
Research Article
Austin J Public Health Epidemiol. 2024; 11(3): 1169.
Short-Term Association Between Air Pollution and Infectious Disease Spectrum in Shanghai, China: A Time-Series Study From 2013 To 2019
Yihan Lin1#; Hao Meng2#; Yong He2; Wenzhuo Liang1; Yiran Niu1; Zhenliang Liu1; Ziying Wang2; Yuan Lei1; Yangyang Tian1; Shiyang Chang1*
¹Department of Histology and Embryology, College of Basic Medical, Hebei Medical University, China
²Department of Pathogenic Biology, College of Basic Medicine, Hebei Medical University, China
*Corresponding author: Shiyang Chang, Department of Histology and Embryology, College of Basic Medical, Hebei Medical University, Shijiazhuang 050017, China. Tel./Fax: +86-0311-86266084 Email: 18901410@hebmu.edu.cn
#These authors have been equally contributed to this article.
Received: August 13, 2024 Accepted: August 30, 2024 Published: September 09, 2024
Abstract
Background: Epidemiological evidence on the association between air pollution and the risks of infectious diseases remained largely lacking. We aimed to examine associations of exposures to fine Particulate Matter (PM2.5) and ozone (O3) with risks of national notifiable infectious diseases in a mega city, shanghai in China.
Methods: We constructed a double-pollutant model for each air pollutant, applying a time-series analysis incorporating both single and Distributed Lag Model (DLM) separately to model the exposure-lag-response relationship with a total of 43 national Notifiable Infectious Diseases (NNIDs) during 2013 to 2019. The model was adjusted for seasonality and log-term trend, mean temperature, relative humidity, and other air pollutant. Analysis was further conducted for NNIDs categories and specific diseases.
Results: The study included 661,267 NNIDs cases. Exposures to PM2.5 and O3 were associated with increased risks of NNIDs but were not associated with the same categories. Each 10 μg/m3 increase in O3 was associated with an increased risk of total NNIDs (Relative Risk [RR] lag 1 month: 1.29, 95% Confidence Interval [CI]: 1.02 to 1.65), vaccine preventable disease (RR lag 1: 1.75, 95% CI: 1.02 to 3.01) and sexually transmitted and bloodborne diseases (RR at lag 2: 1.12, 95% CI: 1.00 to 1.26), while the association for PM2.5 remained inconclusive.
Conclusion: These findings suggested substantial infectious disease burden was associated with exposures to ambient air pollutants, emphasizing the urgent need a complete picture of association between air pollution and notifiable infectious diseases and comprehensive evaluation of the relevant disparity among spectrum of disease.
Keywords: Infectious diseases; Air pollution; Ozone; Fine particulate matter; Time-series study; Distributed lag model
Introduction
China has undergone a rapid epidemiological transition with remarkable progress in the control of infectious diseases. Yet, possibly due to significant reductions in disease burden, infectious diseases are often overlooked as causes of morbidity and mortality in China, and the assessment of climate and air pollution effects and its effect disparity related to infectious diseases [6,18]. In the past few years, the outbreak of a variety of emerging infectious diseases, such as COVID-19 and monkeypox, has raised a new challenge for human health and emerging attention on the climate impact on infectious diseases [3,11,21,22,28].
Ambient air pollutants, such as fine Particulate Matter (PM2.5) and Ozone (O3), could potentially elevate the presence of bacteria, viruses, or other pathogens within the atmosphere. They may also function as an immunosuppressive agent, thereby compromising the typical immune defenses of the human body [23]. However, current epidemiological evidence of the relationship between air pollutants and infectious diseases remains limited and inconclusive, posing a great challenging to draw a reliable conclusion from existing research. Moreover, comprehensive reports of comparison and disparity in the as sociation between air pollution and spectrum of infectious diseases were not identified in China and other countries [7,23]. In this context, the successive infectious disease surveillance system is an opportunity to provide a complete picture of association between air pollution and notifiable infectious diseases and comprehensive evaluation of the relevant disparity among spectrum of disease in the past decade.
To our knowledge, this is the first study to report comprehensively the short-term effect of air pollution on a wide range of notifiable infectious diseases due to 43 causes and evaluate the disparity in association by specific category. The identification of the potential disparity in infectious disease burdens due to air pollution would provide direction for the precise implementation of prevention and control measures.
