein expression is a classic assay of cell proliferation, while NeuN is a neuronal nuclear protein and neuron differentiation marker [30]. Thus, our EE protocol can reverse the MD-induced depression in adolescents through activating VEGF-Flk-1 signaling and subsequent neuron regeneration in the hippocampus.
In conclusion, early maternal deprivation starting in newborn rats induced depressive-like behaviors through negatively regulating VEGF and Flk-1 signaling and reducing hippocampal neurogenesis. Our Environmental Enrichment (EE) protocol can reduce the depressive-like behaviors through positive regulation of VEGF/Flk-1 signaling and subsequent neuron regeneration in the hippocampus.
Author Statements
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No: 81401118) and the ([2020]1Y321).
References
- Birmaher B, Brent D, AACAP Work Group on Quality Issues, et al. Practice parameter for the assessment and treatment of children and adolescents with depressive disorders. J Am Acad Child Adolesc Psychiatry. 2007; 46: 1503-1526.
- Williams JM, Barnhofer T, Crane C, Duggan DS, Shah D, et al. Pre-adult onset and patterns of suicidality in patients with a history of recurrent depression. J Affect Disord. 2012; 138: 173-9.
- Lubell KM, Kegler SR, Crosby AE, Karch D, et al. Suicide trends among by youths and young adults aged 10-24 years—United States, 1990-2004. Morbidity and Mortality Weekly Report. 2007; 56: 905-908.
- Cipriani A, Zhou X, Del Giovane C, Hetrick SE, Qin B, et al. Comparative efficacy and tolerability of antidepressants for major depressive disorder in children and adolescents: a network meta-analysis. Lancet. 2016; 388: 881-90.
- Sale A, Berardi N, Maffei L. Enrich the environment to empower the brain. Trends Neurosci. 2009; 32: 233-239.
- Nithianantharajah J, Hannan AJ. Enriched environments, experience dependent plasticity and disorders of the nervous system. Neurosci. 2006; 7: 699-709.
- Farah MJ, Betancourt L, Shera DM, Savage JH, Giannetta JM, et al. Environmental stimulation, parental nurturance and cognitive development in humans. Dev Sci. 2008; 11: 793-801.
- Xingrui Gong, Yongmei Chen, Jing Chang. Environmental enrichment reduces adolescent anxiety- and depression like behaviors of rats subjected to infant nerve injury. J Neuroinflammation. 2018; 15: 262.
- Saxe MD, Battaglia F, Wang JW, Malleret G, David DJ, et al. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci USA. 2006; 103: 17501-17506.
- Studenski S, Carlson MC, Fillit H, Greenough WT, Kramer A, et al. From bedside to bench: does mental and physical activity promote cognitive vitality in late life? Science Aging Knowledge Environ. 2006; 10: pe21.
- Arnaiz SL, D’Amigo G, Paglia N, Arisendi M, Basso N, Arnaiz MRL. Enriched environment, nitric oxide production and synaptic plasticity prevent the aging-dependent impairment of spatial cognition. Mol. Aspects Med. 2004; 25: 91-101.
- Bennett EL, Diamond MC, Krech D, Rosenweiz MR. Chemical and anatomical plasticity brain. Science. 1964; 146: 610-619.
- Andersen SL, Teicher MH. Stress, sensitive periods and maturational events in adolescent depression. Trends Neurosci. 2008; 31: 183-191.
- Andersen SL, Tomada A, Vincow ES, Valente E, Polcari A, et al. Preliminary evidence for sensitive periods in the effect of childhood sexual abuse on regional brain development. J Neuropsychiatry Clin Neurosc. 2008; 20: 292-301.
- Schoedl AF, Costa MC, Mari JJ, Mello MF, Tyrka AR, et al. The clinical correlates of reported childhood sexual abuse: an association between age at trauma onset and severity of depression and PTSD in adults. J Child Sex Abus. 2010; 19: 156-170.
- Andersen SL, Tomada A, Vincow ES, Valente E, Polcari A, et al. Preliminary evidence for sensitive periods in the effect of childhood sexual abuse on regional brain development. J Neuropsychiatry Clin Neurosci. 2008; 20: 292-301.
- Andersen SL, Teicher MH. Delayed effects of early stress on hippocampal development. Neuropsychopharmacology. 2004; 29: 1988-1993.
- Maurer MH, Tripps WK, Feldmann RE Jr, Kuschinsky W. Expression of vascular endothelial growth factor and its receptors in rat neural stem cells. Neurosci Lett. 2003; 344: 165-168.
- Yang SZ, Zhang LM, Huang YL, Sun FY. Distribution of Flk-1 and Flt-1 receptors in neonatal and adult rat brains. Anat Rec A Discov Mol Cell Evol Biol. 2003; 274: 851-856.
- Heine VM, Zareno J, Maslam S, Joels M, Lucassen PJ. Chronic stress in the adult dentate gyrus reduces cell proliferation near the vasculature and VEGF and Flk-1 protein expression.Eur J Neurosci. 2005; 21: 1304-1314.
- Warner-Schmidt JL, Duman RS. VEGF is an essential mediator of the neurogenic and behavioral actions of antidepressants. Proc Natl Acad Sci USA. 2007; 104: 4647–52
- Jin K, Zhu Y, Sun Y, Mao ZO, Xie L, et al.. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci USA. 2002; 99: 11946–50.
- Cao L, Jiao X, Zuzga DS, Liu Y, Fong DM, et al. VEGF links hippocampal activity with neurogenesis, learning and memory. Nat Genet. 2004; 36: 827-35.
- Crofton EJ, Zhang Y, Green TA. Inoculation stress hypothesis of environmental enrichment. Neurosci Biobehav Rev. 2015; 49: 19-31.
- Richter SH, Zeuch B, Riva MA, Gass P, Vollmayr B. Environmental enrichment ameliorates depressive-like symptoms in young rats bred for learned helplessness. Behav Brain Res. 2013; 252: 287-92.
- Mileva GR, Bielajew C. Environmental manipulation affects depressive-like behaviours in female Wistar-Kyoto rats. Behav Brain Res. 2015; 293: 208-16.
- Seong HH, Park JM, Kim YJ. Antidepressive Effects of Environmental Enrichment in Chronic Stress-Induced Depression in Rats. Biol Res Nurs. 2018; 20: 40-48.
- Gong X, Chen Y, Chang J, Huang Y, Cai M, Zhang M. Environmental enrichment reduces adolescent anxiety- and depression-like behaviors of rats subjected to infant nerve injury. J Neuroinflammation. 2018; 15: 262.
- Doreste-Mendez R, Ríos-Ruiz EJ, Rivera-López LL, Gutierrez A, Torres-Reveron A. Effects of Environmental Enrichment in Maternally Separated Rats: Age and Sex-Specific Outcomes. Front Behav Neurosci. 2019; 13: 198.
- VV Gusel’nikova, DE Korzhevskiy. NeuN As a Neuronal Nuclear Antigen and Neuron Differentiation Marker. Acta Naturae. 2015; 7: 42–47.