
Research Article
Austin J Public Health Epidemiol. 2018; 5(3): 1077.
Determination of Viable Allergenic Fungi in the Documents Repository Environment of the National Archive of Cuba
Borrego SF* and Molina A
Preventive Conservation Laboratory. National Archive of the Republic of Cuba, Cuba
*Corresponding author: Borrego SF, Preventive Conservation Laboratory, National Archive of the Republic of Cuba, Compostela No. 906 esq. a San Isidro, PO Box: 10100, Habana Vieja, Havana, Cuba
Received: September 05, 2018; Accepted: September 26, 2018; Published: October 03, 2018
Abstract
Introduction: Intense exposure to airborne fungal propagules has been associated with the appearance of allergic diseases and mycoses. Indoor environments of archives and libraries are a reservoir of fungal propagules due mainly to abundance of dust, the heterogeneous nature of substrates and overcrowded conditions in repositories, which constitute complex ecosystems. Besides, these environments in tropical countries can be dangerous not only to preserve documents but also for health because having high temperatures and relative humidity.
Objective: The aim of this work was to evaluate the degree of air pollution with allergenic fungi viable in a repository with documents that are manipulated with high frequency (work environment).
Methodology: Air sampling was performed by two methods: active (biocollector) and passive (sedimentation plate). In all cases Petri dishes with Malta Extract Agar supplemented by NaCl (7.5%) was used to isolate the environmental fungi. Fungi were taxonomically identified, and spores were measured to determine their penetrability in the human respiratory tract, and its impact on episodes of allergy.
Results: In terms of concentration and diversity, the repository environment behaved as a fungal propagules reservoir, which showed that there is significant health risk for the staff that manipulates the documents. Some spores were shown to be able to reach the lower respiratory tract when inhaled, which increases their allergenic and pathogenic potential. Aspergillus, Cladosporium, Penicillium and Alternaria, which are referred to as highly allergenic fungi, were prevalent.
Conclusion: Aerobiological studies are a valuable tool for the treatment of persons with allergy to fungi and other disorders that they produce.
Keywords: Anemophilous fungi; Occupational diseases; Allergies; Health; Documentary repository; Archives environments
Introduction
The microbiological quality of indoor air has been related to the appearance of occupational diseases since in certain work environments exposure to biological agents can be intense and persistent [1,2]. The fungal extensive growth has most often been associated with the presence of water in materials or condensation from high humidity, but the environment does not have to be “wet” to support mold associated with health problems. Dampness, which is noted only by minor moisture/condensate, is adequate for some mold, including species of Aspergillus and Penicillium, molds that are thought to be a problem to the health of some building occupants. Other, more hydrophilic molds (Stachybotrys, Fusarium and Acremonium) grow in higher moisture content [2]. For these reason, this phenomenon is increased in areas of tropical climate and buildings with inefficient ventilation or air conditioning systems. Numerous studies have established a close relationship between environmental conditions, the presence of anemophilous fungi and its incidence in the unleashing of respiratory diseases and allergic [3-6]. It is proposed that of 753 allergens officially recognized by the World Organization of Health (WHO) the 16% is of fungal origin and there is sensitization to almost 80 genera [6]. The groups with the highest incidence in type I allergic reactions are the ascomycetes and basidiomycetes [4,7]. In the indoor environments its presence is associated with other pathologies infections (mycosis) mediated by diverse virulence factors that vary according to the groups or taxones [4,7-11].
Allergic responses to fungi are more directly related to the spore than to other fungal propagules, such as mycelium fragments, volatile organic compounds or mycotoxins associated with them. Spores produce allergic reactions due to proteins or glycoproteins that are found on your wall. The answers to each type of spores differ according to the individual, the population and present great variability in their severity [12].
The possibility that a person inhales spores, both in outdoor such as indoor environments, it is elevated, which depends to a large extent on their environmental concentration and physical size [13]. Indoor environments of archives and libraries are a reservoir of fungal propagules due mainly to abundance of dust, the heterogeneous nature of substrates and overcrowded conditions in repositories, which constitute complex ecosystems [14-19].
For this reason, the need to perform quantitative and qualitative follow-up studies and characterization of airborne mycobiota in this type of ecosystem, which will contribute to the improvement of the quality of life of the personnel that works in these institutions or receives systematic services in them.
In Cuba there are few studies of this nature even when the weather conditions are favorable for the development and dispersal of fungi and there is an important sensitization to their spores in the population [20]. The repositories of the National Archive of the Republic of Cuba (NARC) hoard documentation of cultural, historical and scientific interest that hundreds of users request daily. This entails that archivists, stationary and the rest of the personnel involved in the service are exposed to a significant number of fungal allergens for long periods of time (workdays).
