Perspective
AUDs (Alcohol Use Disorders) encompass pathologies with individual traits and manifestations linked to genetic backgrounds, environmental exposures, and family and social dynamics. The search for effective pharmacological treatments has frequently thrown its weight behind a single therapy: a purported “silver bullet” that may cure alcoholism. However, given the complexity of the pathologies associated with AUDs, and the individual variations, this endeavor may fail. Consequently, basic research and clinical trials have contributed strategies targeting specific facets of harmful alcohol consumption.
Experimental evidence with specific receptor antagonists implicates the neurokinin receptor/substance P system (NK-1R/SP) in modulating stress and anxiety associated with alcohol drinking reinforcement, craving, and relapse [1,2]. Given the participation of NK-1R in multiple pathologies (psychiatric disorders, cancer, pain, inflammation), the design of antagonists has been boosted in recent years to attain potent, effective, and selective antagonists of potential interest in medical therapy [3,4]. However, poor outcomes in clinical trials designed to test the efficacy of NK-1R antagonists in major depressive disorder, MDD [5] led to the discontinuation of clinical studies in MDD and other pathologies, including AUD. The few approved NK-1R antagonists for clinical use are only prescribed to treat chemotherapy-induced nausea and vomiting and as an antitussive in patients with lung cancer [4]. Interestingly, new data on antagonist binding to NK-1R showed that effectiveness requires almost 100% receptor occupancy [6]. Receptor occupancy is only accomplished with adequate dosage and sound background knowledge of pharmacodynamic, pharmacokinetic, and safety properties. Additionally, selecting suitable cohorts of individuals is essential for clinical trials [4,7].
Recent structural analysis of the NK-1R revealed the detailed architecture of the binding site of specific antagonists, such as the clinically used aprepitant, to the receptor protein (Figure 1). Weak interactions between the protein and the antagonists define an orthosteric pocket and explain their insurmountable antagonism.
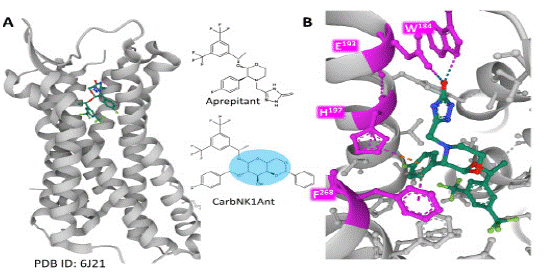
Figure 1: Antagonist aprepitant bound to NK-1 R in a hydrophobic pocket. Panel A represents the NK-1R and the localization of the aprepitant binding site. Panel B depicts a closer view of atomic interactions (dashed lines) of the antagonist with amino acid residues within the receptor protein (pink). The 2D representations of antagonists aprepitant and CarbNK1Ant (a carbohydrate NK-1R antagonist derivative) with a blue area depicting the hydrophilic region of the structure are in between panels. The figures are from the Protein Data Bank [10], PDB Id 6J21 [11], drawn with the free web-based software Mol* [12].
Knowledge of the binding properties of antagonists allows advances in the synthesis of improved drugs interacting with the receptor stably; for example, introducing a galactose carbohydrate moiety (Figure 1, compound CarbNK1 Ant, a drug showing high affinity and potency) in the scaffold formed by two fluorinated phenyl rings. The hydrophilic property added to the molecule secured and improved the interaction with crucial amino acids in the middle part of the hydrophobic cleft [8]. Additionally, allosteric modulation, receptor occupancy, and biased signaling have a say when ascertaining the role of NK-1R antagonism in clinical therapy.
