
Research Article
Austin J Public Health Epidemiol. 2015; 2(2): 1023.
Association Analyses Between Cough Droplet Concentrations and Human Lung Function
Chen SC1,2*, Lin CW¹ and Lei KI¹
¹Department of Public Health, Chung Shan Medical University, Taiwan
²Department of Family and Community Medicine, Chung Shan Medical University Hospital, Taiwan
*Corresponding author: Szu C. Chen, Department of Public Health, Chung Shan Medical University, Taichung, Taiwan
Received: August 13, 2015; Accepted: September 29, 2015; Published: October 23, 2015
Abstract
Purpose: Cough droplets are generated in human airways. Human respiratory tract activities and lung function are linked to a number of processes resulting in the introduction of droplets with infectious content into indoor air. The scope of this work was to measure the droplet experiment and associated with lung function variables.
Methods: A droplet experiment has been performed for measuring the coughed droplet characteristics from 18 nonsmoking healthy volunteers and tested simple lung function variables (Forced Expiratory Volume in 1 second (FEV1), Forced Vital Capacity (FVC), and Peak Expiratory Flow (PEF)) between genders. A 15-channel Grimm dust monitor (Grimm 1.108, Germany) were used to measure the real-time exhaled droplets. Mann-Whitney U tests and independent-sample t test were applied to compare gender-specific differences in particle concentrations and lung function variables. The association analyses between exhaled droplet concentrations and lung function were performed using SPSS, version 17.0.
Results: The average peak concentration was determined to be 60,000 particles/L for total subjects, with a reading of 80,000 and 40,000 particles/L for males and females, respectively. Results showed that droplet concentrations for males were significantly higher than those for females (Mann-Whitney U test, p < 0.05). There was a significant gender difference in FEV1 (L), FVC (L) and PEF (L/sec) variables (Independent-sample t test, p < 0.01).
Conclusions: There was a non-significant relationship between exhaled droplet concentrations and human lung function variables. However, this research could provide an opportunity for understanding how gender-specific, and inter/intra-subject analyses affects exhaled particle concentration and lung function variables.
Keywords: Droplet experiment; Cough; Lung function; FEV1; Influenza
Introduction
Influenza continues to be a major public health concern because of the disease burden and the inherent potential for severe pandemic. Influenza transmission results from virus-exposed susceptible hosts showed that spread could occur by means of either small or large droplets [1,2]. However, human respiratory tract activities, lung function and environmental factors are linked to a number of processes resulting in the introduction of droplets with infectious content into indoor air.
The mechanisms for exhaled particle formation were connected to high air velocities in the airways during exhalation [3]. Dynamic compression of airways occurring during forced expiratory flow, e.g. during cough, may cause instabilities in the layer of Respiratory Tract Lining Fluid (RTLF) and mucus. Subsequently this may produce droplets, presumably being torn off from shaking and vibrating airways walls [3]. In specific, activities such as talking, coughing, sneezing, and breathing could generate different types of respiratory droplets. It was characterized the droplet concentration and size distribution by previously studies [4-9]. Several studies used optical technology (i.e., optical particle counters) to test particle size distribution or concentration for various human respiratory activities [7,10,11], even including new methods to sample and detect virus loads from exhaled respiratory aerosols [12-14]. In an interesting study, Yang et al. [4] measured the size distribution of coughed droplets and nuclei and identified the effect of age and gender on the size distribution and concentration. Results showed that the difference in average droplet size between males and females was insignificant (p > 0.1); however, the variation in droplet concentration between males and females was significant (p < 0.1).
With respect to lung function, assessment of such parameters has gained importance as an index of cardiopulmonary status. Indeed, measurement of Forced Vital Capacity (FVC), forced expiratory volume in 1 second (FEV1), and Peak Expiratory Flow (PEF) are indicators of ventilator capacity within the respiratory system. Lung function may be influenced by several factors, including age and gender [15,16]. Aging causes structural changes to the respiratory system, encompassing modifications that occur to the lungs, rib cage respiratory muscles, and, ultimately, respiratory drive [15]. With respect to gender, there were differences between males and females in terms of inspiratory time, expiratory time, and total time of the respiratory cycle. Spirometry is a well-known and widely used measurement tool in detection, differentiation, and diagnosis of various respiratory diseases. It is also beneficial for monitoring disease progression and improvement following therapeutic intervention [17].