Materials and Methods
Study Design and Infectious Diseases Data
The study is a time-series analysis using secondary data conducted in Shanghai, a megacity in China, during 2013 and 2019. Ethnical approval was not applicable to our research since the data collected in this study is secondary data without any personal information.
Monthly National Notifiable Infectious Diseases (NNIDs) data was collected from the surveillance system, detailed description has been published elsewhere [6]. A total of 43 National Notifiable Infectious Diseases (NNIDs) were included in this study, and were divided into seven categories following the previous categorization approach [9]. Specifically, I. Vaccine Preventable Diseases (11 diseases): This category encompasses seasonal influenza, rubella, pertussis, mumps, measles, hepatitis A, B and D, neonatal tetanus, poliomyelitis and diphtheria. II. Bacterial Diseases (4 diseases): including tuberculosis, scarlet fever, meningococcal meningitis, and leprosy. III. Gastrointestinal and Enterovirus Diseases (5 diseases): This group consists of diseases primarily affecting the gastrointestinal system, such as typhoid and paratyphoid, infectious diarrhea, Hand, Foot, and Mouth Disease (HFMD), dysentery, and acute hemorrhagic conjunctivitis. IV. Sexually Transmitted and Bloodborne Diseases (4 diseases): This category includes syphilis, gonorrhea, HIV/AIDS, and hepatitis C. V. Vectorborne Diseases (7 diseases): This group covers typhus, schistosomiasis, malaria, kala-azar, Japanese encephalitis, dengue, and filariasis. VI. Zoonotic Diseases (9 diseases): brucellosis, hepatitis E, hydatid disease, rabies, anthrax, leptospirosis, H5N1, H7N9, and Severe Acute Respiratory Syndrome (SARS). VII. Quarantinable Diseases (3 diseases): This category encompasses hemorrhagic fever, cholera, and plague.
Air Pollutants and Weather Variables
The two air pollutants included in this study are fine particulate matter (PM2.5) and ozone (O3). The unit of PM2.5 and O3 prediction is μg/m3, aligning with the China ambient air quality standards (GB3095-2012). Monthly average PM2.5 concentrations at surface level were downloaded from a nationwide PM2.5 dataset, with a spatial resolution of 10 km [3,26]. The data set is part of the Tracking Air Pollution in China (TAP, http://tapdata.org.cn/) project. The details of the PM2.5 prediction model have been documented elsewhere [13,15]. Briefly, TAP is a publicly available database with a high temporal and spatial resolution, and the enhanced performance through the integration of multisource-fusion data and machine learning algorithms. Initially, a comprehensive dataset comprising ground PM2.5 measurements from monitoring stations, satellite-derived Aerosol Optical Depth (AOD), meteorological parameters, land use characteristics, population figures, and elevation data, along with information from the Weather Research and Forecasting/Community Multiscale Air Quality Modeling System (WRF/CMAQ), was harmonized into a unified 10 km grid. Subsequently, the estimation of PM2.5 concentration in TAP products was predicted through a two-stage machine learning framework employing a synthetic minority oversampling technique in conjunction with a tree-based gap-filling method. The cross-validation of the prediction model yielded a range of 0.80 to 0.88, indicating comparable performance with existing studies [26].
Maximum 8 h average O3 concentration predictions were collected from TAP dataset, predicted using the three-stage random forest model [27. The three-stage O3 prediction model incorporates a comprehensive set of data sources, including ground measurements in the reference state, CMAQ simulations, Ozone Monitoring Instrument (OMI) satellite O3 profiles (PROFOZ), MERRA-2 meteorological parameters, MODIS Normalized Difference Vegetation Index (NDVI), and National Centers for Environmental Information (NCEI) annual night light data. In the initial stage, two sets of maximum 8-hour average O3 concentration predictions were generated, one incorporating satellite data and the other without, aimed at addressing gaps arising from missing satellite retrievals in the subsequent model. The O3 prediction model that excluded satellite data provided full spatial coverage. In the second stage, we employed an elastic-net regression model to merge random forest predictions from both datasets, ensuring comprehensive O3 predictions. To enhance prediction accuracy, a third-stage model was developed to predict the spatiotemporal distribution of the difference between maximum 8-hour average O3 measurements and random forest predictions, utilizing kriging interpolations. These predicted residuals were then integrated into the second-stage predictions, yielding the final predictions. The 5-fold cross-validation predictions of the O3 prediction model demonstrated an R2 value of 0.70 when compared to ground measurements.
