Review Article
Austin J Public Health Epidemiol. 2014;1(1): 1004.
A Systems Approach to Cancer Health Disparities in Appalachia
Knox SS*, Basu S and Remick S
Department of Epidemiology, West Virginia University, USA
*Corresponding author: Knox SS, Department of Epidemiology, School of Public Health, Mary Babb Randolph Cancer Center, School of Medicine, West Virginia University, Post Box 9190, Morgantown, WV 26508, USA
Received: May 20, 2014; Accepted: June 25, 2014; Published: June 27, 2014
Abstract
The rural area of Appalachia in the U. S. encompasses 13 states that extend along the spine of the Appalachian mountain range. For reasons that are still not fully understood, this region has some of the highest rates of cancer mortality in the U.S. The article discusses cancer as a complex, systemic disease with emergent properties that develops over time through interactions between genetic, biological and environmental factors. The term environment is used broadly to include social, behavioral and toxicological contributors. However, the common denominator uniting many of these factors is low socioeconomic status (SES). The article focuses on the complex pathways through which low SES contributes to cancer in this rural area, highlighting the need for multilevel treatment approaches. It also addresses the inadequacy of traditional statistical methods for interpreting either the multi factorial etiology of cancer or the short and long-term effects of multilevel interventions. To resolve these issues, it suggests greater utilization and development of analytic techniques that can incorporate temporal changes and nonlinear interactions in order to grapple with reducing the disparities. The methodological issues discussed are generalizable to other rural areas.
Keywords: Low socioeconomic status; cancer; dynamical systems; multilevel approaches; Appalachia
Abbreviations
ACCN: Appalachian Community Cancer Network; CDC: Centers for Disease Control; CpG: Island a group of DNA sites characterized by a cytosine nucleotide connected with a guanine nucleotide through a phosphate; EGCG: Epigallocetechin-3-gallate; HPV: Human Papilloma Virus; SES: Socioeconomic Status
Introduction
The region known as Appalachia is about 42 % rural includes 420 counties in 13 states and encompasses approximately 205,000 square miles that stretch along the spine of the Appalachian mountain range from New York to Mississippi [1]. Cancer mortality rates are higher in Appalachia than in the rest of the nation and higher in Appalachian counties than in non-Appalachian counties in states that contain both [2,3]. Although colorectal cancer, lung cancer, female breast cancer and cervical cancer have been identified as having the greatest risk in this region [2], a closer look at the data reveals that mortality from different cancer types is not homogenous within Appalachia but varies from state to state [1,2]. Since cancer is the second leading cause of death in the United States [4], understanding the cancer disparities in Appalachia should be a high priority. More importantly, Appalachia is a rural, low Socioeconomic status (SES) area, and information gleaned from understanding the complex interplay of factors contributing to health disparities in this area will provide important guidelines for approaching health disparities in other rural areas that are also characterized by low SES. Methodological issues relevant to understanding the structure of the systems as well as the relationships among the relevant bio molecular, socioeconomic, environmental factors and pathogenic mechanisms in this underserved population are generalizable also to other underserved groups.
Appalachian research relevant to health care access [3,5-9], the effect of culture on willingness to seek care [3,10-16], and on health related behaviors [17-24], has contributed valuable information for improving health care delivery and prevention, though much remains to be accomplished. An example is the successful utilization of community-based participatory research in bringing about behavior changes related to cancer screening [14-18]. Despite these advances, individual beliefs and behaviors are only part of the complex multi-factor risk profile contributing to cancer health disparities in Appalachia. Given that cancer risk differs between Appalachian and non-Appalachian counties in the same states, and that the magnitude of risk for certain types of cancers varies also among Appalachian states, the probability that the causes are primarily genetic is low. Genetic changes due to population drift occur slowly and these populations are not geographically isolated enough for genetics to be a major contributor to disparities. However, accumulating evidence indicates that the role of gene x environment interactions is extremely important [25,26]. Malignancy is characterized by global gene expression changes such as genome-wide hypomethylation of DNA which can lead to oncogenesis and chromosomal modifications [28] and hypoacetylation of chromatin [27-30]. The fact that these changes are genome-wide and occur before cancer manifests [28], indicates that more than one system (and therefore more than one gene) is affected, and highlights the fact that cancer is not a disease of single cells. In order for uncontrolled growth to occur, the body's defenses against malignancy, e.g., DNA repair, immune surveillance and apoptosis must first cease to function properly [25,26], meaning that cancer is a systemic disease.
Cancer and Epigenetic Regulation
The fact that the same candidate gene can be associated with different phenotypes depending on global gene expression patterns lies at the heart of gene-environment interactions. Metastable epialleles are loci that can be epigenetically modified in a manner that is variable and reversible so that a distribution of phenotypes can be produced from genetically identical cells [28]. This serves the evolutionary function of robustness. The system does not crash because one part of it becomes dysfunctional. Biologically, genes are up and down regulated in a dynamic, ongoing manner based on changing demands and inputs to the system. Data suggest that more loss of tumor suppressor gene function may occur through epigenetically mediated gene transcription repression than through actual gene mutations [29-32]. The epigenetic regulators of gene expression in the microenvironment are strongly influenced not only by health behaviors such as diet and smoking, but by environmental toxicants and psychosocial stressors. A great deal has been written about dietary risk factors and cancer [33], and one of the most important mediating mechanisms is influence of diet on methylation. Folate and vitamin B12 affect the availability of methyl groups that have been experimentally demonstrated to be involved in certain types of carcinogenesis [34-38]. Phytoestrogens present in soy, such as genistein have been shown to inhibit DNA methylation [39] and Epigallocetechin-3-gallate (EGCG), the major catechin (natural phenol and antioxidant) in green tea, inhibits DNA methyl transfers and reactivates expression of epigenetically silenced genes such as RAR-beta2 [40,41]. It has also been demonstrated that treatment of cancer cells with EGCG, can cause the demethylation of CpG islands in promoter regions and reactivation of methylation-silenced genes such as p16 [42], which is one of the most commonly hyper methylated genes in lung cancer [43].