A paucity of studies has examined exhaled droplets as a function of lung parameters in healthy individuals. One study by Schwarz et al. [18] investigated in detail the influence of respiratory variables on exhaled particle number and size distribution during tidal breathing in 21 healthy volunteers. Results showed: (1) increasing tidal volumes dominantly influenced particle number emission while flow rates had little effect; (2) reproducibility within subjects was high, but there was a large variation of particle emission between subjects; and, (3) the ratio of functional residual capacity to total lung capacity was found to correlate with exhaled particle numbers.
Taken together, the first goal of this research was to conduct the droplet experiment for measuring exhaled droplet characteristics of healthy volunteers and test simple lung function variables (FEV1, FVC, and PEF). Secondly, this study aimed to analyze the association between droplet concentration and variables like gender and assess inter-subject variations.
Materials and Methods
Particle measurement
The sampling methods were partly adopted from Xie et al. [5] and Loudon and Roberts [19]. A schematic of the experimental setup is shown in (Figure 1). A small, air-tight box was constructed from Perspex with the dimensions of 36.6 cm × 50.8 cm × 30.5 cm inside [5,19] and placed in a clean room equipped with a central filtration system. An entry hole of 100 mm in diameter was cut for the purpose of respiratory droplet expulsion from a subject’s mouth at nearly twothirds of the height of the front wall (Figures 1A &1B). (Figure 1B) also shows the position of the subject with the sampling hole.
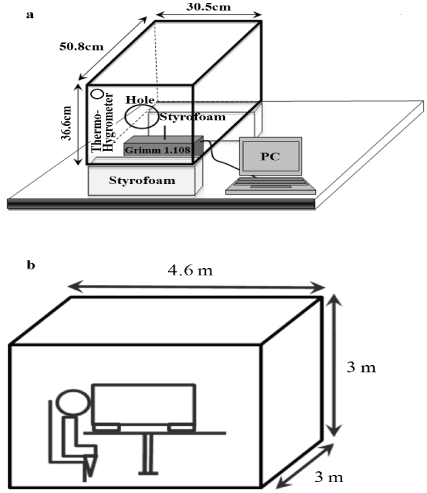
Figure 1: A schematic of the experimental setup. (A) A dust monitor (Grimm
1.108) was used for estimating the particle concentration. An entry hole
of 10 cm in diameter was cut to allow for respiratory droplet expulsion (B)
from a subject’s mouth at about two-thirds of the height of the front wall. The
dimensions of the clean room were 4.6 m × 3 m × 3 m.
In our experiment design, when droplets expelled into the box from the entry hole, large ones would quickly settle or impact on the surfaces wall, while small ones would totally evaporate into droplet nuclei, which could remain suspended in the air. Small droplets or droplet nuclei suspended in the air were measured by a 15-channel Grimm dust monitor (Grimm 1.108, Germany) in order to estimate the real-time size measurements of particles from 0.3 to 20 μm in the sampling box. Size bins of the Grimm 1.108 were separated as 0.3- 0.4, 0.4-0.5, 0.5-0.65, 0.65-0.8, 0.8-1, 1-1.6, 1.6-2, 2-3, 3-4, 4-5, 5-7.5, 7.5-10, 10-15, 15-20, and >20 μm. The sampling flow rate was 1.2 L min-1. The temperature and relative humidity in the box were also recorded before and after the experiments for each subject. Before each experiment, the sampling box was cleaned three times with 75% alcohol and then four times with distilled water.
Experimental design
In this study, 18 non-smoking healthy volunteers (from 19-31 years of age) were recruited. The experiment was conducted between August and September, 2011. Each subject was asked to perform two droplet experiments and one lung function test. At the beginning of the droplet experiments, the dust monitor (Grimm 1.108) were turned on to monitor particle concentrations, and, after waiting for 15 min, each subject was asked to enter the clean room, sit on the chair in front of the box, and wait for another 15 min. Then, the subject performed a coughing activity into the box through the entry hole.