Monthly meteorological data during 2013 and 2019, including average temperature (°C), relative humidity (%), was obtained from the National Meteorological Data Sharing Center (http://data.cma.cn/).
Statistical Analysis
Time Series Regression (TSR) with generalized linear model was applied to explore the short-term effect of individual weather variable on NNIDs categories [16]. To allow for the overdispersion of the NNIDs counts, a Quasi-Poisson model is selected. TSR is widely used for mathematical modelling in environmental epidemiology, as it measures short-term effect (which is the association between monthly variation in exposure and outcome in this study), socioeconomic and demographic levels therefore are assumed to be constant over neighboring months. To control for the long-time trend and seasonality of NNIDs, alternative choices of time adjustment have been performed and compared, including linear trend, time interactions, Fourier terms, and different splines with varied degrees of freedom (df). A Natural Cubic B-Spline (NCS) of time with 8 df per year was selected as the best-fit approach in our analysis (Supplementary Figure 1). For modelling the relationship between air pollutants and NNIDs, two modeling approaches including a single lag model and a Distributed Lag Model (DLM) were employed separately, as the health effect of pollutant variability is usually linear and delayed [1,4]. In particular, the DLM accounts for the impacts of other lag periods and provides cumulative exposure-lag-response associations over multiple lag units. Specifically, a cross-basis function for both exposure and lag dimensions assuming linear relations were introduced into the model [5. For non-infectious disease, a maximum lag of 21 day is commonly used (which approximates to lag1 in this study) [12]. However, given the more complicated casual pathway for infectious disease, wide range of lag months of 2 has been considered, also based on recommendation from previous literatures on infectious immune period, i.e., maximum lag up to 6 months [16,17]. We did the analysis for NNIDs by different categories as well as by specific causes. However, only subgroups with a sample size exceeding 5000 were considered eligible for analysis, ensuring adequate statistical power [2].
Double-pollutant models are applied for each pollutant, adjusting for another pollutant as well as other time-varying weather variables (mean temperature, relative humidity) [5]. The adjustment for each confounding variable is implemented via a NCS with 3 df of moving average of the covariate over the lag period. The effect estimate is reported as Relative Risk (RR) with its 95% Confidence Interval (CI), representing the morbidity risk changes per 10 μg/m3 in PM2.5 or O3 at each lag month, after adjusting for other lagged exposures.
Sensitivity Analysis
Sensitivity analyses were performed to check the robustness of the analysis. An extended 3-month lag period to explore a wider range of relationship pattern and lag durations. Additionally, single-pollutant models were employed for comparison with results adjusted for other pollutants. All the analyses were performed in R software (version 4.2.1; https://www.rproject.org/) with “dlnm” package. The significance level was set at 0.05.
Results
Characteristics of NNIDs and Air Pollutants
There were 661,267 incident NNIDs reported in Shanghai from January 2013 to December 2019 (Table 1). The incident cases predominately consisted of vaccine preventable diseases (93,134 cases, 14%), bacteria diseases (73,851, 11%), gastrointestinal and enterovirus diseases (351,464, 53%) and sexually transmitted and bloodborne diseases (137,036 cases, 20%). There were also 447 incident cases for vector borne diseases, 5,300 for zoonotic diseases, and 35 for quarantinable diseases. The time-series plots showed seasonal patterns for each NNIDs category, along with a decreasing trend over time except for vector borne and zoonotic diseases (Supplementary Figure 2).