The aim of this research was to evaluate the degree of air pollution with allergenic fungi viable in a repository with documents that are manipulated with high frequency (work environment).
Materials and Methods
Characteristics of the study deposit and points of sampling
The study was conducted in a repository that protects a much reviewed documents in the NARC. This archive is located in the municipality of Habana Vieja, Havana, Cuba, in an area bordering the port of Havana, characterized by significant levels of pollution of air, as consequence of the industrial activity and movement vehicular. The repository studied is located in the second floor of building and its dimensions are (length x width x height) 15.2 x 6.2 x 2.5 m; it has natural cross-ventilation system through holes in the wall designed especially for that purpose. The repository store treasures documents on paper of high scientific value and historical heritage, sheltered in wood bookshelf. The access to the premises by the staff and the manipulation of these materials is very frequent due to the high number of documents requested by the users.
Measurements of temperature and relative humidity
Although data is collected continuously using a thermo hygrograph, measurements were made during the collection of samples at each point of sampling using a digital thermo-hygrometer Pen TH 8709 (China).
Microbiological sampling of the air
Seven air sampling points were established agreement with Sánchis [21]. In addition, according to Pasquarella [19], a sample of outdoor air was obtained as a control.
The air samples were taken by two methods: one active (indoor and outdoor) and another passive (indoor). For the plate sedimentation method (passive) suggested by Omeliansky [22-25] open Petri dishes were placed of 110 mm in diameter for 5 minutes at 1.5m from floor. With the volumetric method (active) a SAS Super 100TM biocollector (VWR International Srl, Italy) was used for 1 min at 100 L/min to a similar height to the above mentioned.
In both cases the culture medium used was Malta Extract Agar (MEA) supplemented with Sodium Chloride (NaCl) (7.5%) [26] facilitates the growth of halophilic fungi and some xerophilic species; also, it is used to limit the colonies growth of Mucorales. After the samplings, the dishes were incubated inverted for 7 days at 30°C; then the counting and isolation of different colonies was made for later analyzes.
Biometric analysis
The colony forming units per cubic meter of air (UFC/m3) were determined for each of the sampling methods:
1. Passive method, according to the equation described by Omeliansky [22-25]:
Where: N, microbial concentration in UFC/m3; a, number of colonies per Petri dish; b, area of the Petri dish (cm2); t, exposure time (minute)
2. Active method, according to the manufacturer’s instructions of the team [27]:
The Relative Density (RD) of each taxon from the formula proposed by Smith [28]:
RD = (number of colonies of one taxon / total number of colonies) x 100
Student’s t test was used to evaluate differences in the fungal concentrations obtained by both methods.
Taxonomic identification of the isolates
For the identification the characteristics were taken into account cultural and morphological characteristics of the colonies and of the structures by observation to the stereomicroscope and under an optical microscope, depending on convenient. These characteristics were determined from colonies of each isolate obtained by inoculation in the MEA medium. For observation of the morphological characteristics microcultures were mounted according to the technique of Li et al. [29], as well as fresh, semi-permanent preparations with lactophenol. For the observation of structures hyalines were used lactophenolcotton blue [30] or lactophenol-fushina [31].
The observations and measurements were made in a field trinocular biological microscope clear with digital camera coupled.
Taxonomic identification up to the level of gender was performed according to the criteria of Kendrick and Carmichael [32] and Barnett and Hunter [33]. The identification of Aspergillus species were performed according to Raper and Fennel [34]. To identify up species of the isolates of the genus Cladosporium, performed microcultures in MEA media, agar dextrose potato and synthetic nutrient agar; they had consider the criteria of Castañeda [35] and Bensch [36].
In all cases, at least 20 observations were made distributed in several fields of vision, in preparations of both the young part and the mature area of the colony. Measurements of size conidial of each of the strains were taken into account for the analysis of the penetration of conidia in the human respiratory tract.
Results and Discussion
Concentration of fungi in the air of the repository
In order to carry out a more complete study from a qualitative point of view it was complemented an active method of aerial sampling with another passive, which implied differences between the concentrations estimated from each of them, even in the same sampling point. These differences have been informed by several authors [19,37,38], but this passive method was accepted in environments study by Abdel-Hameed, and Abdel-Mawla [22] and Abdel Hameed et al. [24] and used by different authors previously [23,25,26,39,40].
The determination of concentrations of fungal propagules in the repository allowed to estimate general form the level of air pollution by fungi viable (Table 1).
Concentration
UFC/m3 (OME, passive)
UFC/m3 (SAS, active)
T (0C)
RH (%)
Maxim
1982
180 r
25.1
50.3
Minimum
619
10
24.5
46.6
Average
1223ap
50 *a q
24.8 b
48.2 b
Table 1: Concentrations of fungal propagules detected on the indoor environment of the repository by both sampling methods used.
