Abstract
treatment of NAFLD and AD. Discoveries in genetic markers and noninvasive diagnostic tools, such as biomarkers, are leading to earlier detection of these diseases and more personalized treatment approaches that could slow or even reverse disease progression. The increasing instances of NAFLD, particularly its severe consequences, reflect the larger issue of metabolic syndrome prevalence. Similarly, the rise of AD underscores the urgency for neuroprotective treatments. The gut-liver-brain axis, a recently explored realm, could reveal new treatments influenced by gut microbiota that benefit both liver and brain health. Epigenetic factors such as DNA methylation and histone modifications are also being implicated in the development of NAFLD and AD. These modifiable factors contribute to disease expression and progression, offering alternative intervention strategies that could manipulate disease outcomes. Lastly, the future of NAFLD and AD research necessitates a multidisciplinary approach. Integrating various scientific disciplines will be crucial for gaining insight and translating that knowledge into effective treatments and public health policies, demonstrating the ever-growing importance of collaborative research efforts in tackling these complex diseases.
Keywords: Neurodegenerative disease; Alzheimer’s disease; Non-alcoholic fatty liver disease; Novel pathways; Biomarkers; Epigenetic influence.
Introduction
Non-Alcoholic Fatty Liver Disease (NAFLD) and Alzheimer's Disease (AD) are complex and multifactorial conditions that have a significant impact on public health. NAFLD is characterized by the accumulation of fat in the liver and is associated with various metabolic conditions, while AD is a neurodegenerative disorder that leads to cognitive decline and memory loss. Both diseases have a genetic component that influences their onset and progression. Genetic factors play a crucial role in the development of NAFLD. Variants in genes such as PNPLA3, TM6SF2, HSD17B13, GCKR, and MBOAT7 have been associated with an increased risk of NAFLD and its progression to more severe forms such as Non-Alcoholic Steatohepatitis (NASH), fibrosis, and cirrhosis. Similarly, genetic predispositions have been extensively studied in the context of AD, with variants in genes such as CD2AP and APOE e4 being linked to an increased susceptibility to the disease [3]. The prevalence of NAFLD is increasing globally, with estimates ranging from 29.8% to 47% among adults. The disease is associated with a range of metabolic conditions and can lead to severe liver-related consequences such as cirrhosis and hepatocellular carcinoma [1]. Similarly, AD has a substantial global impact, affecting millions of individuals worldwide. The disease is characterized by the presence of amyloid plaques and neurofibrillary tangles in the brain, leading to cognitive impairment and challenges in daily life [24].
Understanding the genetic underpinnings of NAFLD and AD is crucial for the development of targeted therapeutic interventions and risk assessment strategies. Genome-Wide Association Studies (GWAS) have identified several genetic loci associated with both diseases, shedding light on their pathobiology and potential targets for medication development [2]. In this systematic review, we aim to comprehensively analyze the genetic basis of NAFLD and AD, including the role of rare variants, genetic predispositions, and their implications for disease severity and progression. We explore the genetic landscape of both diseases, including the influence of genetic factors on disease pathogenesis, risk assessment, and potential therapeutic targets. Additionally, we examined the biomarkers associated with NAFLD and AD and their epigenetic effects. By synthesizing and critically analyzing the existing evidence, this review aims to contribute to the advancement of knowledge in the field and provide valuable insights for future research and clinical practice.
Fatty Acid Liver Disease
Fatty Liver Disease (FLD) is one of the most common causes of chronic liver diseases worldwide. The onset and course of FLD are influenced by genetic factors. PNPLA3, TM6SF2, HSD17B13, GCKR, and MBOAT7 are among the genetic variants associated with FLD [1-3]. These genes mediate the lipid metabolism and hepatic lipid management. In particular, it has been discovered that PNPLA3 and TM6SF2 risk alleles are associated with an increased risk of liver-related mortality [4]. Genetic variations linked to FLD are believed to play a role in the accumulation of fat in the liver, which, in turn, causes the illness to begin and worsen. Determining possible targets for treatment and preventing its consequences can be aided by the knowledge of the genetic basis of FLD.
Accumulation of fat in the liver is a typical symptom of Non-Alcoholic Fatty Liver Disease (NAFLD). It is associated with several conditions, including type 2 diabetes and obesity. According to estimates ranging from 29.8% to 47% among adults, NAFLD is becoming increasingly common worldwide [5,6]. According to Le et al. (2022), there is regional variation in prevalence, with the Americas and Southeast Asia having the highest prevalence rates. The prevalence was higher in men than that in women. Research indicates that the prevalence has been rising over time, from 26% in studies conducted prior to 2005 to 38% in those conducted in 2016 or later [8]. Pediatric NAFLD is more common in Korea, (8.2% in 2009 to 12.1% in 2018) [9]. The long-term consequences of NAFLD include liver cirrhosis, fibrosis, and cardiovascular disease. More knowledge and practical risk-prevention techniques are required to address the increasing burden of NAFLD.
A prevalent chronic liver disease, Non-Alcoholic Steatohepatitis (NASH), fibrosis, cirrhosis, and hepatocellular carcinoma are all possible progressions of NAFLD. NAFLD and its associated disorders are influenced by sedentary lifestyle, increased calorie intake, and genetic and epigenetic factors. Polymorphisms in NAFLD-related genes have been identified using Genome-Wide Association Studies (GWAS), and alterations in DNA methylation and gene regulation through certain miRNAs have also been noted [10]. NAFLD has been linked to mitochondrial mutations and mitophagy, and urea cycle metabolites related to mitochondria have been suggested as noninvasive indicators [11]. Numerous genetic loci linked to NAFLD have been identified, and GWAS have proven useful in clarifying the genetic components of the disease [12]. Gene variations including PNPLA3, TM6SF2, MBOAT7, GCKR, and HSD17B13 have been associated with the natural history of NAFLD and may have consequences for medication development, risk assessment, and disease pathobiology [13]. The entire spectrum of NAFLD pathology has been demonstrated to be influenced by common variations in PNPLA3, TM6SF2, MBOAT7, and GCKR. Assessment of genetic risk factors may aid in stratifying the risk of extrahepatic and liver-related consequences of the disease [14].