Cumulative burden, low SES and cancer
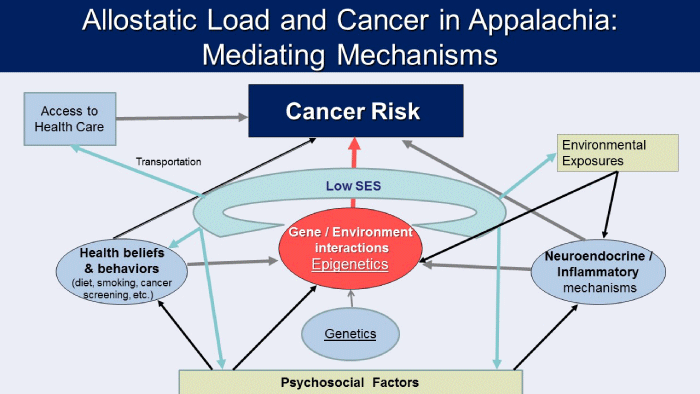
One of the most ubiquitous characteristics of Appalachia is low socioeconomic status. The percent of residents living in poverty in Appalachia is 31% and educational attainment remains lower than in the rest of the country [44]. The fact that the incidence of cancer increases as we age reflects the gradual breakdown of bodily systems over time, based on 'wear and tear'. We know that smoking significantly increases the risk of lung cancer and yet people can smoke for years without contracting cancer because the body's defense systems repair the damage. However, the longer they smoke and the older they are, the higher their risk. So why should rural Appalachia have more cancer than urban low SES areas? Low SES involves many different types of adversity which affect multiple behavioral and physiological pathways. Findings from the McArthur Study of Successful Aging, a longitudinal study of older adults [45] have provided an overview of biological vulnerability in low SES populations. That project revealed that a cumulative index of biological risk explained 35.4% of the disparities between those with higher and lower SES in all-cause mortality even after adjusting for traditional risk factors. The term that they used to describe the biological changes resulting from cumulative wear and tear is 'allostatic load' [46]. Allostasis refers to the dynamic way that the body changes and responds as it adapts to the ever varying demands of its environment [47]. Allostatic load denotes the biological burden that accrues when cumulative demand outstrips the body's ability to compensate, resulting in dysfunction in multiple systems. With each new demand, the body tries to respond in the most optimal manner. However, if an onslaught of multiple demands persists, it becomes increasingly difficult for biological systems to respond optimally. Attempts to compensate for increasing deficiencies in overburdened systems result in eventual dysfunction in feedback mechanisms that maintain physiological balance, resulting in the gradual breakdown of bodily processes related to aging. Given the number of systems that must become dysfunctional for malignancy to manifest, this is a good description of gradual dysfunction leading to cancer. The better one's access to care, the more constructive one's health related behaviors (e.g. smoking, diet, alcohol consumption, physical activity), and social networks; and the lower the exposure to environmental toxins and chronic stress, the more resilience the body will have as it ages. Higher resiliency moderates Allostatic load. However, when demands from the environment are excessive, it becomes difficult for the body's immune and repair mechanisms to handle the load. Its capacity for repair and regeneration diminishes, which accelerates aging and the onset of aging related chronic diseases. Adding to the complexity of understanding how these factors interact, is that there are gender differences in age trajectories of physiological dysregulation related to inflammation and the metabolic syndrome [48]. Accumulating evidence shows that lower SES is associated with poorer trajectories of aging in multiple physiological systems, resulting in an excess of cumulative dysfunction [49,50]. An overview of physiological mechanisms can be seen in (Figure 1). Thus, health disparities related to low SES result from multilevel challenges: individual behaviors, stresses and resources; community-level environmental and social factors, community resources and the impact of state policy decisions and economic conditions over which the individual has no control. According to one review [51], economically disadvantaged areas, i.e., those where economically disadvantaged people can afford to live, are more apt to be at risk due to a higher frequency of pollution sources (and zoning laws which allow them), illegal dumping, poor enforcement of environmental regulations and inadequate response to community complaints. Inadequate public transportation is another community-level factor that can differentially affect low SES individuals, making it difficult for them to get to work or to a proper grocery store to obtain fresh vegetables and fruit. One of the biggest problems in Appalachia is the lack of public transportation systems and the difficulties and expense involved in getting to health care appointments. The distance that people have to travel is often prohibitively expensive for anyone with low incomes and low social cohesiveness in many low SES urban communities is an additional source of stress. State policies related to cigarette taxation and smoke free public environments, access to health care for those who cannot afford it, and policies related to nutrients in school lunches or physical activity in schools also play a major role. At an individual level, poor diet, low physical activity, smoking, alcohol consumption and reluctance to get cancer screening; as well as chronic stress related to poverty, and low social resources to help cope are well known sources of burden. Together these factors result in economically disadvantaged people having more challenges to the biological mechanisms maintaining health and fewer resources with which to cope than higher SES individuals, resulting in a higher Allostatic load.
Social isolation / Social resources and cancer
One of the characteristics that differentiates rural from urban poor is the "micro social system" often present in rural areas, particularly Appalachia [52]. In Appalachia, the economy is often dependent upon a single employer, who rigidly controls who works and who doesn't. This has traditionally created a class system with "haves and have-nots" with almost no middle class. The concept of "social capital", which involves norms of trust, communication networks and mutual cooperation is more common in middle class communities and serves to facilitate mobility and change [52]. Thus the historically rather rigid class structure in Appalachia has led to a dearth of supportive social networks that might improve social/economic conditions. Multiple prospective epidemiologic studies that controlled for relevant covariates and had few losses to follow-up [53], have reported that social isolation increases risk for all-cause mortality [54-61]. Accumulating data also indicate that supportive care increases survival time of cancer patients in the absence of effective targeted therapies [62]. In fact, a meta-analysis of the association of social networks with cancer mortality, reported that in controlled studies, having high levels of perceived social support, larger social network and being married were associated with decreases in relative risk for mortality of 25%, 20% and 12%, respectively [63]. That same study reported that there were stronger associations of social support to leukemia and lymphomas [63]. However, there are also indications that social connections may be different for men and women. One prospective 17 year study of 6,848 adults reported that women who were socially isolated had significantly higher risk of mortality from cancer than women who weren't but that this was not the case with men. Socially isolated men showed significantly poorer cancer survival rates after diagnosis [64]. Both chronic social isolation and chronic social support are associated with neuroendocrine changes in the body. However, the mechanisms seems to vary somewhat. A discussion of some of the physiological mechanisms involved in isolation vs. support, gleaned from both human and animal studies [65], reports that chronic social isolation is associated with sympathetic nervous system over activation, hypothalamic-pituitary-adrenocortical axis dysfunction and endothelial dysfunction. Whereas the buffering effects of social support seem to involve partially different mechanisms, namely the release of oxytocin in the hypothalamus of the brain. Oxytocin is a neuropeptide that differs by only two amino acids from arginine vasopressin [66] and opposes the effects of vasopressin. It is released not only during lactation but also in both men and women during light touch, message, and warm temperature. Since touch is an important aspect of affiliative relationships within families, the cohesiveness in families, which is strained in situations of poverty, is also important on a physical level. Furthermore, shorter telomeres and high telomerase activity have been associated with reduced social support [67]. The presence of telomerase is necessary for unlimited proliferation and is usually found in tumor tissue but not in normal somatic tissue with differentiated cells [68,69]. Together, these extensive data indicate that social isolation can be causally associated with ill health, mortality and cancer, whereas its opposite, social support can serve as a buffer or moderator helping to ameliorate the effect of other chronic burdens.