During the coughing activity, the subject coughed loudly and slowly for 20 repetitions. To control the speed of each activity, we designed a power-point slide to control one cough behavior for every 3 sec. The exhaled activities lasted for 1 min (20 times × 3 sec = 1 min). As soon as this was completed, the subject was asked to wait for 15 min and then leave the clean room. After another 15 min, the dust monitor was turned off to reduce the effects of opening and closing the door, thus maintaining a steady environment particle concentration. Finally, the sampling box was cleaned for the next subject.
Lung function tests
For breathing patterns, the following variables were analyzed: FVC (L), FEV1 (L), and PEF (L/min). FVC is the amount of air that can be forcibly exhaled from the lungs after maximal inspiration. FEV1 is the volume exhaled during the first second of a forced expiratory maneuver after maximal inspiration. PEF expresses the rate with which air is forcefully exhaled. FVC and FEV1 were calculated and PEF was measured after spirometry with a peak flow meter; three measurements were taken and the highest score was recorded.
These tests used Spirolab II (Medical International Research, Rome, Italy). Measurements were made in an upright seated position and we use the method of manual occlusion of nares to avoid confounding [20]. One-way disposable mouthpieces were used to prevent the possibility of infection. Baseline characteristics were collected before testing, and included birth date, height, body weight, and age. Three trials were then performed in accordance with the American Thoracic Society criteria [20].
Data analyses
To analysis the gender-specific difference of droplet concentration, the normality of the data was checked by means of the Kolmogorov-Smirnov test. And Mann-Whitney U tests were applied to compare gender-specific differences in particle concentration. On the other hand, intra-subject variation of droplet concentration, the normality of the data was checked by means of the Shapiro-Wilk test, And Wilcoxon Signed-Rank test or paired t-test were assessed to compare intra-subject variation of droplet concentration. For lung function variable analyses, the normality of the data was checked by means of the Shapiro-Wilk tests. We also used independent-sample t test to compare gender-specific differences of lung function variables. Finally, correlation analyses between exhaled droplet concentrations and lung function were conducted. These analyses were performed using SPSS, version 17.0.
Results
Subjects’ characteristics and exhaled particle concentration
Eighteen subjects, including nine males (M1–M9) and nine females (F1–F9), were recorded. Mean age and age ranges were 24.5 years and 19-31 years, respectively. Sampling temperature and relative humidity were 26.8 ± 1.0oc (mean ± sd) and 50.8 ± 16.0% in all tests. The time-dependent concentrations, experimental timeline (up to 31 min), and design are illustrated in (Figure 2A). Similarly, (Figure 2B) shows the mean time-dependent particle concentrations in total and for male and female subjects. Particle concentrations included a range of particle diameters (0.30-20 μm). Results indicated a peak total concentration of 60,000 particles/L for all subjects as well as 80,000 and 40,000 particles/L for male and female subjects, in that the gray area indicates the respiratory activity period. In particular, particle concentrations increased following the coughing activity (at 15 min) with a 3-4 min time lag and then decreased to background concentrations thereafter.
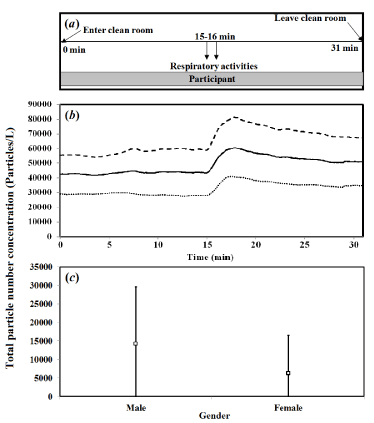
Figure 2: (A) Experimental timeline. The respiratory tract activity (coughing
20 times) was conducted at 15-16 min. (B) Mean time-dependent particle
concentrations of total (solid line), and male (dashed line) and female (dotted
line) subjects. (C) Gender-specific particle numbers are presented. The
values represent the mean ± SD.
