
Research Article
Austin J Public Health Epidemiol. 2015;2(1): 1016.
Seasonal Changes in Birth Weight in a Semi Urban Community in the Gambia: A 4 Year Retrospective Study and Lessons for the Future
Owolabi OA¹*, Marong L², Muhammad AK¹, Townend J¹, Idoko OT¹ and Ota MOC1,3
1Vaccinology Theme, Medical Research Council Unit, Gambia
2Fajikunda Health centre, Gambia
3WHO Regional Office for Africa, Brazzaville, Congo
*Corresponding author: Owolabi OA, Vaccinology Theme, Medical Research Council Unit, PO Box 273,Banjul, Gambia
Received: January 14, 2015; Accepted: April 10, 2015; Published: April 13, 2015
Abstract
Objective: Birth weights are determined by several factors, including seasonal changes in host behavior, environmental and maternal factors. Human birth seasonality can impact on the pattern of diseases and demand on the health system. The objective of this retrospective study was to review the pattern of birth weight of babies born in a community health centre over a period of four years.
Materials and Methods: A four year retrospective study of live singleton births at Fajikunda Health centre from 2007 to 2010. Data that included birth weight, gender, age and parity of mother were obtained from the records of the health centre. Statistical analyses were done using random effects model.
Results: There were 8521 live singletons births over the four years, 4402 (51.7%) males and 4119 (48.3%) females. The proportion of low birth weight (<2500 g) in the population was less than 5% of the total singleton births per year. Strong evidence of monthly variation in birth weight was found after adjusting for year, parity, gender, mother`s age and date of birth (month/ year) specific error terms (p<0.0001). Birth weight was higher from May to September and reached peak in May to July. The average birth weight declined progressively over the four years (p<0.001): from 2007 to 2008 (p=0.99), 2008 to 2009 (p=0.03) and from 2009 to 2010 (p=0.06). Birth weight increased by 127.3 (95% CI: 107.2; 147.6) g per unit increase in parity up to the 5th and the decreased (p<0.0001). Average birth weight of female babies was 114.1 (93.3; 135.0) g lower than the males (p<0.0001). Mother’s age was not associated with birth weight.
Conclusion: We have observed a progressive decline of birth weight with a striking seasonal variation in The Gambia. Further understanding of the reasons behind these changes is required to guide programmes and interventions.
Keywords: Mean birth weight; Maternal age; Parity; Macrosomia; Fajikunda
Introduction
Human populations in different settings show patterns of seasonal variation in reproduction that is reflected typically in the variation of birth rates [1]. The key features of birth seasonality are in the rate of birth, the phase or season of birth, and the birth weight. The season of birth can impact on the subsequent pattern of diseases during the life time [2,3]. The influence of season on pregnancy outcome is likely through a number of factors including human activities/behavior, nutritional pattern, and disease prevalence. The dry season, a period of decreased maternal labor and abundant food stored from previous harvest season, is characterized by favorable pregnancy outcomes as evidenced by birth weights well above 3000 g and maternal weight gains (>0.5 kg/week) comparable to industrialized countries [4-8].
The weight of a baby at birth is an important outcome of pregnancy and a very important factor that subsequently determines infant and childhood morbidity and mortality [9-11]. Increased episodes of diarrhea, pneumonia within the first five years of life and early onset stunting have been associated with birth weight less than 2500g [12-14]. Babies born with weight =4000g are associated with birth injuries, hypoglycemia resulting in neurological insults [15-17] .
In high income countries, the mean birth weight and proportion of macrosomia (= 4000g) have been on the increase. These constituted about 6- 10% of all deliveries, with a yearly increase in birth weight ranging from 1- 5g as reported in National data from Norway, Sweden, Denmark, USA and Canada [18-30]. The observed increase in birth weight and macrosomia have been associated with high Body Mass Index (BMI), pregnancy weight gain, inter- pregnancy weight changes, increasing maternal age and parity, and reduced maternal smoking [18,19,26,31-34].
Studies conducted in the developing countries showed lower pregnancy weight gains and lower birth weights in babies born to mothers whose third trimester occurred in hungry (nutritionally adverse) season [13,35] .