As shown in Figure 1, NAFLD is characterized by the accumulation of fat in the liver and can manifest in a variety of severe forms, including Hepatocellular Carcinoma (HCC), fibrosis, cirrhosis, and NASH [15]. Owing to abnormalities in lipid metabolism, NAFLD is strongly associated with the onset and course of Atherosclerotic Cardiovascular Disease (ASCVD) [16]. Patients with metabolically healthy NAFLD have a favorable biochemical profile but are at risk of worsening the illness to worsen [17]. Metabolic health plays a key role in the development of NAFLD. As both conditions can lead to the development of progressive liver disease, the co-occurrence of NAFLD and Hepatitis B Virus (HBV) infection has drawn attention from researchers [18]. Nutritional recommendations that focus on disease mechanisms, such cutting back on bad macronutrient intake and consuming more of the good kind, can help slow the progression of NAFLD [19]. NAFLD significantly affects overall health, increasing the risk of cardiovascular disease and a number of liver-related problems.
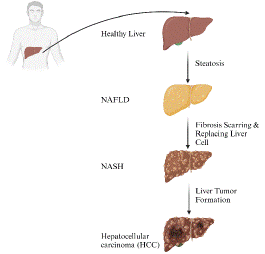
Figure 1: Progression of NAFLD.
Alzheimer’s Disease
Alzheimer's is an intricate and multifaceted ailment that arises from genetic factors. Genetic variants of CD2-Associated Protein (CD2AP) have been identified as predisposing factors for AD; however, their specific mechanisms remain unclear [20]. Moreover, the presence of the APOE e4 gene has been linked to increased susceptibility to Alzheimer's disease [21]. Investigating the genetic underpinnings of Alzheimer's disease is a crucial field of study, because understanding the precise genes implicated can facilitate the creation of focused therapeutic interventions [22]. Nevertheless, a significant amount of knowledge remains to be acquired regarding the consequences of familial mutations and high-risk variations for Alzheimer's [23]. In general, genetic factors have a substantial impact on the onset of Alzheimer's disease, and additional investigations are necessary to comprehensively comprehend the implicated genetic pathways [24].
Alzheimer's disease is a neurodegenerative disease characterized by the presence of amyloid plaques and neurofibrillary tangles in the brain. Dementia is primarily responsible for memory loss, cognitive deterioration, and challenges in daily life. This disease has a substantial global impact and, affects millions of individuals worldwide. Currently, there is no known treatment for Alzheimer's disease, and the underlying causes remain incompletely understood. Nevertheless, scientific studies have indicated that hereditary factors, along with environmental and lifestyle factors, may play a role in its onset. Alzheimer's is projected to become more widespread in the future as a result of the increasing number of elderly individuals. The disease not only has a devastating effect on individuals and their families, but also imposes a substantial economic burden on society [25,26].
Genetic predispositions and risk factors for AD have been thoroughly investigated. A study conducted by the European Alzheimer's & Dementia Biobank Mendelian Randomization (EADB-MR) Collaboration in 2023 discovered that individuals with genetically determined higher levels of high-density lipoprotein cholesterol and increased systolic blood pressure were more likely to develop AD (AD. A separate investigation examined antidiabetic medications and discovered that genetic variability in sulfonylurea targets was linked to a decreased likelihood of developing AD [49]. Furthermore, there is a correlation between genetic changes in the target Glucagon-Like Peptide 1 (GLP-1) analog and a decreased likelihood of developing Alzheimer's disease [27]. Moreover, researchers are currently studying the involvement of specific genetic variations in the molecular characteristics and internal markers of AD, although the effects of these variations on the identification of genes associated with AD risk remain uncertain [28]. Microglia, a specific type of brain cell, have recently been recognized as promising targets for therapeutic development in AD because of their role in the progression of the disease [29].
The precise mechanisms responsible for AD remain unclear. However, numerous factors have been implicated in its pathogenesis and progression. These factors include amyloid-β plaques, neurofibrillary tau tangles, inflammation, mitochondrial dysfunction, oxidative stress, and alterations in protein clearance [30]. β-Amyloid-induced toxicity and aberrant tau protein alteration are recognized as significant causative elements of Alzheimer's disease, as depicted in Figure 2. In addition to the presence of misfolded and aggregated proteins, neuroinflammation is a significant factor in AD development [31]. Additional factors that contribute to the development of Alzheimer's disease include autophagy, mitochondrial degradation, age-related epigenetic alterations, and environmental factors, such as infections, heavy metal ions, nutrition, sleep, stress, and gut microbiota [32]. The pathophysiology of AD is characterized by the involvement of amyloid peptides, cholinergic and glutamatergic neurotransmission, tau proteins, oxidative stress, and calcium dysregulation [33].
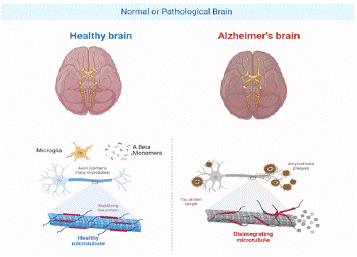
Figure 2: Progression of AD.
Biomarkers
Biomarkers, defined as quantifiable indicators of normal or abnormal processes, are essential for understanding biological processes and their associations with diseases [34]. Nevertheless, the validation and implementation of these methods in clinical practice is gradual and arduous processes [35]. Biomarkers in cancer have potential applications in diagnosis, prognosis, and epidemiology. However, the clinical validation off early disease detection and monitoring remains a challenging task [36]. In the field of environmental epidemiology, biomarkers are used to categorize and measure environmental exposure and its impacts. However, it is crucial to provide a thorough definition of these characteristics [37].
Biomarkers for NAFLD and AD
Extensive research has been conducted on biomarkers for NAFLD and AD. Studies on NAFLD have used metabolomics and lipidomics techniques to identify potential biomarkers associated with these conditions. These biomarkers include changes in amino acid metabolism and other lipid metabolisms [38]. However, only a small number of putative biomarkers have been validated [39]. Advancements in proteomic technology for AD have resulted in the discovery of potential biomarkers, including amyloid and tau species, which can be quantified in the Cerebrospinal Fluid (CSF) or plasma [40]. Furthermore, proteins associated with the degradation of neurons, inflammation in the nervous system, transportation of lipids, and mitochondrial dysfunction have been recognized as potential indicators of disease (disease (AD). Combining CSF biomarkers, including Aβ42, P-tau181, and T-tau, with brain [18F] FDG-PET patterns has demonstrated potential in diagnosing AD [41]. Nevertheless, there may be a lack of agreement between Cerebrospinal Fluid (CSF) and [18F] FDG-PET findings, indicating the existence of atypical AD variations or less progressive neurodegeneration.