n
Meana
SDa
n
Meana
SDa
Vaccine preventable diseases
93,134
1109
1538
Vector borne diseases
447
5.3
4.8
SI
63,728
759
1550
Typhus
-
-
-
Rubella
1,433
17.1
37.3
Schistosomiasis
-
-
-
Pertussis
353
4.2
6.1
Malaria
220
2.6
1.7
Mumps
18,361
219
109
Kala-azar
-
-
-
Measles
2,612
31.1
55.9
JE
9
0.1
0.3
Hepatitis A
1,795
21.4
9.7
Dengue
218
2.6
4.5
Hepatitis B
4,847
57.7
25.2
Filariasis
-
-
-
Hepatitis Db
5
0.1
0.3
Zoonotic diseases
5,300
63.1
23.8
NT
-
-
-
Brucellosis
35
0.4
0.7
Poliomyelitis
-
-
-
Hepatitis E
5,225
62.2
23.8
Diphtheria
-
-
-
HD
6
0.1
0.3
Bacteria diseases
73,851
879
248
Rabies
12
0.1
0.4
TB
48,338
575
95.4
Anthrax
-
-
-
SF
25,475
303
231
Leptospirosis
-
-
-
MM
16
0.2
0.4
H5N1
-
-
-
Leprosy
22
0.3
0.7
H7N9c
22
0.3
1
Gastrointestinal and enterovirus diseases
351,464
4,184.00
2743
SARS
-
-
-
T/P
186
2.2
1.7
Quarantinable diseases
35
0.4
1.1
ID
41,589
495
214
HF
29
0.3
1.1
HFMD
308,266
3670
2714
Cholera
6
0.1
0.3
Dysentery
1,241
14.8
12.9
Plague
-
-
-
AHC
182
2.2
3.1
Totald
661,267
7,872.20
2,837.90
Sexually transmitted and bloodborne diseases
137,036
1,631.00
272
Syphilis
94,688
1,127.00
165
Gonorrhea
37,937
452
136
AIDS
3,889
46.3
18.3
Hepatitis C
522
6.2
4.5
Notes: SI: Seasonal Influenza; NT: Neonatal Tetanus; TB: Tuberculosis; SF: Scarlet Fever; MM: Meningococcal Meningitis; T/P: Typhoid and Paratyphoid; ID: Infectious Diarrhea; HFMD: Hand, Foot, and Mouth Disease; AHC: Acute Hemorrhagic Conjunctivitis; AIDS: Acquired Immune Deficiency Syndrome; JE: Japanese Encephalitis; HD: Hydatid Disease; SARS: Severe Acute Respiratory Syndrome. HF: Hemorrhagic Fever.
a: average and standard deviation (SD) of monthly cases during 2013-2019. b: available from 2016-1. c: available from 2013-12; d: total numbers during 2013-2019. -: no cases.
Table 1: Summary statistics of 43 notifiable infectious diseases by category and specific diseases during 2013-2019 in Shanghai.
Over the study period between 2013 and 2019, the monthly concentrations of PM2.5 and O3 averaged 46.8 and 124.1 μg/m3 (Table 2). For monthly ambient weather variables, the average level of mean temperature and relative humidity was 17.4 °C and 72.8 %, respectively. For the temporal trend, discernible seasonal patterns were observed for all weather exposures (Supplementary Figure 3). It is noteworthy that the mean PM2.5 exposure exhibited a substantial declining trend over time. The pairwise correlations show moderate collinearities between the air pollutant and meteorological variables (Supplementary Figure 4).
PM2.5 (μg/m3)
O3 (μg/m3)
Temperature (°C)
Relative humidity (%)
Minimum
17.4
60.1
4.3
57
10th
25.7
74.4
6.1
65
25th
33
98.5
10.1
68.8
Median
45.1
130.6
18.2
74
Mean
46.8
124.1
17.4
72.8
SD
19.6
33.3
8.3
5.9
75th
56.5
152.5
24.2
77
90th
74.5
161.2
28.3
80
Maximum
118.4
180.8
32
83
Notes: th: percentile of the distribution; SD: standard deviation.
Table 2: Distributions of monthly levels of air pollutants and weather variables during 2013-2019 in Shanghai.
Air Pollutants and NNIDs Categories
Results of the single and distributed lag models are shown in Table 3. PM2.5 exposure at the current month (lag 0) was associated with total NNIDs risk (RR: 1.09, 95% CI: 1.00 to 1.19) and vaccine preventable diseases (RR: 1.22, 95% CI: 1.01 to 1.47), in the single lag analysis in which the analysis without adjustment for other lag months. In the context of DLMs, while most associations were not statistically significant across the exposure lags and categories, exposures to PM2.5 at all lag months and overall cumulative risk were associated with decreased sexually transmitted and bloodborne risk (Table 3).