Figure 1 :
Implications for improving cancer health disparities
In sum, research into health disparities in cancer indicates multi factorial etiology consisting of individual level factors, family level factors, neighborhood or community factors and health policy issues. Known environmental carcinogens include microbial agents (hepatitis virus, Human PapillomaVirus (HPV), human herpes virus, T-cell leukemia virus, and H. pylori) and environmental factors (ultraviolet 6 light, tobacco products, asbestos, radon gas, vinyl chloride and toxic chemicals) [70]. Endogenous mediators include (but are not limited to) inflammation, genetic replication, metabolic dysfunction [70] and epigenetics. Because cancer is a complex disease involving multiple exposure and effective pathways, multi-level approaches towards prevention and intervention are important. Furthermore, the simple linear models typical in the biological sciences are not sufficient for explaining these complex interactions. Environmental factors and health habits influence gene expression at a molecular level and lead to emergent properties that result in a 'whole' which is more than the sum of its parts [25]. Historically, the primary focus of prevention efforts has been directed towards the individual, especially towards educating people about health behaviors, (e.g. cancer screening, smoking, diet) and trying to induce behavior change through community level interventions. These efforts are important and community level intervention has increased their effectiveness but they do not go far enough. Even if we could eliminate all individual lifestyle factors, there would still be multiple 'environmental' (term used broadly to include social and biological, as well as physical) factors left to address [70].
Multi-level interventions
It is obvious from the foregoing discussion that complex etiological factors require multi-level interventions aimed at reducing risk. Historically, the primary focus has been on educating individuals to change non-constructive health behaviors such as smoking or lack of cancer screening. These are important but not sufficient for achieving a comprehensive reduction of health disparities. Some of the issues related to multi-level approaches are listed below.
Health policy-national
The current regulatory approach in the U.S. with respect to environmental toxins is "reactionary rather than precautionary" [71]. That means that only when a chemical is incontrovertibly proven to be a hazard, can it be regulated. Uncertainty as to a chemical's toxicity (the precautionary approach) does not warrant required testing. Of the more than 80,000 chemicals used in the U.S. today, only a few hundred have been tested for toxicity [71]. Since the public bears the burden of proving harm (at enormous financial cost), which is difficult to do with cancer due to the fact that risk may accrue with multiple years of cumulative exposure, regulation is effectively non-existent. Environmental exposures come from agriculture (pesticides and insecticides); manufacturing [Bisphenol A (BPA), perfluoroalkyl substances (PFCs)]; modern lifestyle (dry cleaning, cellular communications, air travel, water, emissions from vehicles); medical sources (radiation, drugs); and even natural sources such as radon gas [71]. Many of the sources from manufacturing and agriculture, such as PFCs used in the linings of food containers and stain resistant cookware and fabrics; BPA, used to strengthen plastics and previously used in baby bottles; and DDT used as a pesticide, are persistent in the environment. This means that they remain even if the chemical has not been used for many years. According to the President's Cancer Panel, the solution to this global problem is a comprehensive policy agenda regarding environmental contaminants and protection of human health [72]. The regulations need to take into account the cumulative effect and long latency of some of these chemicals with respect to chronic disease and enforcement of already existing standards (something that is less common in low SES rural areas). The President's report includes a 'call to action' with respect to suspected environmental carcinogens. This call to action includes more scientific research to help us understand these chemicals and prevent harm to human health, enforcing policies and regulations that protect both workers and the public; as well as implementing policy changes that support public health and reduce the burden of cancer [71].
Health policy-state
The Centers for Disease Control and prevention (CDC) has recommended that all girls 11 and 12 years old be vaccinated against HPV to prevent cervical cancer. Yet a review of an immunization database and interviews of health department personnel in Appalachia have shown great variation in HPV vaccine availability, recommendations, cost, policies and educational materials in seven Appalachian states [73]. A report from the Appalachian Regional Commission reports also that Appalachian counties have , in the aggregate, more healthcare cost, coverage, and access disparities than their respective states' or the United States' average [74]. These data indicate that disparities in cancer in Appalachia may be partially due to varying health policy in these states and that formulating and enforcing state policies aimed at protecting regional health would be an important step in reducing the health disparities in Appalachia.
Community level interventions
Because Appalachians are considered to be medically underserved with respect to cancer mortality, The National Cancer Institute set up an Appalachian Community Cancer Network (ACCN) [75] to take concrete steps to improve prevention efforts. The objective of this network is to build community capacity, including establishing culturally appropriate communication, awareness, educational materials, and knowledge concerning cancer and cancer prevention. Conceptually, their intent is to strengthen research infrastructure, enhance health services, and increase organization and activities of communities and coalitions [75]. The purpose is to increase health care delivery and community cancer control through decreased risk behavior; increased screening; greater health promoting policies and increased clinical trial participation. The ultimate goal is to reduce incidence and mortality from breast, cervical and colorectal cancers and those related to tobacco [75]. One example of the initiatives stemming from this collaboration include, the "Faith moves Mountains" project aimed at increasing cervical cancer screening in Appalachia [76], whose objective is a community-based participatory research program implemented with churches, to increase cervical cancer screening (Pap tests). After modification it has succeeded in recruiting and retaining over 400 women and 30 churches [76], as well as increasing follow-up care in women with abnormal Pap test results [77]. Other prevention projects of this coalition in collaboration with communities have been utilized to successfully pick the most culturally appropriate educational materials for physicians' offices [78] and to increase colonoscopies [79].
Building community capacity, whether it be increasing social resources and social cohesiveness, improving educational efforts or availability of care, involves shifting focus from existing deficits to existing strengths on which capacity for improving interventions and services can be built [80]. Toward this end, the use of dynamic systems approaches have received more and more traction [80- 81] as it becomes evident that a plurality of methods are needed and that no single method fits across the board. Communities are complex, dynamic entities involving power structures, social relationships, generational differences and differing race/ethnic/ cultural values [80]. As community capacity and needs change and evolve, prevention and intervention efforts must also follow suit. The intervention itself can also change community dynamics in ways that require additional adjustment. For these reasons, dynamic systems approaches emphasize 'best processes' rather than 'best practices' because processes change as new knowledge, methods and information develop [80].