Diagnosis and Monitoring of NAFLD Progression
Individuals with Nonalcoholic Steatohepatitis (NASH) and hepatic fibrosis are more likely to experience negative outcomes related to liver and cardiovascular health. Noninvasive techniques are required to diagnose, assess risk, and monitor NASH and fibrosis. The potential of serum miRNA-122 expression levels as biomarkers for detecting and monitoring various phases of liver disease in patients with chronic hepatitis C has been previously assessed [42]. Current innovations offer a diagnostic approach for NAFLD by assessing various metabolite levels of metabolites [43]. Biomarkers for the development of Primary Sclerosing Cholangitis (PSC) can be used to assess risk levels and evaluate the impact of treatment. Liquid biopsy, which involves the analysis of circulating tumor cells and circulating tumor DNA, has become a valuable method for detecting hepatocellular carcinoma in its early stages and guiding treatment decisions [44]. These biomarkers facilitate timely identification, diagnosis, and monitoring of NAFLD and its related consequences.
Diagnosis and Monitoring of AD Progression
Biomarkers are used to diagnose and track AD progression. Currently, the primary tools for diagnosing AD are Cerebrospinal Fluid (CSF) measurements and imaging techniques [45]. CSF biomarkers, including Aβ1–42, phosphorylated tau, and total tau levels, are frequently used to diagnose AD. Nevertheless, the pursuit of novel, cost-effective, and easily obtainable biomarkers has prompted the examination of chemicals found in the blood [46]. Proteins, lipids, metabolites, oxidative stress-related compounds, cytokines, miRNAs, and long non-coding RNAs (lncRNAs) have been investigated as potential biomarkers off AD using blood samples. Furthermore, recent research has indicated that vitamins and chemicals associated with the gut microbiome hold potential as innovative options for the identification and monitoring of Alzheimer's [47]. Prompt identification is essential for successful therapy and many biomarkers have been devised to assess, diagnose, and rule out other associated illnesses. Utilizing resilient and informative biomarkers can enable precise identification during the early phases of illness. Moreover, the assessment of lipid peroxidation biomarkers in blood samples has demonstrated promise in assessing the course of Alzheimer's disease.
Potential Overlapping Biomarkers between NAFLD and AD
Therefore, common biomarkers may be shared between NAFLD and AD. Studies have revealed shared biological processes and pathways related to both disorders. Lipid metabolism, which involves changes in fatty acids, triglycerides, phospholipids, and bile acids, is thought to play a role in NAFLD [40] and AD [48]. Furthermore, metabolic dysregulation, including lipotoxicity, oxidative stress, and endoplasmic reticulum stress, play a role in the development of both disorders. Moreover, there is a correlation between amyloid-β pathology in AD and the presence of circulating metabolites, specifically primary fatty amides [49]. These findings indicate that studying common metabolic pathways and dysregulation could help to identify possible biomarkers for both NAFLD and AD. However, additional studies are required to authenticate these indicators and comprehend the exact molecular pathways underlying the shared development of these disorders.
Common Genetic Pathways
Dong et al. (2023) revealed shared genetic pathways between AD and Ischemic Stroke (IS). Furthermore, common genetic variants and pathways are shared between Autism Spectrum Disorder (ASD) and autoimmune illnesses [51]. In addition, it has been discovered that preserved aging pathways have an impact on several age-related illnesses in humans [52]. These findings indicate the presence of shared molecular processes that are responsible for these distinct diseases. Common pathways include inflammation and immunology, G protein-coupled receptors, signal transduction, synaptic integrity, neurotransmitter metabolism, and cell adhesion molecules. These pathways are promising targets for the prevention and treatment of illness.
Identify and Explain Genetic Pathways Common to both NAFLD and AD
Multiple genetic pathways that are shared by NAFLD and AD have been identified. The genes implicated in insulin resistance, atherogenic dyslipidemia, subclinical inflammation, and oxidative stress are listed in Tables 1, 2, and 3, respectively [53]. Danford et al. (2018) established a correlation between certain genetic variations in PNPLA3, TM6SF2, and GCKR with the occurrence and progression of NAFLD, indicating their involvement in the pathogenesis of the disease. These genetic variables have also been linked to the development of NAFLD and NASH as well as disorders related to metabolic syndromes, such as type 2 diabetes, obesity, and cardiovascular disease [55]. Variations in I148M PNPLA3 have been recognized as significant genetic factors in NAFLD development of NAFLD [56]. These genetic pathways offer promising targets for therapeutic intervention in both NAFLD and AD.
Molecular Link
Impact on NAFLD
Insulin Signaling Impairment
Reduces systemic biological response to insulin, worsening glucose uptake.
Adipose Tissue Dysfunction
Impairs glycolipid homeostasis between adipose and hepatic tissues.
Increased Lipolysis
Results in the release of free fatty acids, contributing to liver fat accumulation.
Hepatic Fat Overload
Causes fat overload in hepatocytes, progressing to NAFLD.
Mitochondrial Dysfunction
Leads to incomplete oxidation of fatty acids and worsens hepatic insulin resistance.
Enhanced Gluconeogenesis
Increases glucose production, counteracting insulin-dependent glycogen synthesis.
De Novo Lipogenesis (DNL)
Lack of suppression of hepatic glucose production, crucial for NAFLD development.
Carbohydrate Response Element-Binding Protein (ChREBP) Activation
Induces expression of genes for the glycolytic pathway, increasing metabolic precursors for DNL.
Steatosis and Insulin Resistance
Mutual relationship worsening the clinical picture of NAFLD and systemic IR.
Oxidative Stress and Inflammation
Induces production of ROS and toxic lipids, worsening insulin signaling.
Kupffer Cells Activation
Promotes local inflammation, aggravating the degree of IR.
Table 1: Molecular links between Insulin Resistance (IR) and NAFLD.
Condition Linked with NAFLD
Impact and Mechanism
Insulin Resistance (IR)
- Abnormal cell response to insulin hormone.
- Strongly associated with NAFLD, atherosclerosis, and Metabolic Syndrome (MetS).