Total
Vaccine preventable
Bacteria
Gastrointestinal and enterovirus
Sexually transmitted and bloodborne
Zoonotic
PM2.5
Single Lag Model
Lag0
1.09 (1.00, 1.19)
1.22 (1.01, 1.47)
1.01 (0.92, 1.11)
1.13 (1.00, 1.29)
1.02 (0.96, 1.08)
1.00 (0.87, 1.15)
Lag1
0.90 (0.79, 1.02)
0.77 (0.59, 1.02)
1.01 (0.88, 1.15)
0.85 (0.71, 1.01)
1.00 (0.92, 1.09)
1.02 (0.85, 1.24)
Lag2
0.99 (0.87, 1.11)
1.07 (0.84, 1.36)
0.93 (0.84, 1.04)
0.98 (0.82, 1.16)
0.94 (0.88, 1.00)
0.95 (0.81, 1.11)
Distributed Lag Model
Lag0
0.92 (0.65, 1.29)
1.47 (0.70, 3.11)
0.78 (0.57, 1.07)
0.76 (0.48, 1.19)
0.82 (0.70, 0.97)
0.89 (0.53, 1.47)
Lag1
0.75 (0.43, 1.30)
1.37 (0.39, 4.78)
0.65 (0.39, 1.09)
0.51 (0.25, 1.06)
0.71 (0.54, 0.92)
0.81 (0.35, 1.89)
Lag2
0.86 (0.64, 1.16)
1.28 (0.66, 2.47)
0.75 (0.57, 1.00)
0.71 (0.48, 1.05)
0.79 (0.68, 0.91)
0.85 (0.54, 1.34)
Net effect1
0.59 (0.18, 1.89)
2.58 (0.19, 35.07)
0.38 (0.13, 1.14)
0.28 (0.06, 1.28)
0.46 (0.26, 0.81)
0.61 (0.10, 3.57)
O3
Single Lag Model
Lag0
0.91 (0.85, 0.98)
0.84 (0.72, 0.97)
1.00 (0.91, 1.09)
0.94 (0.85, 1.03)
0.98 (0.94, 1.03)
0.97 (0.87, 1.08)
Lag1
1.05 (0.99, 1.12)
1.20 (1.06, 1.36)
1.01 (0.93, 1.09)
1.05 (0.97, 1.13)
1.01 (0.97, 1.06)
1.02 (0.94, 1.12)
Lag2
1.00 (0.95, 1.06)
0.93 (0.82, 1.05)
1.01 (0.95, 1.08)
1.00 (0.93, 1.07)
1.01 (0.97, 1.05)
1.00 (0.92, 1.07)
Distributed Lag Model
Lag0
1.09 (0.91, 1.30)
1.25 (0.84, 1.86)
1.22 (0.97, 1.55)
1.11 (0.85, 1.43)
1.11 (0.97, 1.27)
1.06 (0.77, 1.45)
Lag1
1.29 (1.02, 1.65)
1.75 (1.02, 3.01)
1.36 (0.98, 1.88)
1.28 (0.90, 1.82)
1.19 (0.99, 1.43)
1.13 (0.73, 1.76)
Lag2
1.18 (1.01, 1.38)
1.29 (0.94, 2.23)
1.22 (0.99, 1.50)
1.16 (0.92, 1.47)
1.12 (1.00, 1.26)
1.08 (0.82, 1.42)
Net effect1
1.67 (0.95, 2.93)
2.84 0.83, 9.75)
2.03 (0.96, 4.28)
1.64 (0.72, 3.75)
1.48 (0.97, 2.25)
1.29 (0.47, 3.53)
Notes: 1cumulative risk per 10 μg/m3 change in each air pollutant. Model adjusted for seasonality and long-term trend, mean temperature, relative humidity, and O3 or PM2.5.
Table 3: Relative risk (and 95% CIs) of monthly number of infectious diseases per unit increase in air pollutants in the double-pollutant model.
Susceptibility among subgroups was mostly consistent across lags and specific diseases (Figure 1). As an example, each 10-μg/m3 increase over lag 0-2 PM2.5 was associated with 56% (95% CI: -76 to -20) and 59% (95% CI: -79, -22) decrease in new diagnoses of Syphilis and Gonorrhea per month, respectively (Supplementary Table 1). In addition, those who exposed to lower exposure at lag 2 month had a higher risk of tuberculosis (RR: 0.77, 95% CI: 0.60 to 0.99).