Family level interventions
When an individual receives a diagnosis of cancer, it affects the entire family. They become involved in care, support or lack of it, and are sources of information and advice. In Appalachia, families tend to be the first resources that patients go to for advice [82]. This means that it is important to involve the family in treatment decisions. In Appalachia, where resources are already extremely limited, a family member's illness may lead to disruption and even dysfunction, due to the inability of a key family member to continue their usual functional role or due to a loss of income when the ill member cannot work. Thus, interventions to support families whose coping skills may already be taxed to the limit can be very important. Marital status and family income have been shown to be significantly associated with following guidelines for cervical cancer screening (1). Another type of example that demonstrates the importance of family in cancer treatment and prevention involves diet. There is wide agreement that nutrition is a key factor in cancer prevention [33]. The nature, quality, quantities and proportions of different foods and drinks are important, as well as their frequency of consumption [33]. In Appalachia, it has been shown that dinner vegetable variety was very limited because food preparers served only what family members liked to eat, deferring to the male partner and children [83]. That study [83] reported that even removing barriers to access and affordability was not enough to change behavior. The study concluded that role expectations were central to resistance to change and that teaching negotiation techniques could strengthen community interventions targeting families [83]. The use of smokeless tobacco in Appalachian boys has also been shown to be strongly influenced and even instigated by male family members as well as peer networks [84]. This has been intensified by advertising and easy access. Bringing about change in this area necessitates changing not only norms related to masculinity and Appalachian identity but also health policy aimed at reducing tobacco marketing [84].
Individual level factors
Some of the most important individual level behaviors have already been mentioned, as they are often the focus of community level interventions. Cancer screening (Pap test) and prevention such as HPV vaccination are examples. Barriers to both have been identified in Appalachian Kentucky women. Cost is an issue for poor women for whom preventive services are just not a priority [16]. Other barriers include privacy (being seen entering a reproductive clinic in a small town), lack of understanding of the need for three doses of HPV vaccine, and normative influences from peers and family [16], as well as the belief that cervical cancer has symptoms so that if you don't have symptoms, you don't have cancer [85]. However about half of Appalachian women who took part in an HPV study knew that HPV vaccine could prevent cervical cancer [86] even though only 31% of women would get the vaccine for themselves. In older women, the primary reason for not getting it was that they were older than the recommended age. However in women 18-26 years of age, the primary reason for not getting it was that it was 'not safe' [86]. It has been demonstrated that nutrition can affect HPV infection risk and that high antioxidant intake can boost the body's defense against it [87]. However, food insecurity is high in poverty areas like Appalachia, meaning that a healthy diet is out of reach for many people. Unfortunately, food insecurity in Appalachia has also been shown to be significantly associated with high risk sexual behavior [87]. Mobile mammography screening is a method that has been utilized to improve individual access to cancer screening. One such program, "Bonne's Bus" has been used to take breast cancer screening to remote areas of Appalachia with the aim of eliminate the most common barriers to access [88]. However, utilization of these services varies according to individual level factors such as a patient's perception of risk, educational level and socioeconomic status.
Other well-known individual level risk factors for cancer, such as nutrition mentioned earlier, obesity and smoking also have unique features in the rural Appalachian population. Smoking during pregnancy in Appalachia women has been reported to be associated with both low educational level and weight concerns [89]. Similarly, low socioeconomic position and depressive symptoms have been positively associated with smoking in rural Appalachian women [90]. The prevalence of another of the most well-known cancer risk factors, obesity, is high in Appalachia [91,92]. Unfortunately, primary care physicians in southern Appalachia play a limited role in the prevention or intervention of overweight and obesity in childhood [91], even though they believe it is important. They do discuss physical activity with parents of these children but rarely give the parents tools that are needed to make changes [91]. Most of the individual level risk factors have been extensively addressed in the literature but achieving change is still a major challenge.
Genetics is one of the most important individual level risk factors associated with cancer but seems to be of primary importance in creating host susceptibility to environmental insult, (i.e., there is rarely a one-to-one association between genotype and phenotype). DNA sequence is static and does not change as we age, but epigenetic modifications, because they are influenced by changing environmental inputs, are dynamic and fluctuate in response to the body's needs. Cancer increases with age and cumulative burden. The factors constituting to cumulative strain on the system range from stress to health behaviors to environmental toxins, all of which are more frequent in low SES populations and all of which are associated with gene expression changes [26]. These functional importance of the accumulating gene expression changes cannot be underestimated [25,26]. Before tumors manifest, the epigenome is characterized by global changes including genome wide hypomethylation of DNA and site-specific hypermethylation [93] as well as hypoacetylation of chromatin [94-97]. Because the changes are wide spread, they are an indication that multiple systems are involved before tumors manifest. Mutations cannot survive in healthy systems because there are multiple DNA repair, immune and apoptosis mechanisms to combat them [25,26].
Methodological Issues
What should be obvious from the previous discussion is that there are multiple causal factors contributing to cancer in this rural, low SES population and that they interact with each other in complex, nonlinear ways. Furthermore there are multiple possible levels of intervention and a health care delivery system that consists of governmental and non-governmental agencies, as well as the private sector. This means multiple interacting systems, each of which is dynamic. Traditional, linear statistical methods cannot begin to adequately address this complexity. In response to this need, new approaches to systems modeling and systems thinking are beginning to emerge. Below we discuss some of the methodological issues and approaches for addressing them.
Temporal component
In diseases of cumulative burden like cancer, not only are their multiple causal factors, but the timing and duration of exposure are extremely important. For instance, toxic exposures or diet during gestation can have effects on gene expression that sometimes last into adulthood [98-103]. This is also the case for other developmental windows such as post-birth, where it has been demonstrated that maternal care can be a mediator of the effects of environmental adversity on neural development [104-106]. Depending on the gene and the environmental factors under investigation, effects may be cumulative or they may be relegated to a window of vulnerability. If timing of exposure is crucial but is not known to the investigator, s/ he risks designing a study in the wrong age group and failing to find an association when one actually exists, what is called a Type 2 error [107]. Unfortunately, the genes most prone to epigenetic regulation and their critical windows of susceptibility have been incompletely defined [108]. To further complicate matters, some of the time varying elements may be influenced by other time varying elements [109].
Observational data
Even though randomized interventions are the gold standard, when it comes to health policy decisions, such as those pertaining to tobacco products, randomization is not always feasible or ethical [109]. On the other hand, others types of interventions, such as those done in the work place (e.g., to reduce obesity (physical activity programs, removal of sugary drinks in vending machines) lend themselves easily to randomization. It has been suggested [109] that one way to get around the dilemma of randomization when it isn't feasible, is to mimic it by comparing people who change and people who don't during a defined study period.
Dynamical systems analysis
The most crucial issue, however, is not only multilevel factors and multiple simultaneous inputs across systems, some of which are synergistic, some of which are additive, and some of which cancel each other out. There is also the issue of changing dynamics in these systems and temporal windows of vulnerability. Methodologically and analytically, integrating multilevel interventions and/or causality is a challenge. It requires analytic tools that facilitate interpretation of outcomes with as little confounding as possible. The interactions illustrated in Figure 1 involve different rates of change in multiple systems over time. The biological interactions between individual components are sometimes nonlinear and there are strong indications that the accumulating dysfunction in the system from multiple demands is also nonlinear. At some, as yet unknown threshold, a tipping point is reached and the system bifurcates, transitioning from a healthy 'attractor' (i.e., a state to which the system keeps returning after temporary changes) to a non-healthy, or cancerous one [26]. A simple analogy would be 'the straw that broke the camel's back'. The system continues to function after a fashion as more and more feedback loops are compromised. At a critical point, it breaks down and feedback loops that earlier supported health begin to function in support of malignancy [26]. The statistical analyses that are most commonly used in current public health research are not appropriate for complex systems whose components form emergent properties that are capable of adapting to changing circumstances [110].