- Alters metabolism of glucose, fatty acids, and lipoproteins.
- Hyperinsulinemia can deteriorate insulin signaling pathways and exacerbate tissue-specific IR.
- Contributes to increased ectopic fat accumulation and CVD risk.Atherogenic Dyslipidemia
- NAFLD, especially in necroinflammatory stages, can lead to dyslipidemia that promotes atherosclerosis.
- Characterized by abnormal lipid profiles increasing Cardiovascular Disease (CVD) risk.Subclinical Inflammation
- Chronic low-grade inflammation marked by elevated inflammatory markers like IL-6, TNF, CRP, and fibrinogen.
- Increased vascular risk due to high levels of inflammatory cytokines.
- Contributes to the risk of atherosclerosis and CVD.Oxidative Stress
- Imbalance between free radicals and antioxidants, characteristic of NAFLD.
- Results in cellular dysfunction and abnormal cytokine release (TNF-a, CRP, IL-6).
- Plays a role in the pathogenesis of CVD in NAFLD patients by affecting endothelial function.
- Contributes to the progression of liver disease from simple steatosis to steatohepatitis.
Table 2: NAFLD linked with insulin resistance, atherogenic dyslipidemia, subclinical inflammation, and oxidative stress.
Condition Linked with AD
Impact and Mechanism
Insulin Resistance (IR)
- IR, characterized by abnormal cellular responses to insulin, is a significant risk factor associated with AD.
- IR can initiate and exacerbate the pathophysiological processes of AD, influencing neuronal health and function.Atherogenic Dyslipidemia
- AD is often accompanied by dyslipidemia, characterized by abnormal lipid levels that contribute to atherosclerosis.
- Dyslipidemia can impact brain health by affecting cerebral blood flow and vascular integrity, thereby influencing AD progression.Subclinical Inflammation
- AD is associated with chronic, low-grade inflammation.
- Increased levels of inflammatory cytokines like IL-6, TNF, and CRP are observed in AD, contributing to neuronal damage and disease progression.
- Inflammatory processes in the brain can exacerbate AD symptoms and increase the rate of cognitive decline.Oxidative Stress
- AD is marked by increased oxidative stress, an imbalance between free radicals and antioxidants.
- Oxidative stress in AD can result from mitochondrial dysfunction, leading to neuronal damage and the progression of cognitive impairment.
- It can also exacerbate other pathological features of AD, such as amyloid-beta accumulation and tau pathology.
Table 3: AD and various metabolic and physiological processes, including insulin resistance, atherogenic dyslipidemia, subclinical inflammation, and oxidative stress.
NAFLD and AD exhibit overlapping genetic pathways. An investigation of Differentially Expressed Genes (DEGs) identified 21 genes that exhibited similar regulation in both NAFLD and AD [57]. ADAMTS1 and CEBPA were identified as hub genes among the analyzed genes. ADAMTS1 was downregulated, whereas CEBPA was elevated in both disorders. Huang et al. (2022) identified two functional modules: one associated with post-translational protein modification and the other associated with the immunological response. An additional investigation involved an integrated examination of gene expression data in the temporal cortex. This research revealed 16 shared Differentially Expressed Genes (DEGs) between AD and Type 2 Diabetes (T2D). These DEGs were highly concentrated in biological processes, such as apoptosis, autophagy, inflammation, and hemostasis. Hu et al. (2020) identified five hub proteins, five central regulatory transcription factors, and six miRNAs, that are common molecular characteristics shared by both AD and Type 2 Diabetes (T2D). These findings offer a valuable understanding of the same molecular processes linking NAFLD and AD as well as AD and T2D.
Analyze Disease Mechanisms and Treatment
The similarities between NAFLD and AD, such as insulin resistance, inflammation, and oxidative stress, indicate the possible shared pathways of illness. These shared characteristics also have consequences for treatment, as they suggest the possibility of integrated management approaches that address both the primary disease target and any accompanying condition [60]. The correlation between NAFLD and cardiovascular disease, together with the possible contribution of NAFLD to the emergence and advancement of cardiac problems, emphasizes the need for comprehensive treatment strategies [61]. The intricate and interconnected networks implicated in the development of NAFLD and AD further underscore the possibility of employing combination therapies that target various pathways [62]. NAFLD has not received sufficient acknowledgment and appreciation as a significant health issue. Additionally, there is a lack of understanding of the underlying mechanisms of this disease, highlighting the importance of raising awareness and conducting further research [63].
Gut-Liver-Brain Axis
The gut-liver-brain axis denotes the reciprocal exchange of information between the gut, liver, and brain, and the impact that these organs have on each other's operations. Studies have been conducted on chronic liver disease, Hepatic Encephalopathy (HE), and cirrhosis. Disruptions in the gut-liver-brain connection can cause symptoms such as tiredness, isolation, changes in mood, problems with thinking, and difficulty with coordination. The gut microbiome significantly influences this axis, affecting immunological response, metabolite composition, and brain response [64].
The gut-liver-brain axis denotes two-way communication and interaction among the gut, liver, and brain. This axis encompasses the intricate interplay between the gut microbiota, the immune system, neuronal responses, and metabolite composition, which can affect the central nervous system. The gut microbiota has a significant impact on the control and stability of immunological, metabolic, and neuroendocrine activities between the gut and the liver. An imbalance in the gut microbiota, known as dysbiosis, has been noted in individuals with liver illnesses, specifically, cirrhosis. Dysbiosis is associated with cognitive and mood-related behaviors. Understanding the connection between the gastrointestinal system, liver, and brain is crucial to investigate possible medical treatments for liver diseases and related neuropsychiatric disorders [65].
Prolonged and excessive alcohol consumption can cause alterations in the makeup and operation of the gut microbiota, intensifying liver inflammation and damage through the gut-liver connection. Cirrhosis is a medical disorder characterized by inflammation and scarring of the liver tissue. This condition is linked to an imbalance in the gut microbiota, which in turn affects cognitive function and mood-related behaviors. The bidirectional link between the gastrointestinal tract and the liver, referred to as the gut-liver-brain axis, plays a pivotal role in facilitating these interactions [66]. The gut microbiota affects the communication between the gut and the liver, gut, brain, brain, and liver. Any disturbances in this connection can lead to reduced liver function and onset of liver disease. Ensuring a balanced and thriving community of microorganisms in the gastrointestinal tract is crucial for maintaining stability in the connection between the gut and liver, and to decrease the likelihood of liver diseases.