The environmental microbiological quality of a local depends on factors like the activity that in it develops, the number of people who access or remain in it, the location of the repositories inside the building, the location of building, the dusting level on the surfaces, the type of ventilation/acclimatization the geographic and climatic zone; and time of the year [14,16,17].
That is why, although work environments with similar characteristics (such as some documents repository) may have similarities in the environmental mycobiota, it cannot be assumed that their composition is homogeneous. To obtain accurate results, timely and frequent studies must be carried out, even more so when it comes to detecting microorganisms with implications for human health.
Although for some years the studies of mycological quality of indoor air in home and work environments have increased worldwide due to the potential risk that the presence of fungi poses for human health, at present there is no international consensus regarding regulations that establish limit values that allow classifying an indoor environment as contaminated or not.
In the literature, reports are available that establish different levels of indoor fungal contamination [3,41-43]. In relation to this study, some countries of the European Union as Italy and France proposed that 150 and 170 CFU/m3 respectively were indicative of an environment no contaminated (air indoor quality) in the heritage institutions [44,45]. It is valid to highlight that in Cuba there is no standards that regulates the microbiological air quality in archives, libraries or other public institutions of similar characteristics; hence it is necessary to compare the obtained values with international references. In this case, the average value obtained by the active method (SAS) indicates that the repository environment is not contaminated according to Cappitelli et al., [44] and Roussel et al. [45]. However the average value obtained by the passive method indicates to contrary, the repository environment is contaminated according to Borrego et al., [23] because the obtained values were significantly higher. It was reported previously that the obtained fungal concentrations with Omeliansky method were higher than those obtained with SAS [46].
As a general criterion, it can be assumed that the microbiological quality of an indoor environment is good if the microbial concentrations in it are lower or equal to those existing in the surrounding outdoor areas [1,46,47]. This in turn has been shown to be related to the temperature, relative humidity, ventilation and dusting degree [48,49]. Taking into account the average value obtained in outdoor areas was 80 CFU/m3, the Indoor/Outdoor ratio (I/O) was determined and the obtained value was 0.6, lower than 1; it is indicative that the repository environment is not contaminated according to Stryjakowska-Sekulska et al. [47].
In spite of it, in this study with SAS were discovered fungal concentrations significantly superior in some points mainly located in the center of the repository (180 and 155 CFU/m3); similarly with Omeliansky methods in the same points the concentrations were very higher (1982 and 1850 CFU/m3) in relation to the other points. The above is indicative of the existence of microenvironments or “amplification sites” [50] caused by gradients of temperature, relative humidity and ventilation in different areas of the repository. This is indicative that the natural cross-ventilation system is inefficient in this area of the repository because it does not guarantee a good air circulation. Therefore these microenvironments constitute reservoirs of spores and other propagules that can have a marked allergenic character and become an important risk factor considering the high dose of allergens and the long exposure time of the individuals who access the repository during the working day.
The overreaction of the immune system is acquired after prolonged exposures to high concentrations of antigens for months or years. However, once the immune system has been sensitized, the hypersensitivity reaction is triggered by exposure to minimal amounts of the specific allergen [4,12,51].
Thus, individuals already sensitized with the allergens characteristic of some spores or propagules could present episodes of allergy in the future if they come in contact with a small amount of these, even outside the work environment. If one takes into account that most of these fungi are anemophilous and abundant in outdoor environment, it is evident that such contact is highly probable.
The values of temperature and relative humidity in the repository (Table 1) are within the established for the preservation of the documentary supports that are preserved in it [52]. However, in the face of prolonged exposure they can cause dry air to affect the mucosal moisture of the upper respiratory or conjunctive tract [3]. This facilitates the passage of antigens through the membranes of the mucous, triggering subsequent hypersensitivity reactions. That is why in the institutions of this type it is a requirement to stay as short as possible within the repositories [52].
Taxonomic and strains identification with allergenic potential
If a rigorous environmental microbiological diagnosis is to be carried outdoor and indoor environments, it is necessary not only to quantify the microorganisms, but also to identify and characterize them [8,17]. In order to assess the risk of environmental fungi on health, it is necessary to know the genus and the species, since the effects can be severe in an environment with the presence of a highly allergenic or toxigenic agent, even at low concentrations [8,46,53].
At the site, a great diversity of fungi was detected, which were located in 19 genera between ascomycetes, basidiomycetes and zygomycetes (16 filamentous fungi and three yeasts), as well as three types of non-sporulated mycelia (Figure 1). The comparison with the air sample taken outside of the building, where only 9 genera were detected (Alternaria, Aspergillus, Cladosporium, Chrysosporium, Fusarium, Itersonilia, Nigrospora, Nodulisporium, Penicillium), showed great differences. It was evident that both for the concentration and in terms of diversity, the place behaved as an important reservoir of fungal propagules and, consequently, of allergens of different characteristics.