Thus, systems' thinking is conceptually focused on interrelationships between parts as well as their relationships to the functioning 'whole' [111]. Measuring the effect of an individual intervention is relatively easy but measuring the relative and combined effects of multiple interventions (e.g., community, individual and/or health policy) is complicated. This complexity also requires taking into consideration additional factors such as how short-term effects or efforts can translate into long-term outcomes [112]. Systems analytic approaches expand the set of statistical tools used in experimentation, with computational modeling and simulation. For instance, one approach that is capable of incorporating nonlinearity into systemic interactions uses differential equations that simulate what happens to the system when different parameters and feedback loops (e.g., differential growth patterns, oscillatory activity, etc.) change [110,113]. These methods allow predictions about whether the system settles into equilibrium, changes in repeating cycles or varies in more complex ways [111]. Such approaches allow for the use of time delays to facilitate modeling of temporal dynamics and utilize an iterative process of generating hypotheses, diagramming, quantification, reliability testing and perhaps policy analysis until the model meets requirements of robustness, realism and flexibility [113]. It also allows for the inclusion of variables that are hypothesized to be important but for which quantitative measurements are lacking [113]. Because dynamical systems are very sensitive to initial conditions, sensitivity testing in the model is important. What it reveals is which parts of the system are robust to changes and which are sensitive [113], allowing people in a decision making role (e.g., policy makers) to decide where it makes most sense to invest in improvement.
Network analyses
Network analyses are utilized to describe the structure and function of relationships between members (whether they be people in a community or genes and proteins in a signaling pathway) based upon certain criteria [113-118] and can be utilized to combine networks on multiple levels. So for instance, in an attempt to identify a source of a tuberculosis outbreak in British Columbia [118], a complicated social network analysis was done to establish contacts and latency of contacts between people. Then the complete genomes of 32 Mycobacterium tuberculosis outbreaks were genotyped as well as 4 historical isolates from the region that had been taken before the outbreak. The result was the identification of two genetically distinct lineages of M. tuberculosis, suggesting two concomitant outbreaks. Integrating this data with the social-network analyses revealed key transmission events [118]. Another use for network analyses is the identification of multilevel physiological pathways which can be mapped by superimposing 'omics' data from different hierarchical levels (e.g., proteomics data on gene expression data and metabolomics data on top of that) to identify pathophysiological processes. One such Interactive analysis program, Ingenuity Pathway Analysis [119-121], utilizes an interactive database combined with analytic tools that allow the user to incorporate microarray analyses into functional or disease associated networks.
Conclusions
Cancer health disparities in Appalachia require urgent attention. Utilizing multi-disciplinary research and systems analytic approaches to resolve them could not only help facilitate prevention efforts by identifying early warning signs for risk at a stage where cancer is still preventable, they could contribute to improving multi-level treatment approaches by providing comprehensive data on which to base decisions concerning health care priorities and policy. The knowledge gained has the potential to be instrumental in providing methodological tools for integrating cultural, biological and environmental data with social determinants of health that can be utilized to resolve similar issues in other rural populations.
References
- Paskett ED, Fisher JL, Lengerich EJ, Schoenberg NE, Kennedy SK, Conn ME, et al. Disparities in underserved white populations: the case of cancer-related disparities in Appalachia. Oncologist. 2011; 16: 1072-1081.
- Blackley D, Behringer B, Zheng S. Cancer mortality rates in Appalachia: descriptive epidemiology and an approach to explaining differences in outcomes. J Community Health. 2012; 37: 804-813.
- Behringer B, Friedell GH, Dorgan KA, Hutson SP, Naney C, Phillips A, et al. Understanding the challenges of reducing cancer in Appalachia: Addressing a place-based health disparity population. Calif J Health Promotion 2007; 5: 40-49.
- Hoyert DL, Xu J. Deaths: Preliminary data for 2011. Nat Vital Stat Reports. 2012; 61.
- PDA Inc. & The Cecil B Sheps Center for Health Services Research UNC. Health care costs and access disparities in Appalachia. Prepared for : Appalachian Regional Commission.
- Elnicki DM, Morris DK, Shockcor WT. Patient-perceived barriers to preventive health care among indigent, rural Appalachian patients. Arch Intern Med. 1995; 155: 421-424.
- Smith LH, Holloman CH. Health status and access to health care services: a comparison between Ohio's rural non-Appalachian and Appalachian families. Fam Community Health. 2011; 34: 102-110.
- Raghavan D. Slow progress in cancer care disparities: HIPAA, PPACA, and CHEWBACCA... but we're still not there! Oncologist. 2011; 16: 917-919.
- Huttlinger K, Schaller-Ayers J, Lawson T. Health care in Appalachia: a population-based approach. Public Health Nurs. 2004; 21: 103-110.
- Beyer KM, Comstock S, Seagren R, Rushton G. Explaining place-based colorectal cancer health disparities: evidence from a rural context. Soc Sci Med. 2011; 72: 373-382.
- Spleen AM, Lengerich EJ, Camacho FT, Vanderpool RC. Health care avoidance among rural populations: results from a nationally representative survey. J Rural Health. 2014; 30: 79-88.
- Pakettt ED, McLaughlin JM, Reiter PL, Lehman AM, Rhoda DA, Katz ML et al. Psychosocial predictors of adherence to risk-appropriate cervical cancer screening guidelines: A cross sectional study of women in Ohio Appalachia participating in the Community Awareness Resources and Education (CARE) project. Prev Med 2010; 50: 74-80.
- McAlearney AS, Oliveri JM, Post DM, Song PH, Jacobs E, Waibel J,et al. Trust and distrust among Appalachian women regarding cervical cancer screening: a qualitative study. Patient Educ Couns. 2012; 86: 120-126.
- Schoenberg NE, Howell BM, Fields N. Community strategies to address cancer disparities in Appalachian Kentucky. Fam Community Health. 2012; 35: 31-43.
- Scarinci IC, Garcia FA, Kobetz E, Partridge EE, Brandt HM, Bell MC, et al. Cervical cancer prevention: new tools and old barriers. Cancer. 2010; 116: 2531-2542.
- Head KJ, Cohen EL. Young women's perspectives on cervical cancer prevention in Appalachian Kentucky. Qual Health Res. 2012; 22: 476-487.