Liver Brain Axis
Hyperammonemia, which is associated with liver problems, can cause disturbances in neurological functions. Conversely, under specific circumstances, ketone body synthesis may be a safeguard. Liver illnesses impair the organ's capacity to eliminate Amyloid-β (Aβ), which plays a role in the development of AD (Figure 3). The proinflammatory state of commonly linked to liver illnesses worsens this scenario, resulting in neuroinflammation.
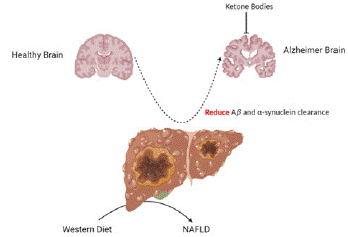
Figure 3: The role of the liver in the metabolism of toxic compounds and in Aβ and a-synuclein clearance is related to AD.
Research Linking Gut Microbiota Changes to NAFLD and AD
Correlation between gut microbiota alterations and the occurrence of NAFLD and AD. Studies have demonstrated a reduction in the prevalence of specific gut bacteria, namely Prevotella, Lactobacillus, and Bacteroides, and a decrease in the amount of equol, a metabolite formed by microbes, in individuals with NAFLD (Dhami et al., 2023). Alterations in the composition and metabolites of gut microbiota have been shown to play a role in the emergence and advancement of NAFLD. Interventions aimed at modifying the gut microbiota have demonstrated the potential to relieve symptoms associated with NAFLD. Furthermore, in the context of AD, there has been a documented imbalance in the gut microbiota, resulting in increased permeability of the intestines, triggering immune system activation and neurodegeneration [68]. Probiotics have been proposed as a therapeutic approach to regulate the gut microbiota and mitigate symptoms resembling those of Alzheimer's disease [69].
Epigenetic Influences
Epigenetic factors, which are influenced by both internal and external stimuli, have a substantial impact on gene transcription, and can cause structural or functional changes. Epigenetic aberrations play a significant role in medical processes, leading to the development of numerous illnesses that affect disease management [70]. Epigenetic mechanisms in pediatric research have been demonstrated to interact with environmental factors and susceptibility genes, influencing the development of the immune system and presentation of diseases. In addition, it has been discovered that epigenetic mechanisms, specifically DNA methylation, play a crucial role in evolution by affecting genomic DNA and remaining consistent during long periods of evolutionary history [71].
What is Epigenetics?
Epigenetics pertains to inheritable modifications of gene expression and functionality that occur without changes in gene sequences. This process encompasses DNA methylation, histone modifications, and non-coding RNAs. Epigenetic pathways are essential for the development of diseases such as Cardiovascular Disease (CVDs), cancer, AD, and endometriosis. Epigenetics plays a role in the emergence and advancement of various illnesses related to Cardiovascular Diseases (CVDs), including hypertension, atrial fibrillation, atherosclerosis, and heart failure. Epigenetic alterations play a role in the variation of phenotypes and inheritance of epigenetic expression in cancer, rendering it a promising candidate for therapeutic interventions [72]. Epigenetic alterations, including DNA methylation, histone modification, and miRNAs, offer novel therapeutic interventions for AD. Epigenetics are also a significant factor in the onset and progression of endometriosis.
Epigenetics and Fatty Acid Liver Disease
Epigenetic alterations have been detected in NAFLD and have significant ramifications in the advancement and emergence of this illness. The involvement of DNA methylation, histone changes, and non-coding RNAs in NAFLD development has been suggested. Research has demonstrated modifications in DNA methylation patterns in NAFLD, characterized by variations in the methylation levels of certain genes, as depicted in Figure 4 [73]. Histone modifications such as acetylation and methylation are involved in preserving chromatin structure and regulating gene expression. Disruptions in these modifications have been linked to Nonalcoholic Fatty Liver Disease (NAFLD). In addition, non-coding RNAs, such as microRNAs, do not function properly in NAFLD and may potentially be used as indicators of the severity of the illness [74].
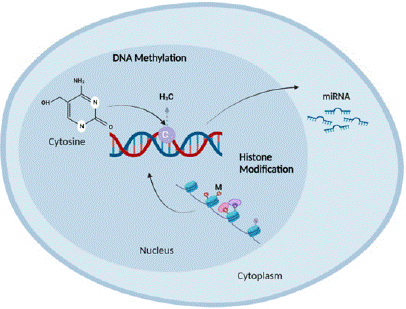
Figure 4: Role of Epigenetic in NAFLD Progression.
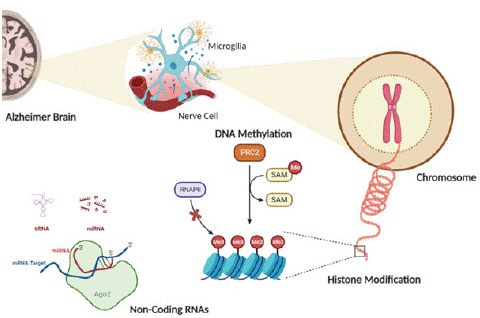
Figure 5: Role of Epigenetic in AD Progression.
Epigenetics and Alzheimer
Epigenetic alterations, such as DNA methylation, histone changes, and regulation by non-coding RNA, have a substantial impact on the advancement and progression of AD, as depicted in Figure 2.5 [75]. These alterations are linked to atypical protein synthesis, inflammation, and memory decline, which are distinctive in AD. Estrogen and androgen have been recognized as the primary catalysts of epigenetic modifications in the brain, which may have an impact on Alzheimer's disease [77]. Moreover, epigenetic modulation can directly or indirectly affect the control of tau phosphorylation, which is a crucial element in AD progression. Researchers are currently investigating the possibility of epigenetic alterations as indicators and therapeutic targets for AD.
Future Prospects
Advancements in the study of the genetics and epigenetics of NAFLD and AD have the potential to greatly enhance our understanding and management of both diseases. PNPLA3, TM6SF2, and MBOAT7 are among the major genetic variations that have been linked to both NAFLD and AD through genetic investigations. These genetic modifiers are associated with the development and progression of liver steatosis, fibrosis, and Hepatocellular Carcinoma (HCC).