We recalculated the real-time particle number concentration by original measurement concentration (average particle numbers from 15 min to 17 min) minus the background concentration (average particle numbers from 0 min to 15 min). Thus, for all subjects, the average background concentration was nearly 57, 601 particles/L. Overall; the average particle concentration was 10,267 particles/L. Gender-specific particle concentrations are presented in (Figure 2C). Evidently, droplet concentrations for males were significantly higher than those for females (Mann-Whitney U test, p < 0.05). Amongst male and female subjects, particle concentrations were 14,161 ± 15,406 (mean ± sd) and 6,373 ± 10,073 particles/L, respectively.
Size distribution and individual variation
(Figure 3A) shows the size characteristics of exhaled particles, including particle diameters from 1-20.0 μm. Particles in the submicron range (0.3-0.65 μm) constituted a predominant proportion of the concentration as compared to large diameter particles (> 1 μm). (Figures 3B & 3C) illustrate the intra-subject variation of exhaled droplet concentrations among all subjects. Statistical analyses showed that droplet concentrations of six of the nine male subjects and six of the nine female subjects were significantly different (p < 0.05) between the first and second test. For all 18 subjects, intra-subject variation in the exhaled particle concentrations was found to be 66.6% significantly different (12 of 18) and 33.3% non-significantly different (6 of 18) by paired t-test analysis and Wilcoxon Signed-Rank test. Hence, intra-subject variation in the exhaled particle concentration was a contributing factor in male and female groups.
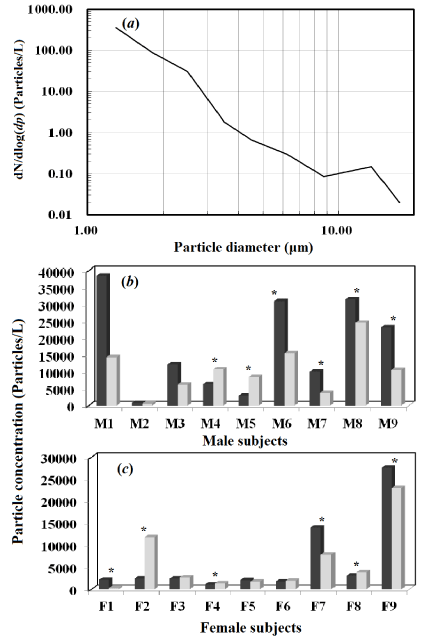
Figure 3: (A) Size distribution of particle concentrations for all subjects. (B-C)
Intra-subject variation analysis for exhaled droplet concentration. Symbols *
express the statistical significance (p < 0.05). Black and grey bars represent
the first and second tests, respectively.
Association analyses between exhaled concentration and lung function
(Table 1) presents physical characteristics, lung function and average exhaled particle concentration among males and females. Age, height, weight and Body Mass Index (BMI) were reported. Results showed that there was a significant gender difference in FEV1 (L) (independent-sample t test, p < 0.01), FVC (L) (p < 0.01) and PEF (L/sec) (p < 0.01) variables. Hence, the correlation analyses between the lung function variables and average droplet concentration for specific individual were presented in (Figure 4). Results show male (square) and female (circle) variables and the best-fitted equations (line). For all subjects, a non-significant correlation was observed for all the variables relating to the average particle concentration. The correlation coefficient (R) was 0.02, 0.014, and 0.12 for average particle concentrations with FVC, FEV1, and PEF, respectively.
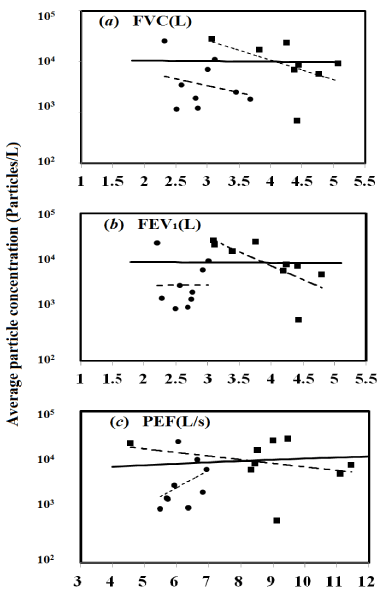
Figure 4: Correlation analysis between lung function variables of (A) FVC,
(B) FEV1, and (C) PEF and average droplet concentrations. The square (■)
and circle (•) represent male and female subjects, respectively. The solid
lines illustrate the linear fitted equation for all data.