The birth weight in the rural areas of The Gambia has previously been determined [7], but an equivalent in the urban area has not until now. The birth weight of an individual is one of the known risk factors predicting the occurrence of communicable and non-communicable diseases in childhood and beyond [36-41]. A study of the birth weight of newborn in our environment is critical to our health care planning in preparedness for the evolution of diseases thought to be alien in our context in adulthood and beyond. This will equally afford the opportunity to determine the mean birth weight in the urban area as a comparator to what is already known in the rural setting in The Gambia. The knowledge from this study will contribute to determining the mean birth weight from different parts of the world, particularly the developing world, prompting investigations into potential causes for changes and seasonal variability in The Gambia. Therefore, a more detailed understanding of the pattern of birth rate and birth weight is needed to guide maternal and child health programmes and practices, as well as predict the associated consequences in the future.
Materials and Methods
This was a retrospective study at the Fajikunda Health Center (Western Region, The Gambia) for the years 2007 to 2010. This health centre is one of the six major health centers in the country, and provides primary health care for a catchment area that comprises of 10 settlements located around the health centre with a population of about 200,000 (2003 national census). The health center runs regular under-5 welfare clinics, antenatal clinics with Voluntary Counseling and Testing (VCT) for HIV and treats acute illnesses for both adults and children. It has 16 beds for admission of acute pediatric and adult cases, a delivery ward of 5 beds and a standby 24- hour ambulance services for transportation of severe or complicated cases to the only tertiary health facility that is about 20 km south of the health center. During the period of the study the health center was being run by an average of 19 registered nurses/ midwives and 12 nurse attendants. The most experienced nurse was in charge as no doctor was assigned.
Participants` data were obtained from the birth registers and independently entered into an open access spreadsheet by two trained nurse/ field workers and verified. Data on singleton live births were used for the purpose of analysis in this study. Twins and infants (singleton) with missing data on birth weight, gender, maternal age and parity were excluded. All weights were measured using TANITA BD-585 digital baby scale with maximum capacity of 20kg and readability of 10g, manufactured by TANITA Corporation Tokyo, Japan. The scale was regularly calibrated by the nurse/ midwives twice daily for the purpose of consistency of measurements, using a standard weight. The birth weight was reported in kilograms but for the purpose of analysis this was reverted to grams. Information on gestational age in completed weeks as determined by the first day of the last menstrual period or ultrasound in early pregnancy was generally unavailable because of the high illiteracy rate of the mothers and lack of required equipment at the facility. Maturity scoring of the newborns was also not routinely done at the facility. Maternal age was documented in years and the birth order (parity) was calculated from the number of previous live births of the mothers.
Statistical Methods
Statistical analyses were carried out using STATA 12.1. A descriptive analysis was performed with the use of chi-square test or Kruskal- Wallis test where applicable. We fitted a random effects model with date of birth (month/ year) as cluster to assess the seasonal variations in birth weight. Babies born in the same month of the same year belonged to the same cluster. Each month/ year of birth had its own error component, which remained constant across births within that date. Month and year were categorical, parity was continuous, quadratic term of parity, gender and age as continuous were used as explanatory variables. Mothers with parity higher or equal to 6 were pooled together because very few mothers (less than 1%) had parity higher than 6. Parity in the following: 0, 1, 2, 3, 4, 5, and 6 or above was used as continuous variable in the model but the ungrouped parity (0 to 12) was used for descriptive purpose. Restricted Maximum Likelihood (REML) estimation method was used to fit the random effects model. We used contrast with Bonferroni`s correction to assess the yearly variation in birth weight.
Results
Birth counts and maternal characteristics
A total of 8995 births were observed during the study period, with the following data missing; parity 19 (0.2%), maternal age 53 (0.6%), newborn gender 63 (0.7%), birth weight 39 (0.4%). The number of still births and multiple births over the four year of study were 151 (1.7%) and 146 (1.6%) respectively. As shown in Table 1, the total singleton live births over the 4 year period studied was 8521 (94.8%), and the yearly proportion of singleton live births ranged from 93.7% to 96.0%.