Epigenetic factors, specifically DNA methylation and histone changes, are crucial for the development of NAFLD and AD. Abnormal DNA methylation patterns have been detected in patients with NAFLD, and these changes may play a role in disease progression and HCC formation [78]. Understanding the genetic and epigenetic pathways that underlie NAFLD and AD can offer a useful understanding of the disease processes and prospective targets for therapeutic intervention. Additional investigations in this area may reveal new diagnostic indicators and therapeutic strategies for these illnesses.
Integration of multiple disciplines is essential for expanding our understanding of NAFLD and AD. Lucas et al. (2018) emphasized the significance of continuous research on NAFLD, specifically in the advancement of novel treatments. Research emphasizes the intricate nature of NAFLD and the necessity for comprehensive techniques, including several tissues and omics networks, to identify disease-causing genes and identify potential therapeutic solutions. Trojanowski et al. (2012) proposed a paradigm in the field of AD that aims to enhance patient care and accelerate the discovery of new therapies through interdisciplinary research. These results emphasize the crucial need for interdisciplinary collaboration to further our understanding of NAFLD and AD.
Conclusion
In conclusion, the interconnectedness of genetic and epigenetic factors in NAFLD and AD presents a complex but enlightening frontier in global health research. Rapid advancements in genomic and biomarker research are catalyzing progress in early detection, monitoring, and therapy development for these conditions.
A shared understanding of disease mechanisms highlights potential for unified therapeutic strategies. Epigenetic insights offer novel, modifiable targets for treatment. Cross-disciplinary approaches are integral for translating this wealth of scientific knowledge into clinical practice and public health advancements, promising significant strides in the management of NAFLD and AD, and ultimately, improving patient outcomes worldwide.
Author Statements
Funding
This research received no external funding.
Conflict of Interest Declaration
The authors declare that they have NO affiliations with or involvement in any organization or entity with any financial interest in the subject matter or materials discussed in this manuscript.
Author Contributions
Muhammad Saad developed a major research plan. Khizra J wrote manuscripts. Muhamad Saad helped to collect data and references. All authors contributed to the article and approved the submitted version.
References
- Gellert-Kristensen H, Tybjærg-Hansen A, Nordestgaard BG, Ghouse J, Fuchs A, Jørgen Tobias Kühl Sigvardsen PE, et al. Genetic risk of fatty liver disease and mortality in the general population: A Mendelian randomization study. Liver International. 2023; 43: 1955–1965.
- Mann JP, Romeo S, Valenti L. Lipid droplets as the genetic nexus of fatty liver. Liver International. 2022; 42: 2594–2596.
- Xie S, Wei S, Ma X, Wang R, He T, Zhao Z, et al. Genetic alterations and molecular mechanisms underlying hereditary intrahepatic cholestasis. Frontiers in Pharmacology. 2023; 14.
- Romeo S, Sanyal A, Valenti L. Leveraging Human Genetics to Identify Potential New Treatments for Fatty Liver Disease. Cell Metabolism. 2020; 31: 35–45.
- Teng ML, Ng CH, Huang DQ, Chan KE, Tan DJ, Lim WH, et al. Global incidence and prevalence of nonalcoholic fatty liver disease. Clinical and molecular hepatology. 2023; 29: S32–S42.
- Riazi K, Azhari H, Charette JH, Underwood FE, King JA, Afshar EE, et al. The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. The lancet. Gastroenterology & Hepatology. 2022; 7: 851–861.
- Le MH, Yeo YH, Li X, Li J, Zou B, Wu Y, et al. 2019 Global NAFLD Prevalence: A Systematic Review and Meta-analysis. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. 2022; 20: 2809–2817.e28.
- Khan A, Ross HM, Parra NS, Chen SL, Chauhan K, Wang M, et al. Risk Prevention and Health Promotion for Non-Alcoholic Fatty Liver Diseases (NAFLD). Livers. 2022; 2: 264–282.
- Song K, Kim HS, Chae HW. Nonalcoholic fatty liver disease and insulin resistance in children. Clinical and experimental pediatrics. 2023; 66: 512–519.
- Jonas W, Schürmann A. Genetic and epigenetic factors determining NAFLD risk. Molecular metabolism. 2021; 50: 101111.
- Dabravolski SA, Bezsonov EE, Baig MS, Popkova TV, Nedosugova LV, Starodubova AV, et al. Mitochondrial Mutations and Genetic Factors Determining NAFLD Risk. International journal of molecular sciences. 2021; 22: 4459.
- Kim DY, Park JY. Genetic risk factors associated with NAFLD. Hepatoma Research. 2020; 6: 85.
- Trépo E, Valenti L. Update on NAFLD genetics: From new variants to the clinic. Journal of hepatology. 2020; 72: 1196–1209.
- Valenti L, Pelusi S. The Natural History of NAFLD: Environmental vs. Genetic Risk Factors. Non-Alcoholic Fatty Liver Disease. Springer, Cham. 2020.
- Abu Mohammad Asaduzzaman. IDDF2022-ABS-0216 Does PNPLA3 RS738409 impact on NAFLD progression? A systematic review. BMJ Journals. 2022: 71.
- Wang Z, Ye M, Zhang XJ, Zhang P, Cai J, Li H, et al. Impact of NAFLD and its pharmacotherapy on lipid profile and CVD. Atherosclerosis. 2022; 355: 30-44.
- Boulouta A, Aggeletopoulou I, Kanaloupitis S, Tsounis EP, Issaris V, Papantoniou K, et al. The impact of metabolic health on non-alcoholic fatty liver disease (NAFLD). A single center experience. Clinics and research in hepatology and gastroenterology. 2022; 46: 101896.
- Tourkochristou E, Assimakopoulos SF, Thomopoulos K, Marangos M, Triantos C. NAFLD and HBV interplay - related mechanisms underlying liver disease progression. Frontiers in Immunology. 2022; 13: 965548.
- Berná G, álvarez-Amor L, Franz Martin. Geometry of Nutrition: Nutrients and NAFLD Progression. Springer EBooks. 2020: 49-67.
- Calabrò M, Crisafulli C. Perspective Chapter: Alzheimer - A Complex Genetic Background. IntechOpen. 2022.
- Zhang C. Genetic Basis of Alzheimer’s Disease and Its Possible Treatments Based on Big Data. 2020 International Conference on Big Data and Social Sciences (ICBDSS). 2020.