Variables a
Male (n = 9)
Female (n = 9)
Age (Years)
24.8±5.5 b
24.2±5.2
Height (m)
1.74±0.06
1.61±0.07
Weight (Kg)
72.2±8.6
51.8±12.9
BMI (Kg/mē)
23.8±3.1
19.9±3.9
FEV1 (L)
3.93±0.62
2.63±0.27
FEV1 % pred
93.78
85.78
FVC (L)
4.23±0.59
2.93±0.44
FVC % pred
85.70
83.06
PEF (L/Second)
8.9±2.0
6.2±0.5
PEF % pred
96.40
91.52
Average Particle Concentration (Particles/L)
14161±15406
6373±10073
Table 1: Physical characteristics, lung function and average exhaled particle concentrations of the participants.
Factors affect the exhaled particle concentrations
In our study, exhaled particle concentrations for males were significantly higher as compared to females (Mann-Whitney U test, p < 0.05). Yang et al. [4] indicated that the difference in concentration was likely due to males having a larger cough flow rate as compared to females. In their study, a linear correlation existed between droplet concentrations and cough flow rates for males (R² = 0.95) and females (R² = 0.96). Previously, Schwarz et al. [18] found a greater exhaled particle number in subjects who had a lower ratio of residual capacity to total lung capacity (RC/TLC). In the current study, however, cough flow rate and RC were both not be measured to valid our results. For all 18 subjects, intra-subject variation in the exhaled particle concentrations was found to be 66.6% significantly different (12 of 18) and 33.3% non-significantly different (6 of 18) by Wilcoxon Signed-Rank test or paired t-test analysis. And, we found that intersubject variations were also significant.
Our result indicates that the total particle number concentration for all subjects was 10,267 particles/L. To compare our experiment result with previous studies on coughed droplet concentrations, we think it was acceptable. Chao et al. [11] used the Interferometric Mie Imaging (IMI) technique to measure the droplet concentration. The estimated total number of droplets expelled ranged from 947 – 2085 per cough and 112 – 6720 for speaking (counting 1-100). Coughed droplets may not have the same formation mechanism as droplets that are exhaled. Airway closure is considered the mechanism responsible for exhaled droplet formation [7,8,21,22]. This can occur in different generations of the airways for different individuals. Also, elderly subjects reach airway closure at an earlier stage of exhalation than younger individuals [23,24].
Exhaled particles from the lung were measured previously and reported in the submicron range with a diameter between 0.3 and 0.8 μm [4,6,10] indicated that the total average size distribution of the droplet nuclei was 0.58-5.42 μm, and 82% of droplet nuclei were in the range of 0.74-2.12 μm. Our particle size distribution ranged between 0.3 and 0.65 μm and this showed agreement with other studies. Literature also suggests that droplets expelled during coughing and sneezing often are larger from 1-16 μm [4,11,25]. Hence, from those data show that the variation of droplet size were partly due to the different experimental design and sampling methods.
However, the impacts of environmental conditions (relative humidity and temperature) to particle size distribution were not quantified. From the droplet origin (mouth) to the sampling position, a droplet would evaporate and its size would shrink. So the droplet size at the sampling position is smaller than the droplet size at the origin. These points are our experimental limitations, unless we can estimate the “residence time” for droplet and roughly calculated the droplet size at the origin [5]. Nicas et al. [26] also suggested that exhaled particles shrink to about half their size upon entering ambient air due to water loss. On the other hand, we used dust monitor (Grimm 1.108) that had optical particle counter uncertainty. The uncertainty is related to sizing errors. Sizing ability is sensitive to the optical properties of the aerosol, especially in the size range close to the instrument’s wave length (810 nm for Lasair II-110). Studies show that the number size distribution of exhaled particle peak close to the lower detection limit of the dust monitor [8,21].