Efforts and investment in more basic and clinical research will improve the rational use of NK-1R antagonists in AUDs and other pathologies. In preclinical research, the following topics require further study: 1) Organic synthesis and analysis of affinity and efficacy of new antagonists based on structure refinements (polarity, isomers, and size) of the chemical group that links the two fluorinated phenyl rings (Figure 1), considering dynamic and multiple active states of NK-1R, 2) Structural determination of atomic interactions of antagonists with NK-1R and definition of the orthostatic site for different antagonists, 3) Analysis of dosage, administration routes, pharmacological interactions, formulation of compounds, tissue penetration, safety, pharmacodynamic and pharmacokinetics in cell cultures and animal models. Topics worth considering when setting clinical trials must cover: a) The selection of suitable individuals, considering the complexity and variability of clinical disorders (stress-induced psychiatric disorders, cancer, or inflammatory diseases), b) The application of doses to attain receptor occupancy close to 100%, within the safety range, and c) The combination with other drugs to reinforce searched for effects (including opioid antagonists, GABA B receptors agonists, 5HT3 receptor antagonists or glutamate system regulators) [9].
It may sound like we start from point zero, but this is not the case. Investment in a new vision and focus on this field may bring unseen and beneficial uses of NK-1R antagonists in AUDs and other pathologies, as they have been repurposed to use as effective antitumoral drugs alone or together with radiotherapy and chemotherapy [3]. The task is challenging, but the results may provide new effective and safe drugs to alleviate human suffering.
There is no “silver bullet” for the time being to cure AUDs. Considering and studying NK-1 R antagonists with a new, open, and fresh perspective is a priority to reinforce the toolkit for the pharmacological treatment of AUD.
References
- Coveñas R, Muñoz M. Involvement of the Substance P/Neurokinin-1 Receptor System in Cancer. 2022; 14: 3539.
- PDB Protein Data Bank (PDB). https://pdb101.rcsb.org. 2022. Accessed 26 December 2022.
- Muñoz M, Muñoz ME, Morell F, Coveñas R. Why Use Aprepitant Only as a Cough Suppressant in Lung Cancer when at Higher Doses it Could also Exert an Antitumor Action? Archivos de Bronconeumología. 2022; 58: 727-728.
- Rodríguez FD, Coveñas R. Involvement of the Substance P/Neurokinin-1 Receptor System in Alcohol Use Disorders (Chapter 6). In L. V. Berhardt (Ed.), Advances in Medicine and Biology, volume 194 (pp. 151-172). New York: Nova, Medicine and Health. 2022.
- Schank JR, Heilig M. Substance P and the Neurokinin-1 Receptor: The New CRF. International Review of Neurobiology. 2017; 136: 151-175.
- Recio R, Lerena P, Pozo E, Calderón-Montaño JM, Burgos-Morón E, et al. Carbohydrate-Based NK1R Antagonists with Broad-Spectrum Anticancer Activity. Journal of Medicinal Chemistry. 2021; 64: 10350-10370.
- Carvalho AF, Heilig M, Perez A, Probst C, Rehm J. Alcohol Use Disorders. Lancet (London, England). 2019; 394: 781–792.
- Chen S, Lu M, Liu D, Yang L, Yi C, Ma L, et al. Human Substance P Receptor Binding Mode of the Antagonist Drug Aprepitant by NMR and Crystallography. Nature Communications. 2019; 10: 638.
- Domi E, Domi A, Adermark L, Heilig M, Augier E. Neurobiology of Alcohol-Seeking Behavior. Journal of Neurochemistry. 2021; 157: 1585-1614.
- Keller M, Montgomery S, Ball W, Morrison M, Snavely D, et al. Lack of Efficacy of the Substance P (Neurokinin1 Receptor) Antagonist Aprepitant in the Treatment of Major Depressive Disorder. Biological Psychiatry. 2006; 59: 216-223.
- Rupniak NMJ, Kramer MS. NK1 Receptor Antagonists for Depression: Why a Validated Concept Was Abandoned. Journal of Affective Disorders. 2017; 223: 121-125.
- Sehnal D, Bittrich S, Deshpande M, Svobodová R, Berka K, et al. Mol Viewer: Modern Web App for 3D Visualization and Analysis of Large Biomolecular Structures. Nucleic Acids Research. 2021; 49: W431-W437.