2007
2008
2009
2010
Total
p
Births
Number of births
2442 (100)
2251 (100)
2079 (100)
2221 (100)
8995 (100)
Number of singleton live births, n (%)
2309 (95.6)
2132 (94.7)
1948 (93.7)
2132 (96.0)
8521 (94.8)
0.008a
Singleton live births characteristics
Mean birth weight in grams (SD)c
3344 (513)
3342 (527)
3289 (506)
3250 (478)
3308 (508)
0.0001b
Birth weight group
< 2500 g
80 (3.5)
99 (4.6)
93 (4.8)
80 (3.8)
352 (4.1)
<0.0001a
2500 g - 2999 g
309 (13.4)
252 (11.8)
285 (14.6)
357 (16.7)
1203 (14.1)
3000 g – 3499 g
814 (35.3)
754 (35.4)
763 (39.2)
858 (40.2)
3189 (37.4)
3500 g – 3999 g
823 (35.6)
749 (35.1)
618 (31.7)
670 (31.4)
2860 (33.6)
= 4000 g
283 (12.3)
278 (13.1)
189 (9.7)
167 (7.8)
917 (10.8)
Mean mothers age (SD)
24.9 (5.3)
24.7 (5.2)
25.7 (5.5)
25.7 (5.6)
25.2 (5.4)
0.0001b
Mean mothers parity (SD)
2.0 (1.7)
2.0 (1.7)
2.1 (1.8)
2.2 (1.8)
2.1 (1.7)
0.0001b
Newborns gender, n (%)
Female
1152 (49.9)
1009 (47.3)
945 (48.5)
1013 (47.5)
4119 (48.3)
0.30a
Male
1157 (50.1)
1123 (52.7)
1003 (51.5)
1119 (52.5)
4402 (51.7)
Season
Dry (January-May and November-December)
1378 (59.7)
1313 (61.6)
1253 (64.3)
1298 (60.9)
5242 (61.5)
0.02a
Wet (June-October)
931 (40.3)
819 (38.4)
695 (35.7)
834 (39.1)
3279 (38.5)
Table 1 Describes the yearly birth counts from 2007 to 2010, and includes the mean birth weight, gender proportion of new births per year, number of births per birth weight category and season of delivery
aChi-square test.
bKruskal-Wallis test.
cStandard deviation
Table 1: Yearly singleton births, mean birth weight, gender, maternal age and parity with season.
There were a total of 4402 male and 4119 female singleton live births in the cohort. The proportion of males remained slightly higher than females throughout the four year period at an average of 51.7% (Table 1). The mean maternal age over the 4 year period increased from 24.9 in 2007 to 25.7 in 2010 (p< 0.0001).The mean (± S.D.) parity was on average 2.1 (±1.7) over the 4 year period and did not change substantially, although there was evidence of overall difference over the years (p<0.0001). The mean birth weight increased non-linearly with parity (Figure 1).The mean (± S.D.) birth weight over the four year period was 3308 (± 508)g and there was strong evidence of variation in the mean birth weight between years (p=0.0001).

Figure 1: Relationship between parity of mother and birth weight. Depicts the
mean birth weights with maternal parity over the four year period.
The proportions of low birth weights (<2500 g) in the population of the singleton births per year were (3.5%, 4.6%, 4.8% and 3.8% from 2007 to 2010, respectively). Over the four years, 10.7% of singleton live births had birth weight 4000g and above (macrosomia), with the lowest proportions observed in 2010 at 7.8% and highest in 2008 at 13.1% (Table 1).
Multivariable analysis for seasonal variations of birth weight:
After adjusting for month, parity, gender, and mother`s age specific error terms, there was strong evidence of variation in birth weight over the years (p<0.0001), (Table 2). Using the contrasts with Bonferroni`s correction (Table not shown), there was no evidence of variation in birth weight from 2007 to 2008 (p=0.99) but there were some evidence of decrease in birth weight from 2008 to 2009 (p=0.03) and from 2009 to 2010 (p=0.06).