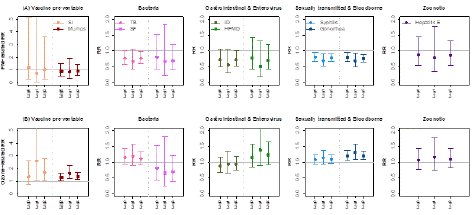
Figure 1: Relative risk (and 95% CIs) of air pollutant related infectious disease per 10 μg/m3 increase in PM2.5 and O3 for each category of NNIDs. The double-pollutant DLM model was adjusted for seasonality and long-term trend, other air pollutant, mean temperature, and relative humidity.
Exposures to O3 at lag 1 and 2 months were statistically and significantly associated with increased total NNIDs risk in DLMs but not observed for in single lag models. Each 10-μg/m3 increase in lag 1 and 2 O3 was respectively associated with 29% (95% CI: 2 to 65) and 18% (95% CI: 1 to 38) risk increase in new cases per month. For NNIDs categories, those who experienced higher O3 concentration at lag 1 month is associated with a higher risk of vaccine preventable diseases in both single lag model (RR: 1.20, 95% CI: 1.06 to 1.36) and DLM (RR: 1.75, 95% CI: 1.02 to 3.01) (Table 3).
In the DLM analysis, a higher sexually transmitted and bloodborne risk was also associated with a higher level of O3 exposure at lag 2 month (RR: 1.12, 95% CI: 1.00 to 1.26). These associations were consistent for specific diseases in the further subgroup analyses with all the effect sizes larger compared with those in the single lag models, particularly for seasonal influenza, mumps, scarlet fever and gonorrhea (Figure 1, Supplementary Table 1).
Sensitivity Analysis
Sensitivity analyses showed that the association estimates were generally robust given the altered conditions adjustment of covariates (Supplementary Table 2). In addition, the association estimates changed only slightly after excluding adjustment for other air pollutant in the single-pollutant model. When we applied a longer maximum lag of up to 3 months, the DLM results were mostly inconsistent across lag durations and NNIDs categories for PM2.5 exposure, and the associations for O3 attenuated with increasing lag periods (Supplementary Table 3).
Discussion
This study represents the most comprehensive study using the infectious disease surveillance system to offer a holistic assessment of the association between ambient air pollution and notifiable infectious diseases. Our evaluation also allows for a thorough evaluation of disparities across the spectrum of diseases. Our findings reveal a potential association between PM2.5 and O3 and total NNIDs, and the associations with susceptible categories and causes may vary for different air pollutants. Few studies have explored the relationship between air pollution and infectious diseases, often focusing on specific diseases, which hinders comparisons. Our study reveals no significant impact of PM2.5 and O3 on Tuberculosis (TB), aligning with recent meta-analyses [23,25]. However, a prior literature review has reported a conflicting finding indicating a positive association between PM2.5 and TB [20]. More extensive studies, encompassing a broader range of infectious diseases, are warranted for comprehensive understanding of the impact of air pollution on infectious diseases.
The observed associations may stem from the hypothesis that air pollutants could potentially increase the presence of bacteria, viruses, or other pathogens in the ambient air (Frontera et al. 2020). This phenomenon may be attributed to impacts due to specific constituents in urban PM2.5, chemical reactions of air pollutants (such as pH levels and heavy metals), temperature and humidity and other meteorological factors [10,19,24]. Furthermore, ambient air pollutants might play an immunosuppressive role that potentially compromising the normal immune system in human health in the human body [8,14].
Interestingly, the results suggest that PM2.5 exposure has a more immediate effect on NNIDs, with the peak observed at the current month, but O3 peaked at the lag 1 month, showing a more delayed effect. We hypothesize that their impacts may differ across different infectious stages or through varied pathways apart from inflammation and oxidative stress, but further explorations are needed. In addition, the results from our sensitivity analysis (3 months of lag) indicated that the observed impacts were sensitive to different lags. Overall, we can observe that the higher lags (2, and 3 months of lag) the higher inconsistency of the coefficients.