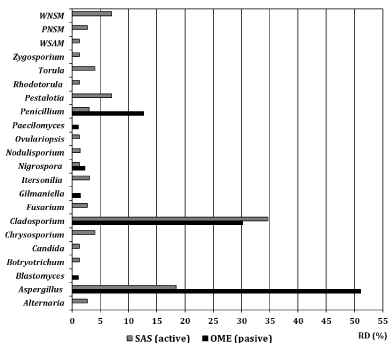
Figure 1: Relative Density (RD) percentages of the genera and mycelia
detected in the repository by two collect methods. OME: Fungi detected
by sedimentation passive on Petri dish with culture medium (Omeliansky
methods); SAS: Fungi detected by the portable volumetric biocollector;
PNSM: Pigmented Non-sporing Septate Mycelium; WNSM: White Nonsporing
Septate Mycelium; WSAM: White Non-sporing Aseptate Mycelium.
It is worth mentioning that the fungal diversity in the environment could be greater, since non-viable fungal propagules were not taken into account, although they are unable to colonize any substrate, they can cause allergic reactions. Prolonged and repeated exposure to such a diversity of spores or other fungal fragments increases the risk of specific allergic reactions against fungal antigens [4,12].
It was evident the predominance of representatives of the genera Aspergillus and Cladosporium, with the highest values of relative density. Penicillium spp. was detected too.
It is widely known that the representatives of Aspergillus spp., Cladosporium spp. and Penicillium spp. are producers of a large quantity of antigens with a marked allergenic character; these can be enzymes, toxins and volatile organic compounds (Table 2). On the other hand, some components of the cell wall of spores such as glycoproteins and certain cross-reactive proteins of phylogenetically highly conserved structure can exert a negative effect even without active growth or viability [6,53].
Genera
Main allergens
Main affectations to the health
Alternaria
Alt a 1 - 6
Common allergen. It produces type I allergies (immediate hypersensitivity or allergic rhinitis followed by asthma attacks, are the case of hay fever and asthma) and Type III (delayed hypersensitivity, is the case of hypersensitivity to pneumonia). It also produces nasal, subcutaneous, ocular and nail lesions. It can produce toxins harmful to health and others that have mutagenic properties. They can produce severe fungal infections.
Opportunistic infections primarily of skin, eye and nose.
Aspergillus
Asp f 1
Asp f 2
Asp f 3
It is a common allergen. It produces type I and III allergies. It can produce allergic bronchopneumonia, aspergillosis and allergic fungal sinusitis. Producer of potent toxins such as aflatoxins B1 and B2, gliotoxins, fumigotoxins, etc., which can cause eye infections.
Also cause superficial infection of nose, skin, ears and nails.
Cladosporium
Cla h 1
Cla h 2
It is a powerful allergen. It produces type I and III allergies. It produces toxins that cause serious effects in man. It can produce severe mycosis, infection of skin, nails and cornea.
Penicillium
Pen c 3
Pen c 22
It is a common allergen. It produces type I and III allergies. It produces harmful toxins to man. It produces volatile organic compounds that give a strong smell of mold and that are irritating. Some species can produce infections to man.
Also cause superficial infection of nose, skin, ears and nails.
Paecilomyces
x
It produces type I and III allergies. It produces toxins that cause serious effects in man. It can produce severe mycosis.
Nigrospora
X
It produces type I and III allergies.
Itersonilia
x
Produce It produces type I and III allergies. Fruiting body causing hypersensitivity.
Candida
enolasa
It produces type IV allergies (delayed hypersensitivity of cell type, probable cause of allergic alveolitis).
Table 2: Fungi detected in this study whose propagules are reported to cause allergies.
Although certain allergenic properties can be attributed to a fungal genus, there are great differences between species and even between strains of the same species. In this study we detected species of the mentioned genera that are reported as recognized causes of allergies: Aspergillus niger, Aspergillus flavus, Cladosporium cladosporioides, Penicillium oxalicum and Penicillium chrysogenum [6,53-55].
Another genus detected of great importance for allergists was Alternaria. Although its concentration in the air is generally lower than that of others such as Cladosporium spp., there are more people allergic to Alternaria and the responses to its allergens are much more severe [4,6,20,53]. Cladosporium usually produces a mild allergic reaction due to its high concentrations in the environment, important cause of inhalant allergy and allergic asthma in humans [10,54-56]. Both fungal genera cause hypersensitivity reactions, which can be immediate and affect the upper respiratory system causing rhinitis and asthma [9]; some authors have shown that there is a cross reactivity between these types of spores. This fact and the high concentrations reached by the representatives of Cladosporium spp. in the air, enhances the immune response of people sensitive to Alternaria spp. [10,54,57].