Estimated effect on mean birth weight in grams (95% CI)
p
Fixed effects component
Intercept Month (Ref: January)
3179.7 (3097.1; 3262.3)
<0.0001
February
31.8 (-44.1; 107.6)
0.41
March
28.6 (-46.1; 103.3)
0.45
April
16.0 (-60.5; 92.5)
0.68
May
120.7 (42.3; 199.0)
0.003
June
153.2 (72.6; 233.8)
<0.001
July
152.0 (70.2; 233.9)
<0.001
August
110.5 (32.2; 188.9)
0.006
September
73.6 (-0.5; 147.6)
0.05
October
36.2 (-37.7; 110.1)
0.34
November
-18.5 (-93.2; 56.3)
0.63
December
4.3 (-70.6; 79.1)
0.91
Year (Ref.: 2007)
2008
-1.9 (-46.4; 42.6)
0.93
2009
-62.2 (-107.4; -17.0)
0.007
2010
-115.9 (-160.4; -71.4)
<0.0001
Parity
127.3 (107.2; 147.6)
<0.0001
Parity squared
-15.1 (-18.7; -11.6)
<0.0001
Gender (Ref.: Male)
Female
-114.1 (-135.0; -93.3)
<0.0001
Mothers age
0.9 (-1.8; 3.7)
0.50
Random effects component
Standard deviation of random intercept
41.7 (27.2; 64.0)
-
Standard deviation of residuals
489.1 (481.8; 496.6)
-
Log restricted-likelihood = -64797.56; Wald chi2(18) = 556.89
Table 2: Factors influencing seasonal variations of singleton live birth weight.
There was strong evidence of monthly variation in birth weight after adjusting for year, parity, gender, mother`s age and date of birth (month/ year) specific error terms (p<0.0001), (Table 2). Birth weight was higher from May to September and reached peak in May to July (Figure 2 & Table 2).
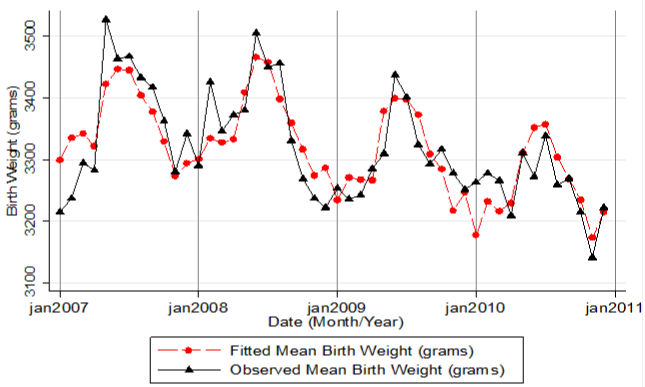
Figure 2: Pattern of Mean Birth weight from 2007 to 2010. Shows the average
monthly birth weight over the four year period.
The mothers’ parity was classified from 0 to 6 and above Controlling for month, year, gender, mother`s age and date of birth (month/ year) specific error terms, birth weight increased by 127.3 (95% CI: 107.2; 147.6) g per unit increase in parity up to the fifth parity then decreased (p<0.0001), (Figure 1 & Table 2). As shown in Table 2, there was no evidence of association between mother`s age and birth weight after adjusting for month, year, parity, gender, and date of birth (month/ year) specific error terms (p=0.50), but female birth weight was 114.1 (93.3; 135.0) g lower than the average males (p<0.0001).
Discussion
This study found that amongst 8521 singleton live births delivered at Fajikunda health centre during the period of 2007 to 2010, the mean birth weight was 3308g (S.D. ± 508g), higher than that reported in Lesotho, Nigeria, India [42-44], but was comparable to that described in the developed world [45].
Although the mean birth weight over the four year period is comparable to that seen in developed countries [23,24,45], there was a decreasing trend in the mean birth weight over years. The reason for this is not exactly known, but could be related to environmental/ climatic changes and/ or the global economic down turn leading to poor maternal nutrition and antenatal care. Maternal nutrition and antenatal care are major factors that impact on the fetal outcome, of which birth weight is a major parameter [12].
We observed that the average maternal age at delivery increased over the period of study. This trend might be explained by increasing female education with time in the population. Increase in female education will lead to relatively late marriages and increased use of family planning methods for child spacing. These will contribute to a gradual shift from young to older women still having babies.
The proportion of births in this study that were low birth weight (<2500 g) remained fairly constant over the four year period at less than 5% of births per year. This rate was lower than that observed in studies from different parts of Africa and Asia where the bulk of the low birth weight resides globally and compatible with what obtained in the industrialized areas of the world [45,46]. The proportion of low birth weight seen in this study could indicate the effects of malaria control programs in place and the possibility of regional differences within a country having some regions with higher percentage of low birth weight than the others.
Our observation of a significant difference of males weighing 115g more than females on the average is consistent with most studies [47]. Other studies have found males heavier at birth by 126.4g in the developed country and 93.1g in the developing country [47]. It is thought that this difference in weight could be due to difference in level of sex hormone exposure in uteri at the point of sex differentiation [48].