- Hewage S, Griswold H, Sergeev A, Gerome J, Hamilton A, Holben D. Food security, produce intake/behaviors, and cervical health of adult women living in rural Appalachia: a pilot study. The FASEB J 2014; 28.
- Studts CR, Tarasenko YN, Schoenberg NE, Shelton BJ, Hatcher-Keller J, Dignan MB. A community-based randomized trial of a faith-placed intervention to reduce cervical cancer burden in Appalachia. Prev Med. 2012; 54: 408-414.
- Brown LJ, Wenrich TR. Intra-family role expectations and reluctance to change identified as key barriers to expanding vegetable consumption patterns during interactive family-based program for Appalachian low-income food preparers. J Acad Nutr Dietetics. 2012; 112: 1188-1200.
- Wewers ME, Katz M, Paskett ED, Fickle D. Risky behaviors among Ohio Appalachian Adults. Preventing Chronic Disease. 2006; 3: A127.
- Kruger TM, Swanson M, Davis RE, Wright S, Dollarhide K, Schoenberg NE. Formative research conducted in rural Appalachia to inform a community physical activity intervention. Am J Health Promot. 2012; 26: 143-151.
- Fisher JL, Engelhardt HL Stephens JA, Smith BR. Haydu GG, Indian RW, Paskett ED. Cancer-related disparities among residents of Appalachia Ohio. J of Health Disparities Res Pract. 2008; 2: 61-74.
- Wewers ME, Salsberry PJ, Ferketich AK, Ahijevych KL, Hood NE, Paskett ED. Risk factors for smoking in rural women. J Womens Health (Larchmt). 2012; 21: 548-556.
- Klein EG, Liber AC, Kauffman RM, Berman M, Ferketich AK. Local smoke-free policy experiences in Appalachian communities. J Community Health. 2014; 39: 11-16.
- Knox SS. From 'omics' to complex disease: a systems biology approach to gene-environment interactions in cancer. Cancer Cell Int. 2010; 10: 11.
- Knox SS, Ochs MF. Implications of systemic dysfunction for the etiology of malignancy. Gene Regul Syst Bio. 2013; 7: 11-22.
- Callinan PA, Feinberg AP. The emerging science of epigenomics. Hum Mol Genet. 2006; 15 Spec No 1: R95-101.
- Feinberg AP, Ohlsson R, Henikoff S. The epigenetic progenitor origin of human cancer. Nat Rev Genet. 2006; 7: 21-33.
- Baylin SB, Chen WY. Aberrant gene silencing in tumor progression: implications for control of cancer. Cold Spring Harb Symp Quant Biol. 2005; 70: 427-433.
- Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet. 2002; 3: 415-428.
- Jones PA, Baylin SB. The epigenomics of cancer. Cell. 2007; 128: 683-692.
- Herman JG, Baylin SB. Gene silencing in cancer in association with promoter hypermethylation. N Engl J Med. 2003; 349: 2042-2054.
- World Cancer Research Fund / American Institute for Cancer Research. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Washington DC: AICR, 2007.
- Liu Z, Choi SW, Crott JW, Smith DE, Mason JB. Multiple B-vitamin inadequacy amplifies alterations induced by folate depletion in p53 expression and its downstream effector MDM2. Int J Cancer. 2008; 123: 519-525.
- Brunaud L, Alberto JM, Ayav A, Gérard P, Namour F, Antunes L, et al. Effects of vitamin B12 and folate deficiencies on DNA methylation and carcinogenesis in rat liver. Clin Chem Lab Med. 2003; 41: 1012-1019.
- Harnack L, Jacobs DR Jr, Nicodemus K, Lazovich D, Anderson K, Folsom AR. Relationship of folate, vitamin B-6, vitamin B-12, and methionine intake to incidence of colorectal cancers. Nutr Cancer. 2002; 43: 152-158.
- Ross SA. Diet and DNA methylation interactions in cancer prevention. Ann N Y Acad Sci. 2003; 983: 197-207.
- Choi SW, Mason JB. Folate status: effects on pathways of colorectal carcinogenesis. J Nutr. 2002; 132: 2413S-2418S.
- Lyn-Cook BD, Blann E, Payne PW, Bo J, Sheehan D, Medlock K. Methylation profile and amplification of proto-oncogenes in rat pancreas induced with phytoestrogens. Proceedings Of The Society For Experimental Biology And Medicine. Society For Experimental Biology And Medicine. 1995; 208: 116-119.
- Fang MZ, Chen D, Sun Y, Jin Z, Christman JK, Yang CS. Reversal of hypermethylation and reactivation of p16INK4a, RARbeta, and MGMT genes by genistein and other isoflavones from soy. Clin Cancer Res. 2005; 11: 7033-7041.
- Fang MZ, Wang Y, Ai N, Hou Z, Sun Y, Lu H, et al. Tea polyphenol (-)-epigallocatechin-3-gallate inhibits DNA methyltransferase and reactivates methylation-silenced genes in cancer cell lines. Cancer Res. 2003; 63: 7563-7570.
- Yang CS, Fang M, Lambert JD, Yan P, Huang TH. Reversal of hypermethylation and reactivation of genes by dietary polyphenolic compounds. Nutr Rev. 2008; 66: S18-20.
- Belinsky SA. Gene-promoter hypermethylation as a biomarker in lung cancer. Nat Rev Cancer. 2004; 4: 707-717.
- Gebremariam GH, Gebremedhin TG, Schaeffer PV. Employment, income, and migration in Appalachia: A spatial simultaneous equations approach. J Reg Sci. 2011; 51: 102-120.
- Seeman TE, Crimmins E, Huang MH, Singer B, Bucur A, Gruenewald T, Berkman LF. Cumulative biological risk and socio-economic differences in mortality: MacArthur studies of successful aging. Soc Sci Med. 2004; 58: 1985-1997.
- Korte SM, Koolhaas JM, Wingfield JC, McEwen BS. The Darwinian concept of stress: benefits of allostasis and costs of allostatic load and the trade-offs in health and disease. Neurosci Biobehav Rev. 2005; 29: 3-38.
- McEwen BS. Sex, stress and the hippocampus: allostasis, allostatic load and the aging process. Neurobiol Aging. 2002; 23: 921-939.
- Yang Y, Kozloski M. Sex differences in age trajectories of physiological dysregulation: inflammation, metabolic syndrome, and allostatic load. J Gerontol A Biol Sci Med Sci. 2011; 66: 493-500.
- Seeman T, Epel E, Gruenewald T, Karlamangla A, McEwen BS. Socio-economic differentials in peripheral biology: cumulative allostatic load. Ann N Y Acad Sci. 2010; 1186: 223-239.