Lung function estimations
The result for lung function variables was that females had significantly lower values than males for FEV1, FVC, and PEF (independent-sample t test, p < 0.01). Parreira et al. [16] indicated that such results could be attributed to physical differences between males and females. Ostrowski and Barud [27] mentioned that much factors influencing or related to human lung function. Since lung function values are already well linked to gender, sex, height, weight and race of an individual [28]. Height linearly correlates with lung size [29]. The predictor, age, seems likely a confounding factor. Studying age-dependent changes of lung function through the lifespan reveals distinct differences: FEV1 and FVC keep increasing from birth to the age of 25 years, then remain stable for 5-10 years or more, and start declining in later adulthood. A non-linear dependence between age and lung function is complex [27]. Turner et al. [30] found that an increase in functional residual capacity/total lung capacity (FRC/ TLC) ratio with increasing age, which the age ranged of 20-60 years, and indicated that the changes in lung elasticity are primarily the results of alterations in the contribution of elastin to the overall volume-pressure behavior of lungs. Calogero and Sly [31] provided that the lung function growth continues throughout childhood reaching peak adult values earlier in girls than in boys, and lung function reach optimal condition in age 18-20 years, then go slowly decreased after 50 years old.
Exhaled droplet associated with human lung function
Association between exhaled droplet concentrations and lung function were assessed in the current study. We did not find a significant relationship for total subjects between FVC, FEV1, and PEF and exhaled particle concentration (Figure 4). The reasons could be due to a large variation of particle concentrations contributed to different patterns for male and female subjects. Chalupa et al. [32] mentioned that there was no significant relationship between FEV1 and the deposition fraction of inhaled ultrafine particles; however, the deposition fraction increased with increasing tidal volume. We think, however, that there was a need to determine other lung function parameters such as functional residual capacity in order to correlate to exhaled particle concentrations [18].
Conclusion
This study measured the exhaled particle concentration and size distribution for 18 healthy subjects. At the same time, lung function variables for specific individuals were also analyzed. Difference analyses of gender specific were performed by statistical methods, and correlations between particle concentrations and lung function variables were assessed. In conclusion, there was a non-significant relationship between exhaled droplet concentrations and lung function variables for all subjects. However, this research could provide an opportunity for understanding how gender-specific, and inter/intra-subject analyses affects exhaled particle concentration and lung function variables.
References
- Brankston G, Gitterman L, Hirji Z, Lemieux C, Gardam M. Transmission of influenza A in human beings. Lancet Infect Dis. 2007; 7: 257–265.
- Han K, Zhu X, He F, Liu L, Zhang L, Ma H, et al. Lack of airborne tranmission during outbreak of pandemic (H1N1) 2009 among tour group members, China. Emerg Infect Dis. 2009; 15: 1578–1581.
- Moriarty JA, Grotberg JB. Flow induced instabilities of a mucus–serous bilayer. J Fluid Mech. 1999; 397: 1–22.
- Yang S, Lee GW, Chen CM, Wu CC, Yu KP. The size and concentration of droplets generated by coughing in human subjects. J Aerosol Med. 2007; 20: 484–494.
- Xie X, Li Y, Sun H, Liu L. Exhaled droplets due to talking and coughing. J R Soc Interface 2009; 6: 703–714.
- Morawska L, Johnson GR, Ristovski ZD, Hargreaves M, Mengersen K, Corbett S, et al. Size distribution and sites of origin of droplets expelled from the human respiratory tract during expiratory activities. J Aerosol Sci. 2009; 40: 256–269.
- Johnson GR, Morawska L. The mechanism of breath aerosol formation. J Aerosol Med Pulm D. 2009; 22: 229–237.
- Holmgren H, Ljungström E, Almstrand AC, Bake B, Olin AC. Size distribution of exhaled particles in the range from 0.01 to 2.0m. J Aerosol Sci. 2010; 41: 439–446.
- Gralton J, Tovey E, McLaws ML, Rawlinson WD. The role of particle size in aerosolised pathogen transmission: A review. J Infect. 2011; 62: 1–13.
- Papineni RS, Rosenthal FS. The size distribution of droplets in the exhaled breath of healthy human subjects. J Aerosol Med. 1997; 10: 105–116.