Our study has some strengths. First, the present study included more than 6 million infectious disease cases due to 43 causes over 7 years in a mega city setting. This sample size provides high statistical power and enhances the generalizability of our findings to the urban population with similar climates. Second, air pollutants and weather variables often display lagged effects, requiring flexible models that account for the exposure-lag-response relationship. Here we used a modeling method that flexibly describes potential linear and lagged effects of air pollution. The effects of time-varying confounding factors were considered and controlled, including time trend (seasonality and long-term trend) and meteorological variables. Third, in the absence of a universally adopted classification for infectious diseases, we divided NNIDs into seven categories based on a categorization approach based on prior research. This enabled a thorough examination of the links between air pollution and a range of notifiable infectious diseases, facilitating comprehensive comparisons and disparity assessments for the disease spectrum. Our findings from such categorization held potential for targeted interventions and further investigations into the distinctive pathogenic pathways underlying these diseases. This study also exhibits several limitations that should be interpreted with caution. First, we assume that all the morbidity cases have the same monthly exposure level of air pollutants and weather that were averaging at the city level. Such measurements do not fully represent spatial variations of exposure among populations living in urban and sub-urban areas in this mega city. Secondly, the study resolution is monthly average, which could influence the adequacy of the statistical models and the results. However, in single-city series investigations employing Poisson distribution, total number of event and the variation (SD) of exposure are the two dominant factors determining the precision and power of the model, irrespective of the time resolution or duration [2]. Thirdly, we were unable to explore the association with air pollution for some specific categories of NNIDs due to limited sample size (<5000), such as vector borne diseases. Further studies should focus on identifying specific diseases within each classification with larger sample sizes and more precise resolution. Moreover, we did not consider multicollinearity issue that arise from immune population and strong autocorrelation by disease transmission [16]. Meanwhile, other time-varying confounders were not considered, i.e., behavioral change and public health policy change.
Conclusions
In conclusion, our findings suggest that ambient air pollution is associated with infectious diseases, particularly the disparities observed within the spectrum of diseases and across pollutants. These results have important implications for policymakers, as they can help inform interventions and mitigation measures to reduce the adverse effects of air pollution on infectious disease in Shanghai. Overall, our study contributes to the lacked evidence highlighting the urgent need for evaluation and action to address the serious challenges for substantial air pollution-attributable infectious disease burdens.
Author Statements
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Competing Interests
The authors declare no actual or potential conflicts of interest.
Data Availability
The datasets generated and used in our study were obtained from the official website, and are not publicly available due to the data-sharing agreement required by the website.
Authors Contributions
Yihan Lin and Hao Meng performed data analysis. Yong He, Wenzhuo Liang, and Yiran Niu participated in the methodology and data curation. Zhenliang Liu and Ziying Wang prepared material and collected data, and helped validate the results. Yuan Lei, Yangyang Tian, and Shiyang Chang contributed to the conceptualization, design, and supervision. The first draft of the manuscript was written by Yihan Lin and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
References
- Armstrong B. Models for the relationship between ambient temperature and daily mortality. Epidemiology. 2006; 17: 624-31.
- Armstrong BG, Gasparrini A, Tobias A, Sera F. Sample size issues in time series regressions of counts on environmental exposures. BMC Med Res Methodol. 2020; 20: 15.
- Barouki R, Kogevinas M, Audouze K, Belesova K, Bergman A, Birnbaum L, et al. The COVID-19 pandemic and global environmental change: Emerging research needs. Environ Int. 2021; 146: 106272.
- Bell ML, McDermott A, Zeger SL, Samet JM, Dominici F. Ozone and short-term mortality in 95 US urban communities, 1987-2000. JAMA. 2004; 292: 2372-8.
- Bhaskaran K, Gasparrini A, Hajat S, Smeeth L, Armstrong B. Time series regression studies in environmental epidemiology. Int J Epidemiol. 2013; 42: 1187-95.
- Chen L, Wang L, Xing Y, Xie J, Su B, Geng M, et al. Disparity in spectrum of infectious diseases between in-school and out-of-school children, adolescents, and youths in China: findings from a successive national surveillance from 2013 to 2021. Lancet Reg Health West Pac. 2023; 38: 100811.
- Chen R, Yang J, Zhang C, Li B, Bergmann S, Zeng F, et al. Global Associations of Air Pollution and Conjunctivitis Diseases: A Systematic Review and Meta-Analysis. Int J Environ Res Public Health. 2019; 16: 3652.
- Ciencewicki J, Jaspers I. Air pollution and respiratory viral infection. Inhal Toxicol. 2007; 19: 1135-46.