- Gruenewald TL, Karlamangla AS, Hu P, Stein-Merkin S, Crandall C, Koretz B, et al. History of socioeconomic disadvantage and allostatic load in later life. Soc Sci Med. 2012; 74: 75-83.
- Gee GC, Payne-Sturges DC. Environmental health disparities: a framework integrating psychosocial and environmental concepts. Environ Health Perspect. 2004; 112: 1645-1653.
- Duncan CM. Understanding persistent poverty: Social class context in rural communities. Rural Sociol 1996; 61: 103-124.
- Berkman LF. The role of social relations in health promotion. Psychosom Med. 1995; 57: 245-254.
- Berkman LF, Syme SL. Social networks, host resistance, and mortality: a nine-year follow-up study of Alameda County residents. Am J Epidemiol. 1979; 109: 186-204.
- Blazer DG. Social support and mortality in an elderly community population. Am J Epidemiol. 1982; 115: 684-694.
- House JS, Robbins C, Metzner HL. The association of social relationships and activities with mortality: prospective evidence from the Tecumseh Community Health Study. Am J Epidemiol. 1982; 116: 123-140.
- Kaplan GA, Salonen JT, Cohen RD, Brand RJ, Syme SL, Puska P. Social connections and mortality from all causes and from cardiovascular disease: prospective evidence from eastern Finland. Am J Epidemiol. 1988; 128: 370-380.
- Orth-Gomér K, Rosengren A, Wilhelmsen L. Lack of social support and incidence of coronary heart disease in middle-aged Swedish men. Psychosom Med. 1993; 55: 37-43.
- Schoenbach VJ, Kaplan BH, Fredman L, Kleinbaum DG. Social ties and mortality in Evans County, Georgia. Am J Epidemiol. 1986; 123: 577-591.
- Seeman TE, Berkman LF, Kohout F, Lacroix A, Glynn R, Blazer D. Intercommunity variations in the association between social ties and mortality in the elderly. A comparative analysis of three communities. Ann Epidemiol. 1993; 3: 325-335.
- Welin L, Tibblin G, Svärdsudd K, Tibblin B, Ander-Peciva S, Larsson B, et al. Prospective study of social influences on mortality. The study of men born in 1913 and 1923. Lancet. 1985; 1: 915-918.
- Spiegel D. Mind matters in cancer survival. JAMA. 2011; 305: 502-503.
- Pinquart M, Duberstein PR. Associations of social networks with cancer mortality: a meta-analysis. Crit Rev Oncol Hematol. 2010; 75: 122-137.
- Reynolds P, Kaplan GA. Social connections and risk for cancer: prospective evidence from the Alameda County Study. Behav Med. 1990; 16: 101-110.
- Knox SS, Uvnäs-Moberg K. Social isolation and cardiovascular disease: an atherosclerotic pathway? Psychoneuroendocrinology. 1998; 23: 877-890.
- Berecik KH. Role of vasopressin in central cardiovascular regulation. In: Kunos G and Ciriello J (Eds.). Central Neural Mechan in Cariovasc Reg. Vol. 1. Birkhauser Basel, pp. 1034.
- Zalli A, Carvalho LA, Lin J, Hamer M, Erusalimsky JD, Blackburn EH, et al. Shorter telomeres with high telomerase activity are associated with raised allostatic load and impoverished psychosocial resources. Proc Natl Acad Sci U S A. 2014; 111: 4519-4524.
- Shay JW, Bacchetti S. A survey of telomerase activity in human cancer. Eur J Cancer. 1997; 33: 787-791.
- Shay JW, Wright WE. The reactivation of telomerase activity in cancer progression. Trends Genet. 1996; 12: 129-131.
- Kripke ML. Reducing death from cancer: what will it take? Tumour Biol. 2012; 33: 1275-1278.
- Reuben SH for the President's Cancer Panel. Reducing environmental cancer risk: what we can do now. U.S. Department of Health and Human Services, National Cancer Institute, Diane Publishing. 2010.
- Charo RA. Politics, parents, and prophylaxis--mandating HPV vaccination in the United States. N Engl J Med. 2007; 356: 1905-1908.
- Katz ML, Reiter PL, Kluhsman BC, Kennedy S, Dwyer S, Schoenberg N, et al. Human papillomavirus (HPV) vaccine availability, recommendations, cost, and policies among health departments in seven Appalachian states. Vaccine. 2009; 27: 3195-3200.
- Appalachian Regional Commission, Report prepared by PDA, Inc. and the Cecil B Sheps Center for Health Services Research, UNC, Chapel Hill. Health Care Costs and Access Disparities in Appalachia. 2012.
- Lengerich EJ, Wyatt SW, Rubio A, Beaulieu JE, Coyne CA, Fleisher L, et al. The Appalachia Cancer Network: cancer control research among a rural, medically underserved population. J Rural Health. 2004; 20: 181-187.
- Schoenberg NE, Hatcher J, Dignan MB, Shelton B, Wright S, Dollarhide KF. Faith Moves Mountains: an Appalachian cervical cancer prevention program. Am J Health Behav. 2009; 33: 627-638.
- Dignan M, White C, Scheonberg N, Shelton B, Feltner F, Stacey S, et al. Effectiveness of an intervention for adherence to follow-up recommendations for abnormal pap test in Appalachian Kentucky. Health Behav Policy Rev. 2014; 1: 6-15.
- Davis RE, Armstrong DK, Dignan M, Norling GR, Redmond J. Evaluation of educational materials on colorectal cancer screening in Appalachian Kentucky. Prev Chronic Dis. 2006; 3: A43.
- Dignan M, Shelton B, Slone SA, Tolle C, Mohammad S, Schoenberg N, et al. Effectiveness of a primary care practice intervention for increasing colorectal cancer screening in Appalachian Kentucky. Prev Med. 2014; 58: 70-74.
- Trickett EJ, Beehler S, Deutsch C, Green LW, Hawe P, McLeroy K, et al. Advancing the science of community-level interventions. Am J Public Health. 2011; 101: 1410-1419.
- Midgley G. Systemic intervention for public health. Am J Public Health. 2006; 96: 466-472.
- Marshall CA, Larkey LK, Curran MA, Weihs KL, Badger TA, Armin J, et al. Considerations of culture and social class for families facing cancer: the need for a new model for health promotion and psychosocial intervention. Fam Syst Health. 2011; 29: 81-94.
- Brown JL, Wenrich TR. Intra-family role expectations and reluctance to change identified as key barriers to expanding vegetable consumption patterns during interactive family-based program for Appalachian low-income food preparers. J Acad Nutr Diet. 2012; 112: 1188-1200.
- Nemeth JM, Liu ST, Klein EG, Ferketich AK, Kwan MP, Wewers ME. Factors influencing smokeless tobacco use in rural Ohio Appalachia. J Community Health. 2012; 37: 1208-1217.