The isolation of Alternaria cinerariae in the outdoor of the building and the low concentration found in the indoor could explain its origin and its occasional detection in the premises. Other genera of potentialities were isolated to cause allergic reactions (Table 2).
The size of fungal propagules as a virulence factor
The individual capacity of a microorganism to cause diseases depends on a series of mechanisms known as virulence factors or pathogenic attributes [8,58-60]. In filamentous fungi these are diverse and vary according to the group, the genus or the species [11,61].
In the case of hypersensitivity reactions, asthma or invasive mycosis in the respiratory tract, the size of the spores can certainly be considered as an important pathogenic attribute. The respiratory tract functions as a complex network for the distribution of inspired air, in which the ducts branch out and decrease in diameter progressively until reaching the alveoli, so it acts as a trap, filtering and retaining most of the particles that accompany the more than 8000 L of air that daily mobilize the lungs [1,13,62].
When they are inhaled in large quantities, the spores of small size are dragged to the lower respiratory tract and can reach the alveoli. In this study, the measurement of the spores of the sporulated isolates, in addition to being used for taxonomic identification, made it possible to determine their potential to penetrate the respiratory tract (Table 3).
Genera
Size (length x width, μm)
RT
Alternaria
22 - 95 x 8 - 19
A
Aspergillus
2 - 10
A, B y C
Blastomyces
2 - 7 x 2 - 4.5
A, B y C
Botryotrichum
10 - 13 x 15 - 21
A
Chrysosporium
6 - 7 x 4 - 6
A y B
Cladosporium
3 - 25 x 2 - 8
A, B y C
Fusarium
30 - 60 x 3 - 5
A
Gilmaniella
7 - 10
A
Itersonilia
18 - 21.7 x 8 - 10.5
A
Nigrospora
12 - 15
A
Nodulisporium
10 - 13
A
Ovulariopsis
18.5 - 60 x 6 - 13
A
Paecilomyces
2.5 - 3 x 2 – 3.2
A, B y C
Penicillium
2.2 - 4
A, B y C
Pestalotia
17 - 20 x 5 - 8
A
Zygosporium
9 - 15 x 6 - 9
A
Torula
4 - 6
A y B
Candida
3.8 x 2 - 7
A y B
Rhodotorula
7 - 30 x 3 - 8
A
Table 3: Size of the spores of the different taxa detected. Respiratory Tract level (RT) to which the spores can penetrate.
In the upper respiratory tract 100% of the detected propagules can penetrate; however, 58% cannot pass to the rest of the respiratory tracts because their size exceeds 10 μm. Among the spores that are retained at this first level are those of the genera Alternaria, Botryotrichum, Fusarium, Gilmaniella, Itersonilia, Nigrospora, Nodulisporium, Ovulariopsis, Pestalotia, Zygospirium and Rhodotorula. The spores of these fungi can come into contact with the nasopharyngeal mucosa and trigger allergies or infections.
The rest of the spores (42%) are able to pass to trachea, bronchi and bronchioles. They stand out in this group, genera detected with high RD as Aspergillus, Cladosporium and Penicillium. Only spores with a size smaller than 3 μm can penetrate the alveoli (26%).
Rodríguez et al., [63] in a study aimed at identifying fungal genera isolated from the nasal and pharyngeal mucosa of patients with allergic rhinitis, detected spores of the genera Cladosporium, Penicillium, Aspergillus, Alternaria and Fusarium at the nasal level and spores of Cladosporium, Aspergillus and Penicillium at the pharyngeal level, result that supports the analysis we perform. It should also be considered that the species of these last 3 genera are identified with high frequency and almost always predominantly in the air and on the surfaces of studied work environments in Cuba [15,16,23,46,64].
Impact of aeromycological studies on allergology
Aeromycological studies are a valuable tool in the fight against allergies to fungi and other problems that they cause. The best treatment for allergic processes is based on avoiding exposure to the allergen that triggers the reaction [12]. Although this treatment is almost never possible in full, through systematic environmental studies could elucidate the dynamics of the mycobiota characteristic of certain premises or buildings, which would result in a better prophylactic work in the prevention of allergies.
One of the biggest problems in the study of fungal allergies is the standardization of antigenic extracts because there are important antigenic variations related to the environmental conditions of growths such as temperature, pH, or substrates in which they develop [55,65].
In the absence of specific information on the qualitative composition and antigen concentration in the atmosphere and without the use of uniform extracts of antigens, it is difficult to establish a cause-effect relationship.
Indoor and outdoor aerobiological studies together with the characterization of the isolates of certain geographical areas, buildings, homes or workplaces could facilitate the banks of specimens that allow the elaboration of extracts with strains to which a probability is most likely to be exposed population or group determined. On the other hand, they will allow locating possible sources of spores or deposition zones of certain propagules.