- Dong Y, Wang L, Burgner DP, Miller JE, Song Y, Ren X, et al. Infectious diseases in children and adolescents in China: analysis of national surveillance data from 2008 to 2017. BMJ. 2020; 369: m1043.
- Fernstrom A, Goldblatt M. Aerobiology and its role in the transmission of infectious diseases. J Pathog. 2013; 2013: 493960.
- Ford JD, Zavaleta-Cortijo C, Ainembabazi T, Anza-Ramirez C, Arotoma-Rojas I, Bezerra J, et al. Interactions between climate and COVID-19. Lancet Planet Health. 2022; 6: e825-e833.
- Gasparrini A. Modelling Lagged Associations in Environmental Time Series Data: A Simulation Study. Epidemiology. 2016; 27: 835-42.
- Geng G, Xiao Q, Liu S, Liu X, Cheng J, Zheng Y, et al. Tracking Air Pollution in China: Near Real-Time PM2.5 Retrievals from Multisource Data Fusion. Environ Sci Technol. 2021; 55: 12106-12115.
- Glencross DA, Ho TR, Camiña N, Hawrylowicz CM, Pfeffer PE. Air pollution and its effects on the immune system. Free Radic Biol Med. 2020; 151: 56-68.
- Han X, Cao M, Pan Z, Guo J, Huang D, Sun W, et al. Association between long-term exposure to PM2.5 constituents and electrocardiographic abnormality: A nationwide longitudinal study in China. Environ Int. 2023; 178: 108130.
- Imai C, Armstrong B, Chalabi Z, Mangtani P, Hashizume M. Time series regression model for infectious disease and weather. Environ Res. 2015; 142: 319-27.
- Leung CC, Yew WW, Chan TY, Tam CM, Chan CY, Chan CK, et al. Seasonal pattern of tuberculosis in Hong Kong. Int J Epidemiol. 2005; 34: 924-30.
- Levy K, Smith SM, Carlton EJ. Climate Change Impacts on Waterborne Diseases: Moving Toward Designing Interventions. Curr Environ Health Rep. 2018; 5: 272-282.
- Nairz M, Weiss G. Iron in infection and immunity. Mol Aspects Med. 2020; 75: 100864.
- Popovic I, Soares Magalhaes RJ, Ge E, Marks GB, Dong GH, Wei X, et al. A systematic literature review and critical appraisal of epidemiological studies on outdoor air pollution and tuberculosis outcomes. Environ Res. 2019; 170: 33-45.
- Shao L, Cao Y, Jones T, Santosh M, Silva LFO, Ge S, et al. COVID-19 mortality and exposure to airborne PM2.5: A lag time correlation. Sci Total Environ. 2022; 806: 151286.
- Sidell MA, Chen Z, Huang BZ, Chow T, Eckel SP, Martinez MP, et al. Ambient air pollution and COVID-19 incidence during four 2020-2021 case surges. Environ Res. 2022; 208: 112758.
- Song X, Guo X, Hu X, Zhang Y, Wei D, Hu Y, et al. Human exposure risk assessment for infectious diseases due to temperature and air pollution: an overview of reviews. Environ Sci Pollut Res Int. 2023; 30: 88272-88280.
- Wooldridge KG, Williams PH. Iron uptake mechanisms of pathogenic bacteria. FEMS Microbiol Rev. 1993; 12: 325-48.
- Xiang K, Xu Z, Hu YQ, He YS, Dan YL, Wu Q, et al. Association between ambient air pollution and tuberculosis risk: A systematic review and meta-analysis. Chemosphere. 2021; 277: 130342.
- Xiao Q, Geng G, Cheng J, Liang F, Li R, Meng X, et al. Evaluation of gap-filling approaches in satellite-based daily PM2.5 prediction models. Atmos Environ. 2021; 244: 117921.
- Xue T, Zheng Y, Geng G, Xiao Q, Meng X, Wang M, et al. Estimating Spatiotemporal Variation in Ambient Ozone Exposure during 2013-2017 Using a Data-Fusion Model. Environ Sci Technol. 2020; 54: 14877-14888.
- Zhang X, Tang M, Guo F, Wei F, Yu Z, Gao K, et al. Associations between air pollution and COVID-19 epidemic during quarantine period in China. Environ Pollut. 2021; 268: 115897.