- Ibanez L, Miller JB. Editorial for the Genetics of Alzheimer’s Disease Special Issue: October 2021. Genes. 2021; 12: 1794.
- Floriddia E. Glia heterogeneity in AD. Nat Neurosci. 2022; 25: 531.
- Vandal M, Gunn C, ádám Institóris, Bourassa P, Mishra R, Govind Peringod, Belzil C, et al. The Alzheimer risk factor CD2AP causes dysfunction of the brain vascular network. BioRxiv (Cold Spring Harbor Laboratory). 2020.
- Carrillo MC, Thies W, Bain LJ. The Global Impact of Alzheimer’s Disease. S. Karger AG EBooks. 2012; 1–14.
- Dhingra H, Choudhari SG. Alzheimer’s Disease: Understanding Its Novel Drug Delivery Systems and Treatments. Cureus. 2022; 14: e31394.
- European Alzheimer’s & Dementia Biobank Mendelian Randomization (EADB-MR) Collaboration, Luo J, Thomassen JQ, Bellenguez C, Grenier-Boley B, de Rojas I, et al. Genetic Associations Between Modifiable Risk Factors and Alzheimer Disease. JAMA network open. 2023; 6: e2313734.
- Ma Y, Vardarajan BN, Bennett DA, Fornage M, Seshadri S, Destefano AL, et al. Alzheimer’s disease GWAS weighted by multi- omics and endophenotypes identifies novel risk loci. Alzheimer’s & Dementia. 2020; 16: e043977.
- Takatori S, Wang W, Iguchi A, Tomita T. Genetic Risk Factors for Alzheimer Disease: Emerging Roles of Microglia in Disease Pathomechanisms. In: Guest, P. (eds) Reviews on Biomarker Studies in Psychiatric and Neurodegenerative Disorders. Advances in Experimental Medicine and Biology. Springer, Cham. 2019; 1118: 83-116.
- Sheppard O, Coleman M. Alzheimer’s Disease: Etiology, Neuropathology and Pathogenesis. In X. Huang (Ed.), Alzheimer’s Disease: Drug Discovery. Exon Publications. 2020.
- Si ZZ, Zou CJ, Mei X, Li XF, Luo H, Shen Y, et al. Targeting neuroinflammation in Alzheimer’s disease: from mechanisms to clinical applications. Neural regeneration research. 2023; 18: 708–715.
- Kim H, Chung JY. Pathobiolgy and Management of Alzheimer’s Disease. Chonnam medical journal. 2021; 57: 108–117.
- Sanabria-Castro A, Alvarado-Echeverría I, Monge-Bonilla C. olecular Pathogenesis of Alzheimer’s Disease: An Update. Annals of neurosciences. 2017; 24: 46–54.
- Nuthalapati NS. An Overview of Biomarkers. 2020.
- Frangogiannis NG. Biomarkers: hopes and challenges in the path from discovery to clinical practice. Translational research: the journal of laboratory and clinical medicine. 2012; 159: 197–204.
- Mishra A, Verma M. Cancer biomarkers: are we ready for the prime time?. Cancers. 2010; 2: 190–208.
- Grandjean P. Biomarkers in epidemiology. Clinical chemistry. 1995; 41: 1800–1803.
- Hawksworth J, Fernández E, Gevaert K. A new generation of AD biomarkers: 2019 to 2021. Ageing research reviews. 2022; 79: 101654.
- Masoodi M, Gastaldelli A, Hyötyläinen T, Arretxe E, Alonso C, Goggini M, et al. Metabolomics and lipidomics in NAFLD: biomarkers and non-invasive diagnostic tests. Nat Rev Gastroenterol Hepatol. 2021; 18: 835–856.
- Lakshman R, Shah R, Reyes-Gordillo K, Varatharajalu R. Synergy between NAFLD and AFLD and potential biomarkers. Clinics and research in hepatology and gastroenterology. 2015; 39: S29–S34.
- Lukiw WJ, Vergallo A, Lista S, Hampel H, Zhao Y. Biomarkers for AD and the Application of Precision Medicine. Journal of personalized medicine. 2020; 10: 138.
- Pasha H. Biomarkers in Liver Disease: From Diagnosis to Prognosis. Afro-Egyptian Journal of Infectious and Endemic Diseases. 2020; 10: 332-334.
- McGlinchey AJ, Govaere O, Geng D, Ratziu V, Allison M, Bousier J, et al. Metabolic signatures across the full spectrum of non-alcoholic fatty liver disease. JHEP reports : innovation in hepatology. 2022; 4: 100477.
- de Vries EM, Beuers U, Ponsioen CY. Biomarkers for disease progression of primary sclerosing cholangitis. Current opinion in gastroenterology. 2015; 31: 239–246.
- Wang Shu. The Progression of Current Biomarkers for the Diagnosis of Alzheimer’s Disease. Highlights in Science, Engineering and Technology. 2023; 36: 621-627.
- Mahaman YAR, Embaye KS, Huang F, Li L, Zhu F, Wang JZ, et al. Biomarkers used in Alzheimer’s disease diagnosis, treatment, and prevention. Ageing research reviews. 2022; 74: 101544.
- Cox T, Bourgeat P, Doré V, Doecke JD, Fripp J, Chatterjee P, et al. Comparing the longitudinal progression of CSF biomarkers with PET Amyloid biomarkers for Alzheimer’s disease. Alzheimer’s & Dementia. 2022; 18: e068082.
- Di Costanzo A, Paris D, Melck D, Angiolillo A, Corso G, Maniscalco M, et al. Blood biomarkers indicate that the preclinical stages of Alzheimer’s disease present overlapping molecular features. Scientific reports. 2020; 10: 15612.
- Lin H, Tang S, Liang L, Chen L, Zou C, Zou D. Exploring Early Physical Examination Diagnostic Biomarkers for Alzheimer’s Disease Based on Least Absolute Shrinkage and Selection Operator. Computational and mathematical methods in medicine. 2022; 2022: 3039248.
- Dong W, Huang Y. Common Genetic Factors and Pathways in Alzheimer’s Disease and Ischemic Stroke: Evidences from GWAS. Genes. 2023; 14: 353.
- Rodriguez-Gomez DA, Garcia-Guaqueta DP, Charry-Sánchez JD, Buitrago ES, Blanco M, Velez-van-Meerbeke A, et al. A systematic review of common genetic variation and biological pathways in autism spectrum disorder. BMC Neurosci. 2021; 22: 60.