Conclusion
In the analyzed documents repository, a considerable concentrations of propagules were detected corresponding to viable fungal species and species of marked allergenic nature, which evidenced that there is an important risk for the health of the work team taking into account the permanence in the same during the workday.
An important part of the spores of the aerial mycobiota can reach the lower respiratory tract and reach the alveoli, which accentuates its allergenic and pathogenic potential.
It is necessary as an essential prophylactic measure, to demand the use of the means of personal protection (especially for respiratory tract) by all the personnel who have access to the repository.
The generalization and systematization of aerobiological studies of this type in work and home environments in Cuba can contribute significantly to the prophylaxis, treatment and research in the field of allergology.
Acknowledgments
The authors thank the Assistance Program for Archives of Latin America, ADAI (Project 064/2012) the grants received to finance part of this work.
Contributors
The authors contributed substantially to the study conception and design, data collection and analysis, and drafting and revision of the article. All approved the final version to be published.
Conflict of Interest
The authors declare that there is no conflict of interests or financial interests.
References
- Storey E, Dangman KH, Schenck P, DeBernardo RL, Yang CS, Bracker A, et al. Guidance for clinicians on the recognition and management of health effects related to mold exposure and moisture indoors. USA: University of Connecticut Health Center. Center for Indoor Environments and Health. 2004.
- Molina A, Borrego SF. Aerobiología y biodeterioro del género Aspergillus Link. en depósitos de tres instituciones patrimoniales cubanas. Bol Micol. 2016; 31: 2-18.
- Nevalainen A, Morawska L. Biological agents in indoor environments. Assessment of health risks. Work conducted by a WHO Expert Group between 2000-2003. 2009.
- Haleem-Khan AA, Mohan-Karuppayil S. Fungal pollution of indoor environments and its management. Saudi J Biol Sci. 2012; 19: 405-426.
- Cabral JP. Can we use indoor fungi as bioindicators of indoor air quality?. Historical perspectives and open questions. Sci Total Environ. 2010; 408: 4285-4295.
- Crameri R, Garbani M, Rhyner C, Huitema C. Fungi: The neglected allergenic sources. Allergy. 2014; 69: 176-185.
- Kauffman HF, van der Heide S. Exposure, sensitization, and mechanisms of fungus-induced asthma. Curr Allergy Asthma Rep. 2003; 3(5): 430-437.
- Guarro J. Taxonomía y biología de los hongos causantes de infección en humanos. Enferm Infecc Microbiol Clin. 2012; 30: 33-39.
- Bonifaz A. Micología médica básica. México: McGraw-Hill Education. 2012.
- Haber L, Thran B, Chaisson C. Review of the health risks of mold, health effects of molds and mycotoxins. Final Report on Toxicology Excellence for Risk Assessment (TERA), Perspective Scientific Consulting and Lifeline Group. Completed Under Contract CPSC-D-12-0001. Task Order 0013. 2015.
- Woodcock A. Moulds and asthma: time for indoor climate change ? Thorax 2007; 62: 745-746.
- Green BJ, Tovey ER, Sercombe JK, Blachere FM, Beezhold DH, Schmechel D. Airborne fungal fragments and allergenicity. Med Mycology 2006; 44: 245–255.
- Reponen T, Grinshpun SA, Conwell KL, Wiuest J, Anderson M. Aerodynamic versus physical size of spores: Measurement and implication for respiratory deposition. Grana 2001; 40: 119-125.
- Grbic ML, Stupar M, Vukojevic J, Maricic I, Bungur N. Molds in museum environments: Biodeterioration of art photographs and wooden sculptures. Arch Biol Sci. 2013; 65: 955-962.
- Borrego S, Molina A, Santana A. Fungi in archive repositories environments and the deterioration of the graphics documents. EC Microbiology 2017; 11: 205-226.
- Borrego S, Perdomo I. Airborne microorganisms cultivable on naturally ventilated document repositories of the National Archive of Cuba. Environ Sci Pollut Res. 2016; 23: 3747–3757.
- Molina A, Borrego SF. Análisis de la micobiota existente en el ambiente interior de la mapoteca del Archivo Nacional de la República de Cuba. Bol Micol. 2014; 29: 2-17.
- Okpalanozie OE, Adebusoye SA, Troiano F, Cattò C, Ilori MO, Cappitelli F. Assessment of indoor air environment of a Nigerian museum library and its biodeteriorated books using culture-dependent and –independent techniques. Int Biodeter Biodegr. 2018.
- Pasquarella C, Saccani E, Sansebastiano GE, Ugolotti M, Pasquariello GY, Albertini R. Proposal for a biological environmental monitoring approach to be used in libraries and archives. Ann Agr Environ Med. 2012; 19: 209-212.