The increasing birth weight with parity has been demonstrated in other studies with variable influence of increasing parity on mean birth weights; in Thailand from parity 0 to Parity 2+ [49], Indonesia till parity 3+ [50], Morocco till parity 4+ [51] and the Iraq till 7+ [52].
The reasons for the variations of birth weights with season are not very clear, particularly as pregnancy lasts over a period of nine months during which a number of factors would have contributed to the outcome. However, the temporal events with the number of deliveries and birth weight are likely to be related to the climatic, nutritional and socio-cultural activities. Nevertheless, seasonal variations in birth weight have been documented by several studies with heterogeneous results [4,5,7,8,53,54]. The factors implicated have been annual seasonal fluctuations in temperature, sunlight exposure, and humidity, social and economic variability [55-58].
Our study demonstrated an increase in birth weights during the months of May to September that coincides with the hunger (raining) season in contrast to what was obtained in a randomised controlled trial conducted in the rural setting in The Gambia [7], Burkina Faso [4] and Lesotho [12]. The difference between the findings in our study and those of others is likely due to the fact that these studies were in rural communities that are heavily dependent on direct farming proceeds for feeding. In contrast, our study population is in an urban area that has the opportunity of availability of food items in the market pooled from different regions, and therefore do not have much variation in food availability with season. There are likely to be a number of other factors that are yet unknown, which may be acting singly or in combination to cause the striking variations in birth weight observed with season.
The absence of comprehensive socio- demographic characteristics and pre-pregnancy weights, the malaria morbidity and mortality pattern of the mothers in this study, the gestational age of the newborn at birth, data from other clinics and the retrospective nature of the data used are limitations to this study. However, the observed repetitiveness of the findings over the study period of four years was a strong compelling factor that led credence to this study and set a good platform for future study, which with appropriate study design could validate our findings and unravel the factors responsible.
In conclusion, we have observed a progressive decline of birth weight over the four year period of study, and importantly a striking variation of birth weight with seasons of the year in The Gambia. These data are relevant for programmes and interventions, and stimulate further research to identify the factors responsible for these changes.
References
- Lam DA, Miron JA. The effects of temperature on human fertility. Demography. 1996; 33: 291-305.
- Dorélien AM, Ballesteros S, Grenfell BT. Impact of birth seasonality on dynamics of acute immunizing infections in Sub-Saharan Africa. PLoS One. 2013; 8: e75806.
- Martinez-Bakker M, Bakker KM, King AA, Rohani P. Human birth seasonality: latitudinal gradient and interplay with childhood disease dynamics. Proceedings Biological sciences. The Royal Society. 2014; 281.
- Wendl-Richter HU. Birthweight distribution in rural north-west Burkina Faso. Trop Med Int Health. 1997; 2: 404-408.
- Kinabo J. Seasonal variation of birth weight distribution in Morogoro, Tanzania. East Afr Med J. 1993; 70: 752-755.
- Aitken IW. Determinants of low birthweight among the Mendi of Sierra Leone: implications for medical and socio-economic strategies. Int J Gynaecol Obstet. 1990; 33: 103-109.
- Sana MC, Andrew MP, Timothy JC, Frances F, Lawrence TW, Elizabeth MEP, et al. Effect on birth weight and perinatal mortality of maternal dietary supplements in rural Gambia: 5 year randomized controlled trial. BMJ. 1997; 315: 786-790.
- Bantje H. Seasonal variations in birthweight distribution in Ikwiriri village, Tanzania. J Trop Pediatr. 1983; 29: 50-54.
- De Onis M, Blössner M, Villar J. Levels and patterns of intrauterine growth retardation in developing countries. Eur J Clin Nutr. 1998; 52: S5-15.
- Ashworth A. Effects of intrauterine growth retardation on mortality and morbidity in infants and young children. Eur J Clin Nutr. 1998; 52: S34-41.
- Saleem T, Sajjad N, Fatima S, Habib N, Ali SR, Qadir M. Intrauterine growth retardation--small events, big consequences. Ital J Pediatr. 2011; 37: 41.
- Mathule MSL KT, Gates G, Spicer MT. Predictors of birthweight in healthy women attending a rural antenatal clinic. African Journal of Food Agriculture and Nutritional Development. 2005; 5: 1-19.