- Hatcher J, Studts CR, Dignan MB, Turner LM, Schoenberg NE. Predictors of cervical cancer screening for rarely or never screened rural Appalachian women. J Health Care Poor Underserved. 2011; 22: 176-193.
- Ruffin MT 4th, Hade EM, Gorsline MR, DeGraffinreid CR, Katz ML, Kobrin SC, et al. Human papillomavirus vaccine knowledge and hypothetical acceptance among women in Appalachia Ohio. Vaccine. 2012; 30: 5349-5357.
- Hewage S, Griswold H, Sergeev A, GermeJ, Hamilton A, Holben D. Food security, produce intake/behaviors, and cervical health of adult women living in rural Appalachia: a pilot study (805.16). The FASEB J 2014; 28: 1 Supplement.
- LeMasters T, Muadhave S, Atkins E, Vyas A, Remick S, Vona-Dais L. "Don't know" and accuracy of breast cancer risk perceptions among Appalachian women attending a mobile mammography program: Implications for educational interventions and patient empowerment. J Cancer Ed. 2014.
- Correll JA, Dalton WT, Bailey B. Weight concerns, body image, and smoking continuation in pregnant women in rural Appalachia. Am J Health Behav. 2013; 37: 734-744.
- Wewers ME, Salsberry PJ, Ferketich AK, Ahijevych KL, Hood NE, Paskett ED. Risk factors for smoking in rural women. J Womens Health (Larchmt). 2012; 21: 548-556.
- Slack T, Myers CA, Martin CK, Heymsfield SB. The geographic concentration of us adult obesity prevalence and associated social, economic, and environmental factors. Obesity (Silver Spring). 2014; 22: 868-874.
- Berlin KS, Hamel-Lambert J, DeLamatre C. Obesity and overweight status health disparities among low-income rural Appalachian preschool children. Children's Health Care. 2013; 42: 15-26.
- Callinan PA, Feinberg AP. The emerging science of epigenomics. Hum Mol Genet. 2006; 15 Spec No 1: R95-101.
- Baylin SB, Chen WY. Aberrant gene silencing in tumor progression: implications for control of cancer. Cold Spring Harb Symp Quant Biol. 2005; 70: 427-433.
- Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet. 2002; 3: 415-428.
- Jones PA, Baylin SB. The epigenomics of cancer. Cell. 2007; 128: 683-692.
- Feinberg AP, Ohlsson R, Henikoff S. The epigenetic progenitor origin of human cancer. Nat Rev Genet. 2006; 7: 21-33.
- Bernal AJ, Dolinoy DC, Huang D, Skaar DA, Weinhouse C, Jirtle RL. Adaptive radiation-induced epigenetic alterations mitigated by antioxidants. FASEB J. 2013; 27: 665-671.
- Guerrero-Bosagna C, Savenkova M, Haque M, Nilsson E, skinner MK. Environmentally induced epigenetic transgenerational inheritance of altered sertoli cell transcriptome and epigenome: Molecular etiology of male infertility. PLOS/one. 2013; 8: 59922.
- Dolinoy DC, Weidman JR, Jirtle RL. Epigenetic gene regulation: linking early developmental environment to adult disease. Reprod Toxicol. 2007; 23: 297-307.
- Jirtle RL, Skinner MK. Environmental epigenomics and disease susceptibility. Nat Rev Genet. 2007; 8: 253-262.
- Waterland RA, Jirtle RL. Transposable elements: targets for early nutritional effects on epigenetic gene regulation. Mol Cell Biol. 2003; 23: 5293-5300.
- Schmidt CW. IOM issues report on breast cancer and the environment. Environ Health Perspect. 2012; 120: 60-61.
- Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu Rev Neurosci. 2001; 24: 1161-1192.
- Francis DD, Champagne FA, Liu D, Meaney MJ. Maternal care, gene expression, and the development of individual differences in stress reactivity. Ann N Y Acad Sci. 1999; 896: 66-84.
- Weaver IC, Szyf M, Meaney MJ. From maternal care to gene expression: DNA methylation and the maternal programming of stress responses. Endocr Res. 2002; 28: 699.
- Knox SS, Echeveria D. Methodological issues related to longitudinal epidemiological assessment of developmental trajectories in children. J Epidemiol Community Health. 2009; 63: 1-3.
- Weidman JR, Dolinoy DC, Murphy SK, Jirtle RL. Cancer susceptibility: epigenetic manifestation of environmental exposures. Cancer J. 2007; 13: 9-16.
- Glass TA, Goodman SN, Hernán MA, Samet JM. Causal inference in public health. Annu Rev Public Health. 2013; 34: 61-75.
- Luke DA, Stamatakis KA. Systems science methods in public health: dynamics, networks, and agents. Annu Rev Public Health. 2012; 33: 357-376.
- Trochim WM, Cabrera DA, Milstein B, Gallagher RS, Leischow SJ. Practical challenges of systems thinking and modeling in public health. Am J Public Health. 2006; 96: 538-546.
- Galea S, Riddle M, Kaplan GA. Causal thinking and complex system approaches in epidemiology. Int J Epidemiol. 2010; 39: 97-106.
- Homer JB, Hirsch GB. System dynamics modeling for public health: background and opportunities. Am J Public Health. 2006; 96: 452-457.
- Blanchet K, James P. How to do (or not to do) a social network analysis in health systems research. Health Policy Plan. 2012; 27: 438-446.
- Movahedi S, Van de Peer Y, Vandepoele K. Comparative network analysis reveals that tissue specificity and gene function are important factors influencing the mode of expression evolution in Arabidopsis and rice. Plant Physiology. 2011; 156: 1316-1330.
- Gregson J, Sowa M, Flynn HK. Evaluating form and function of regional partnerships: applying social network analysis to the Network for a Healthy California, 2001-2007. J Nutr Educ Behav. 2011; 43: S67-74.
- Scott J. Social network analysis: developments, advances and prospects. SOCNET. 2011; 1: 21-26.
- Gardy JL, Johnston JC, Ho Sui SJ, Cook VJ, Shah L, Brodkin E, et al. Whole-genome sequencing and social-network analysis of a tuberculosis outbreak. N Engl J Med. 2011; 364: 730-739.
- Azmi AS, Wang Z, Philip PA, Mohammad RM, Sarkar FH. Proof of concept: network and systems biology approaches aid in the discovery of potent anticancer drug combinations. Mol Cancer Ther. 2010; 9: 3137-3144.
- Werner T. Bioinformatics applications for pathway analysis of microarray data. Curr Opin Biotechnol. 2008; 19: 50-54.
- Calvano SE, Xiao W, Richards DR, Felciano RM, Baker HV, Cho RJ, et al. A network-based analysis of systemic inflammation in humans. Nature. 2005; 437: 1032-1037.