- Sánchez K, Almaguer M. Aeromicología y salud humana. Rev Cubana Med Trop. 2014; 66: 322-337.
- Sánchis-Solera J. Los nueve parámetros más críticos en el muestreo microbiológico del aire. Rev Tecn Lab. 2002; 276: 858-862.
- Abdel-Hameed AA, Abdel-Mawla HF. Sedimentation with the Omeliansky formula as an accepted technique for quantifying airborne fungi. Pol J Environ Stud. 2012; 21: 1539-1541.
- Borrego S, Guiamet P, Gómez de Saravia S, Battistoni P, Garcia M, Lavin P, et al. The quality of air at archives and the biodeterioration of photographs. Int Biodeter Biodegr. 2010; 64: 139-145.
- Abdel-Hameed AA, Ayesh AM, Abdel-Razik-Mohamed M, Abdel-Mawla HF. Fungi and some mycotoxins producing species in the air of soybean and cotton mills: a case study. Atmos Pollut Res. 2012; 3: 126-131.
- Bogomolova EV, Kirtsideli I. Airborne fungi in four stations of the St. Petersburg underground railway system. Int Biodeter Biodegr. 2009; 63: 156-160.
- Rojas TI, Martínez E, Aira MJ, Almaguer M. Aeromicota de ambientes internos: Comparación de métodos de muestreo. Bol Micol. 2008; 23: 67-73.
- SAS Super 100™. Microbiological monitoring of the environment. Instruction manual. Milan, Italy: International Pbi Spa. 2001.
- Smith G. Ecology and field biology. New York: Harper and Row. 1980.
- Li DW, Yang CS, Harrington F, Yang CS, Heinsohn PA. Microscopic analytical methods for fungi. Sampling and analysis of indoor microorganisms. New Jersey: John Wiley & Sons, Inc. 2007; 75-104.
- Johnston A, Booth C. Plant pathologist’s pocketbook. UK: Commonwealth Mycological Institute. 1983.
- Klich MA, Pitt JI. A laboratory guide to the common Aspergillus species and their teleomorphs. UK: Commonwealth Scientific and Industrial Research Organization. 1994.
- Kendrick BW, Carmichael JW. Hyphomycetes. The fungi. An advanced treatise. Volume IVA. Surrey, England: Ainsworth Institute. 1973.
- Barnett HL, Hunter BB. Illustrated genera of imperfect fungi. USA: APS Press. The American Phytopathological Society. 1998.
- Raper KB, Fennel DI. The genus Aspergillus. Maryland, USA: Williams and Wilkins Publisher, Company Baltimore. 1965.
- Castañeda RF, Fabré DE, Parra MPP, Pérez M, Guarro J, Cano J. Some airborne conidial fungi from Cuba. Mycotaxon. 1996; 60: 283-290.
- Bensch K Braun U, Groenewald JZ, y Crous PW. The genus Cladosporium. Stud Mycol. 2012; 72: 1-401.
- Yamamoto N, Schmechel D, Chen BT, Lindsley WG, Peccia J. Comparison of quantitative airborne fungi measurements by active and passive sampling methods. J Aerosol Sci. 2011; 42: 499–507.
- Távora LGF, Gambale W, Heins-Vaccari EM, Arriagada GLH, Lacaz CS, Santos CR, et al. Comparative performance of two air samplers for monitoring airborne fungal propagules. Braz J Med Biol Res. 2003; 36: 613-616.
- Borrego S, Lavin P, Perdomo I, Gómez de Saravia S, Guiamet P. Determination of indoor air quality in archives and the biodeterioration of the documentary heritage. ISRN Microbiology. 2012.
- Guiamet PS, Borrego S, Lavin P, Perdomo I, Gómez de Saravia S. Biofouling and biodeterioration in material stored at Historical Archive of the Museum of La Plata, Argentine and at the National Archive of the Republic of Cuba. Colloid Surface B. 2011; 85: 229-234.
- Radler de Aquino F, De Góes LF. Guidelines for indoor air quality in offices in Brazil. Proc Healthy Build. 2000; 4: 549-553.
- SBM. Building biology evaluation guideline. Alemania: Baubiologie Maes/Institut für Baubiologie/Ökologie IBN. 2008.
- InspectaPedia.com. Mould exposure standards. Levels of allergenic or toxic mould & how much mould means a problem ? Building and Environmental Inspection, Testing, Diagnosis, Repair, Problem Prevention Advice. 2009.
- Cappitelli F, Fermo P, Vecchi R., Piazzalunga A, Valli G, Zanardini E, Sorlini C. Chemical-physical and microbiological measurements for indoor air quality assessment at the Ca´Granada Historical Archive, Milan (Italy). Water Air Soil Pollut. 2009; 201: 109-120.