- Neumann CG, Harrison GG. Onset and evolution of stunting in infants and children. Examples from the Human Nutrition Collaborative Research Support Program. Kenya and Egypt studies. Eur J Clin Nutr. 1994; 48: 90-102.
- Prendergast AJ, Humphrey JH. The stunting syndrome in developing countries. Paediatr Int Child Health. 2014; 34: 250-265.
- Retraction: Low birth weight: causes and consequences. Diabetol Metab Syndr. 2014; 6: 60.
- Maayan-Metzger A, Lubin D, Kuint J. Hypoglycemia rates in the first days of life among term infants born to diabetic mothers. Neonatology. 2009; 96: 80-85.
- Araz N, Araz M. Frequency of neonatal hypoglycemia in large for gestational age infants of non-diabetic mothers in a community maternity hospital. Acta Medica (Hradec Kralove). 2006; 49: 237-239.
- Alberman E. Are our babies becoming bigger? JR Soc Med. 1991; 84: 257-260.
- Kramer MS, Morin I, Yang H, Platt RW, Usher R, McNamara H, et al. Why are babies getting bigger? Temporal trends in fetal growth and its determinants. J Pediatr. 2002; 141: 538-542.
- Surkan PJ, Hsieh CC, Johansson AL, Dickman PW, Cnattingius S. Reasons for increasing trends in large for gestational age births. Obstet Gynecol. 2004; 104: 720-726.
- Odlind V, Haglund B, Pakkanen M, Otterblad Olausson P. Deliveries, mothers and newborn infants in Sweden, 1973-2000. Trends in obstetrics as reported to the Swedish Medical Birth Register. Acta Obstet Gynecol Scand. 2003; 82: 516-528.
- Skjaerven R, Gjessing HK, Bakketeig LS. Birthweight by gestational age in Norway. Acta Obstet Gynecol Scand. 2000; 79: 440-449.
- Wen SW, Kramer MS, Platt R, Demissie K, Joseph KS, Liu S, Sauve R. Secular trends of fetal growth in Canada, 1981 to 1997. Paediatr Perinat Epidemiol. 2003; 17: 347-354.
- Ananth CV, Wen SW. Trends in fetal growth among singleton gestations in the United States and Canada, 1985 through 1998. Semin Perinatol. 2002; 26: 260-267.
- Schack-Nielsen L, Mølgaard C, Sørensen TI, Greisen G, Michaelsen KF. Secular change in size at birth from 1973 to 2003: national data from Denmark. Obesity (Silver Spring). 2006; 14: 1257-1263.
- Bergmann RL, Richter R, Bergmann KE, Plagemann A, Brauer M, Dudenhausen JW. Secular trends in neonatal macrosomia in Berlin: influences of potential determinants. Paediatr Perinat Epidemiol. 2003; 17: 244-249.
- Meeuwisse G, Olausson PO. [Increased birth weights in the Nordic countries. A growing proportion of neonates weigh more than four kilos]. Lakartidningen. 1998; 95: 5488-5492.
- Nolte E, Koupilová I, McKee M. The increase in very-low-birthweight infants in Germany: artefact or reality? Paediatr Perinat Epidemiol. 2002; 16: 131-140.
- Ørskou J, Kesmodel U, Henriksen TB, Secher NJ. An increasing proportion of infants weigh more than 4000 grams at birth. Acta Obstet Gynecol Scand. 2001; 80: 931-936.
- Wollschlaeger K, Nieder J, Köppe I, Härtlein K. A study of fetal macrosomia. Arch Gynecol Obstet. 1999; 263: 51-55.
- Feleke Y, Enquoselassie F. Maternal age, parity and gestational age on the size of the newborn in Addis Ababa. East Afr Med J. 1999; 76: 468-471.
- Ali M, Lulseged S. Factors influencing adolescent birth outcome. Ethiop Med J. 1997; 35: 35-42.
- Ørskou J, Henriksen TB, Kesmodel U, Secher NJ. Maternal characteristics and lifestyle factors and the risk of delivering high birth weight infants. Obstet Gynecol. 2003; 102: 115-120.
- Villamor E, Cnattingius S. Interpregnancy weight change and risk of adverse pregnancy outcomes: a population-based study. Lancet. 2006; 368: 1164-1170.
- Lawrence M, Coward WA, Lawrence F, Cole TJ, Whitehead RG. Fat gain during pregnancy in rural African women: the effect of season and dietary status. Am J Clin Nutr. 1987; 45: 1442-1450.