- Chao CYH, Wan MP, Morawska L, Johnson GR, Ristovski ZD, Hargreaves M, et al. Characterisation of expiration air jets and droplet size distribution immediately at the mouth opening . J Aerosol Sci. 2009; 40: 122–133.
- Fabian P, McDevitt JJ, DeHaan WH, Fung ROP, Cowling BJ, Chan KH, et al. Influenza virus in human exhaled breath: An observational study. PLoS One 2008; 3: 2691.
- Lindsley WG, Blachere FM, Thewlis RE, Vishnu A, Davis KA, Cao G, et al. Measurements of airborne influenza virus in aerosol particles from human coughs. PLoS One 2010; 5: 15100.
- Yang W, Elankumaran S, Marr LC. Concentrations and size distributions of airorne influenza A viruses measured indoors at a health centre, a day-care centre and on aeroplanes. J R Soc Interface 2011; 8: 1176–1184.
- Verschakelen JA, Demedts MG. Normal thoracoabdomianl motions. Influence of sex, age, posture, and breathe size. Am J Respir Crit Care Med. 1995; 151: 399–405.
- Parreira VF, Bueno CJ, Franca DC, Vieira DSR, Pereira DR. Breathing pattern and thoracoabdominal motion in healthy individuals: influence of age and sex. Res Bras Fisioter Sao Carlos. 2010; 14: 411–416.
- Pierce R. Spirometry: an essential clinical measurement. Aust Fam Phys. 2005; 34: 535–539.
- Schwarz K, Biller H, Windt H, Koch W, Hohlfeld JM. Characterization of exhaled particles from the healthy human lung-a systematic analysis in relation to pulmonary function variables. J Aero Med Pulm Drug Del. 2010; 23: 371–379.
- Loudon RG, Roberts RM. Relation between the airborne diameters of respiratory droplets and the diameter of the stains left after recovery. Nature 1967; 213: 95–96.
- American Thoracic Society. Standardization of Spirometry. 1994 update. Am J Respir Crit Care Med. 1995; 152: 1107–1136.
- Almstrand AC, Bake B, Ljungstrom E, Larsson P, Bredberg A, Mirgorodskaya E, et al. Effect of airway opening on production of exhaled particles. J Appl Physiol. 2010; 108: 584–588.
- Haslbeck K, Schwarz K, Hohlfeld JM, Seume JR, Koch W. Submicron droplet formation in the human lung. J Aerosol Sci. 2010; 41: 429–438.
- Macklem PT. Airway obstruction and collateral ventilation. Physiol Rev. 1971; 51: 368–436.
- Dollfuss RE, Milicemi J, Bates DV. Regional ventilation of the lung studied with boluses of 133xenon. Resp Physiol. 1967; 2: 234–246.
- Johnson GR, Morawska L, Ristovski ZD, Hargreaves M, Mengersen K, Chao CYH, et al. Modality of human expired aerosol size distributions. J Aerosol Sci. 2011; 42: 839–851.
- Nicas M, Nazaroff WW, Hubbard A. Toward understanding the risk of secondary airborne infection: emission of respirable pathogens. J Occup Environ Hyg. 2005; 2: 143–154.
- Ostrowski S, Barud W. Factors influencing lung function: are the predicted values for spirometry reliable enough? J Physiol Pharmacol. 2006; 57: 263–271.
- Quanjer PH, Tammeling GJ, Cotes JE, Pedersen OF, Peslin R. Lung-volumes and forced ventilator flows-report working party standardization of lung-function tests European-community for steel and coal-official statement of the European respiratory society. Eur Respir J. 1993; 16: 5–40.
- Barud W, Ostrowski S, Wojnicz A, Hanzlik JA, Samulak B. Evaluation of lung function in male population from vocational mining schools of the Lublin Coal Basin. Ann Univ Mariae Curie Sklodowska Med. 1991; 46: 39–43.
- Turner JM, Mead J, Wohl ME. Elasticity of human lungs in relation to age. J Appl Physiol. 1968; 25: 664–671.
- Calogero C, Sly PD. Developmental physiology: lung function during growth and development from birth to old age. Paediatr Lung Function 2010; 47: 1–15.
- e particle deposition in subjects with asthma. Environ Health Pers. 2004; 112: 879–882.