- Roussel S, Reboux G, Millon L, Parchas MD, Boudih S, Skana F, et al. Microbiological evaluation of ten French archives and link to occupational symptoms. Indoor Air. 2012; 22: 514-522.
- Rojas TI, Aira MJ. Fungal biodiversity in indoor environments in Havana, Cuba. Aerobiologia. 2012; 28: 367-374.
- Stryjakowska-Sekulska M, Piotraszewska-Pajak A, Szyszka A, Nowicki M, Filipiak M. Microbiological quality of indoor air in university rooms. Polish J of Environ Stud. 2007; 16: 623-632.
- Borrego-Alonso S, Molina-Veloso A. Comportamiento de la aeromicrobiota en dos depósitos del Archivo Nacional de la República de Cuba durante 7 años de estudio. AUMGMDOMUS. 2014; 6: 1-24.
- Rodríguez-García JC, Rodríguez B, Borrego S. Evaluación de la calidad micológica ambiental del depósito de fondos documentales del Museo Nacional de la Música de Cuba en época de lluvia. AUMGMDOMUS. 2014; 6: 123-146.
- Pinzari F. Microbial ecology of indoor environments. The ecological and applied aspects of microbial contamination in archives, libraries and conservation environments. Abdul-Wahab Al-Sulaiman SA, editor. In: Sick building syndrome in public buildings and workplaces. Springer Berlin Heidelberg. 2011; 1-35.
- Ayats J, Martín-Mazuelos E, Pemán J, Quindós G, Sánchez F, García-Rodríguez J, et al. Recomendaciones sobre el diagnóstico de la enfermedad fúngica invasora de la Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica (SEIMC). Enferm Infecc Microbiol Clin. 2011; 29: 39.
- Resolución No. 41/2009 del Ministerio de Ciencia Tecnología y Medio Ambiente (CITMA). Lineamientos para la conservación de las fuentes documentales. Gaceta Oficial de la República de Cuba. 2009.
- Horner WE, Helbling A, Salvaggio JE, Lehrer SB. Fungal allergens. Clin Microbiol Rev. 1995; 8: 161-179.
- Twaroch TE, Curin M, Sterflinger K, Focke-Tejkl M, Swoboda I, Valenta R. Specific antibodies for the detection of Alternaria allergens and the identification of cross-reactive antigens in other fungi. Int Arch Allergy Immunol. 2016; 170: 269-278.
- Zhang Z, Reponen T, Khurana-Hershey GK. Fungal exposure and asthma: IgE and non-IgE-mediated mechanisms. Curr Allergy Asthma Rep. 2016; 16: 86.
- Almaguer-Chávez M, Sánchez K, Rojas TI. El género Cladosporium en la atmósfera del Occidente de Cuba: pasado, presente y futuro. Rev Cub Cienc Biol. 2014; 3: 8-19.
- Venables KM, Tee RD, Hawkins ER, Gordon DJ, Wale CJ, Farrer NM, et al. Laboratory animal allergy in pharmaceutical company. Br J Ind Med. 1988; 45: 660-666.
- da Silva ENB, Cavalcanti MAQ, de Souza-Motta MC. Pathogenicity characteristics of filamentous fungi strains isolated from processed oat. Rev Microbiol. 1999; 30: 377-380.
- Heitman J. Microbial pathogens in the fungal kingdom. Fungal Biol Rev. 2011; 25: 48-60.
- Karkowska-Kuleta J, Rapala-Kozik M, Kozik A. Fungi pathogenic to humans: molecular bases of virulence of Candida albicans, Cryptococcus neoformans and Aspergillus fumigatus. Acta Biochim Pol. 2009; 56: 211-224.
- Rintala H, Pitkäranta M, Täubel M. Microbial communities associated with house dust. Advances in Applied Microbiology, Burlington: Academic Press. 2012; 78: 75-120.
- Hatch TF. Distribution and deposition of inhaled particles in respiratory tract. Bacteriol Rev. 1961; 25: 237-240.
- Rodríguez-Orozco A, Vargas-Villegas E, Tafolla-Muñoz L, Ruiz-Reyes H, Hernández-Chávez LA, Vázquez-Garcidueñas S. Géneros fúngicos aislados de pacientes con rinitis alérgica y su relación con la prueba de hipersensibilidad subcutánea de prick. Rev Mex Micol. 2008; 28: 89-94.
- Anaya M, Borrego SF, Gámez E, Castro M, Molina A, Valdés O. Viable fungi in the air of indoor environments of the National Archive of the Republic of Cuba. Aerobiologia. 2016; 32: 513–527.
- Carnés J. Los extractos como fármacos. En: Laboratorios Leti. Inmunoterapia como herramienta clínica moderna. Barcelona: Laboratorios Leti. 2009.