- Franco MC, Oliveira V, Ponzio B, Rangel M, Palomino Z, Gil FZ. Influence of birth weight on the renal development and kidney diseases in adulthood: experimental and clinical evidence. International journal of nephrology. 2012; 2012: 5.
- Harder T, Dudenhausen JW, Plagemann A. Letter by Harder et al regarding article, "Low birth weight, a risk factor for cardiovascular diseases in later life, is already associated with elevated fetal glycosylated hemoglobin at birth". Circulation. 2007; 115: e462.
- Pfab T, Slowinski T, Godes M, Halle H, Priem F, Hocher B. Low birth weight, a risk factor for cardiovascular diseases in later life, is already associated with elevated fetal glycosylated hemoglobin at birth. Circulation. 2006; 114: 1687-1692.
- Bertagnon JR, de Mattos Segre CA, Dall Colletto GM. Weight-for-length relationship at birth to predict neonatal diseases. Sao Paulo Med J. 2003; 121: 149-154.
- Donma MM, Donma O. Low birth weight: a possible risk factor also for liver diseases in adult life? Med Hypotheses. 2003; 61: 435-438.
- Szathmari M, Vasarhelyi B, Tulassay T. [Correlations of low birth weight and diseases in adulthood. The hypothesis, the facts and the doubts]. Orvosi hetilap. 2002; 143: 2221-2228.
- Tjon A Ten WE, Kusin JA, De With C. Birthweight distribution in the Mantsonyane area: Lesotho. Trop Geogr Med. 1986; 38: 131-136.
- Swende TZ. Term birth weight and sex ratio of offspring of a Nigerian obstetric population. Int J Biol Med Res. 2011; 2: 531- 532.
- Athavale VB. Examination of the newborn infant: external features. Gupta S, Editor. In: A Textbook of pediatrics. Vileas Publishing House, New Delhi. 1989:111- 117.
- Lahmann PH, Wills RA, Coory M. Trends in birth size and macrosomia in Queensland, Australia, from 1988 to 2005. Paediatr Perinat Epidemiol. 2009; 23: 533-541.
- United Nations Children Fund. State of the worlds children report. 2003.
- Kramer MS. Determinants of low birth weight: methodological assessment and meta-analysis. Bull World Health Organ. 1987; 65: 663-737.
- de Zegher F, Devlieger H, Eeckels R. Fetal growth: boys before girls. Horm Res. 1999; 51: 258-259.
- Tuntiseranee P1, Olsen J, Chongsuvivatwong V, Limbutara S. Socioeconomic and work related determinants of pregnancy outcome in southern Thailand. J Epidemiol Community Health. 1999; 53: 624-629.
- Achadi EL, Hansell MJ, Sloan NL, Anderson MA. Women's nutritional status, iron consumption and weight gain during pregnancy in relation to neonatal weight and length in West Java, Indonesia. Int J Gynaecol Obstet. 1995; 48: S103-119.
- Boutaleb Y, Lahlou N, Oudghiri A, Mesbahi M. [Birth weight in an African country]. J Gynecol Obstet Biol Reprod (Paris). 1982; 11: 68-72.
- Ramankutty P, Tikreeti RA, Rasaam KW, Al-Thamery DM, Yacoub AA, Mahmood DA. A study on birth weight of Iraqi children. J Trop Pediatr. 1983; 29: 5-10.
- McGrath JJ, Barnett AG, Eyles DW. The association between birth weight, season of birth and latitude. Ann Hum Biol. 2005; 32: 547-559.
- Gloria-Bottini F, Magrini A, Bottini E. The effect of genetic and seasonal factors on birth weight. Early Hum Dev. 2009; 85: 439-441.
- Chodick G, Flash S, Deoitch Y, Shalev V. Seasonality in birth weight: review of global patterns and potential causes. Hum Biol. 2009; 81: 463-477.
- Buckles K HD. Season on birth and later outcomes: old questions, and new answers. NBER working paper. 2009.
- Darrow LA, Strickland MJ, Klein M, Waller LA, Flanders WD, Correa A, Marcus M. Seasonality of birth and implications for temporal studies of preterm birth. Epidemiology. 2009; 20: 699-706.
- Lokshin M RS. Month of birth and childrens health in india. World Bank Research Working Paper. 2009:1- 40.