- Kreiner E, Waage J, Standl M, Brix S, Pers TH, Couto Alves A, et al. Shared genetic variants suggest common pathways in allergy and autoimmune diseases. The Journal of allergy and clinical immunology, 2017; 140: 771–781.
- Li X, Sui JQ, Lu L, Zhang N, Xu X, Dong QY, et al. Gene polymorphisms associated with non-alcoholic fatty liver disease and coronary artery disease: a concise review. Lipids in Health and Disease. 2016; 15: 53.
- Danford CJ, Yao ZM, Jiang ZG. Non-alcoholic fatty liver disease: a narrative review of genetics. Journal of biomedical research. 2018; 32: 389–400.
- Sookoian S, Pirola CJ. Genetics of Nonalcoholic Fatty Liver Disease: From Pathogenesis to Therapeutics. Seminars in liver disease. 2019; 39: 124–140.
- Eslam M, Valenti L, Romeo S. Genetics and epigenetics of NAFLD and NASH: Clinical impact. Journal of hepatology. 2018; 68: 268–279.
- Shu J, Li N, Wei W, Zhang L. Detection of molecular signatures and pathways shared by Alzheimer’s disease and type 2 diabetes. Gene. 2022; 810: 146070.
- Huang C, Wen X, Xie H, Hu D, Li K. Identification and Experimental Validation of Marker Genes between Diabetes and Alzheimer’s Disease. Oxidative medicine and cellular longevity. 2022; 2022: 8122532.
- Hu Z, Jiao R, Wang P, Zhu Y, Zhao J, De Jager P, et al. Shared Causal Paths underlying Alzheimer’s dementia and Type 2 Diabetes. Sci Rep. 2020; 10: 4107.
- Kanbay M, Bulbul MC, Copur S, Afsar B, Sag AA, Siriopol D, et al. Therapeutic implications of shared mechanisms in non-alcoholic fatty liver disease and chronic kidney disease. Journal of nephrology. 2021; 34: 649–659.
- Mantovani A, Ballestri S, Lonardo A, Targher G. Cardiovascular Disease and Myocardial Abnormalities in Nonalcoholic Fatty Liver Disease. Digestive diseases and sciences. 2016; 61: 1246–1267.
- Singh S, Osna NA, Kharbanda KK. Treatment options for alcoholic and non-alcoholic fatty liver disease: A review. World journal of gastroenterology. 2017; 23: 6549–6570.
- Djordjevic DB, Zdravkovic M, Nagorni A, Manolis A, Tsioufis C, Lovic D. A Critical Approach of Guideline Therapeutic Recommendations for NAFLD. Current vascular pharmacology. 2018; 16: 228–238.
- Nguyen HH, Swain MG. Avenues within the gut-liver-brain axis linking chronic liver disease and symptoms. Frontiers in neuroscience. 2023; 17: 1171253.
- Mohan A, Godugu S, Joshi SS, Shah KB, Vanka SC, Shakil H, et al. Gut-brain axis: altered microbiome and depression - review. Annals of medicine and Surgery. 2023; 85: 1784–1789.
- Smith ML, Wade JB, Wolstenholme J, Bajaj JS. Gut microbiome-brain-cirrhosis axis. Hepatology (Baltimore, Md.). 2023.
- Dhami M, Raj K, Singh S. Relevance of Gut Microbiota to AD: Potential Effects of Probiotic in Management of AD. Aging and Health Research. 2023; 3: 100128.
- Lanthier N, Delzenne N. Targeting the Gut Microbiome to Treat Metabolic Dysfunction-Associated Fatty Liver Disease: Ready for Prime Time?. Cells. 2022; 11: 2718.
- Higarza SG, Arboleya S, Arias JL, Gueimonde M, Arias N. The gut-microbiota-brain changes across the liver disease spectrum. Frontiers in cellular neuroscience. 2022; 16: 994404.
- Tollefsbol TO. An Overview of Medical Epigenetics. Elsevier EBooks. 2016; 3–7.
- Mendizabal I, Keller TE, Zeng J, Yi SV. Epigenetics and Evolution. Integrative and Comparative Biology. 2014; 54: 31–42.
- Carlberg C, Velleuer E, Molnár F. Epigenetics and Disease. In: Molecular Medicine. Springer, Cham. 2023.
- Fu W, Joshi A, Pinney SE, Pheruza Tarapore Wang Z, Green S. RF22 | PSUN318 Hepatocytes Exposed to PFOA Prior to Differentiation Leads to Epigenetic Changes in Genes Linked With NAFLD. Journal of the Endocrine Society. 2022; 6: A449–A449.
- Lyall MJ, Thomson JP, Cartier J, Ottaviano R, Kendall TJ, Meehan RR, et al. NAFLDis associated with dynamic changes in DNA hydroxymethylation. Epigenetics. 2020; 15: 61–71.
- Shierly, Wirawan C. The role of epigenetic modifications in Alzheimer’s disease. International Journal of Research in Medical Sciences. 2020; 11: 294.
- Nikolac Perkovic M, Videtic Paska A, Konjevod M, Kouter K, Svob Strac D, Nedic Erjavec G, et al. Epigenetics of Alzheimer’s Disease. Biomolecules. 2021; 11: 195.
- Kumar R, Fatima F, Yadav G, Singh S, Haldar S, Alexiou A, et al. Epigenetic Modifications by Estrogen and Androgen in Alzheimer’s Disease. CNS & neurological disorders drug targets. 2023; 22: 6–17.
- Habash NW, Sehrawat TS, Shah VH, Cao S. Epigenetics of alcohol-related liver diseases. JHEP reports: innovation in hepatology. 2022; 4: 100466.
- Lucas C, Lucas G, Lucas N, Krzowska-Firych J, Tomasiewicz K. A systematic review of the present and future of non-alcoholic fatty liver disease. Clinical and experimental hepatology. 2018; 4: 165–174.
- Trojanowski JQ, Arnold SE, Karlawish JH, Naylor M, Brunden KR, Lee VM. A model for improving the treatment and care of Alzheimer’s disease patients through interdisciplinary research. Alzheimer’s & dementia: the journal of the Alzheimer’s Association. 2012; 8: 564–573.
