
Research Article
Austin J Reprod Med Infertil. 2016; 3(1): 1038.
Liquid Nitrogen Vapor Sealing of Straw Containers can be Unsafe and Detrimental to Embryo Survival
Schiewe MC¹*, Schiewe E²^, Vu VN³^, Zozula S¹ and Anderson RE1,4
¹Ovation Fertility, ART Laboratory, USA
²University of Southern California, USA
³University of California-Los Angeles, USA
4Southern California Center for Reproductive Medicine, USA
^Summer Science Student Training Program Interns- Summer 2014, Ovation Fertility, Newport Beach, CA, USA
*Corresponding author: Mitchel C. Schiewe, Ovation Fertility, ART Laboratory Newport Beach, CA 92663, USA
Received: June 13, 2016; Accepted: July 22, 2016; Published: July 26, 2016
Abstract
Background: Aseptic closed vitrification (VTF) systems have been proving their clinical effectiveness in recent years. Although the risk of pathogenic contamination between samples in liquid nitrogen storage has been a debatable issue among open VTF systems users, there is growing interest to hybridize systems. In short, some open system users aim to achieve the ultra-rapid cooling rates of direct LN2 exposure and then seal the device into a plastic straw container. Specialized commercial LN2 baths have been developed to assist in these hybrid-device systems. We strived to 1) determine whether LN2 vapor sealing of straws presents safety and reliability concerns that create potentially harmful laboratory practices; and 2) reveal a validation method which verifies the competency of seals as a quality control practice.
Materials and Methods: Using a repeated VTF (rVTF) model on research consented, discard embryos, human blastocysts were randomly assigned to either Control (n=19) or ultra-rapid cooling treatment (UR-TRT; n=22). Standard micro Secure-VTF (μS-VTF) warming of flexipettes was first performed without extraction/elution, then dried with sterile gauze. Re-VTF was performed at 1 min post-warming, by either: 1) Control μS-VTF; or 2) UR-TRT where flexipettes were dipped into LN2 (5 sec), inserted in straws held in LN2 and sealed closed for storage. Subsequently, all straws were warmed using standard μS-VTF procedures and elution in sucrose solutions. Following isotonic equilibration and 24h in vitro culture, blastocyst survival and development, respectively, was assessed.
Results: Following the fatal rupturing of the first 3 UR-TRT straws, warming procedures had to be modified for possible LN2 accumulation inside straws due to incomplete seals. By allowing for 15 sec of N2 out gassing, the remaining 19 straws warmed without incident, yet 6 did reveal evidence of LN2 seepage (41% incomplete seals). No difference in blastocyst survival at 0 h was evident between treatments; however development was reduced in the UR-TRT group at 24 h (86.7% vs. 62.5%).
Conclusion: The hybridization of a UR-closed VTF system has proven to be a potentially unreliable, unsafe and less effective procedure in our rVTF model system. The inability to guarantee complete seals of super-cooled straws or the possible entry of N2 vapors inside a straw upon sealing creates significant risks which are unnecessary quality control variables absent in standard aseptic, closed VTF methods.
Keywords: Vitrification; Straw sealing; Embryos; Liquid nitrogen vapor; Quality control
Abbreviations
BL: Blastocyst; CBS: Cryo Bio Systems; CSS: Cut Standard Straw; DMSO: Dimethyl Sulfoxide; HSV: High Security Vitrification; LN2: Liquid Nitrogen; μS-VTF: Micro Secure Vitrification; N2: Nitrogen; Non-DMSO; BL-VTF: Blastocyst Vitrification with a solution not containing DMSO; rVTF: re-Vitrification; UR-TRT: Ultra Rapid Cooling Treatment; VTF: Vitrification.
Introduction
Following his pioneering embryo vitrification efforts in 1985, Dr. Rall effectively developed a more practical vitrification method for mammalian embryos using a closed straw system containing a less toxic 6.5M glycerol-6% (w/v) bovine serum albumin based solution [1,2]. Over 15 years later, its clinical application with lower molarity, mixed cryoprotective agent solutions began being widely promoted in combination with micro-volume, open device systems [3-5]. A variety of vitrification devices were ultimately introduced into the IVF industry, promoting the concept that ultra-rapid cooling rates in excess of 10,000°C/min were a necessity for vitrification to achieve high survival rates. Ultimately the dogma surrounding the relative importance of cooling rate was put into perceptive by a series of warming rate studies by Seki and Mazur [6-8] clearly exhibiting warming rate as the primary factor influencing vitrification success. Independent of the cooling rate, post-warming survival of embryos can only be optimized when the warming rate exceeds the cooling rate.
Among the various devices developed for clinical vitrification, several systems are closed devices aseptically retained within a securely sealed straw at room temperature, including the cut standard straw (CSS) [9], high security vitrification (HSV) [10], Vitrisafe [11,12], and micro Secure vitrification (μS-VTF) [13]. The primary advantage of the latter closed vitirification systems being the safe and secure storage of human gametes and embryos, eliminating possible risks associated with the transmission of pathogens in LN2, which has been a debated issue [14]. As aseptically closed vitirification systems have now proven to be effective for oocytes and blastocysts [10- 13,15], it would seem that any risk of disease transmissions between open sample specimens should be deemed unacceptable by regulatory agencies and professional societies. Although it is possible to cryostore human bio-products in sterilized LN2 [16], aseptic cryostorage is then dependent on LN2 vapor storage tanks. Unfortunately, LN2 vapor storage is not a widely accepted alternative for most IVF laboratories applying vitrification, due to the temperature sensitive nature of vitrified products.
Based on the growing high-level of success and undeniable security advantages of some aseptic closed systems [10-13], there has been another interesting but potentially disconcerting trend occurring. Some commercial companies, as well as innovative Embryologists, are hybridizing vitirification systems (e.g., Cryotop, CSS, respectively) by attempting to seal a LN2 exposed open devices into plastic straws. Unlike the safety and security of weld-sealing an ionomeric plastic straw under ambient (20-22°C) conditions, the compliance of super-cooled straws to effective heat sealing may be compromised leading to sub-optimal, unsecure closure. Because embryos do survive vitrification with high efficacy, being relatively unchanged post-warming; it is possible to re-vitrify them with similar efficiency. In turn, we are able to utilize patient consented, vitrified, non-viable aneuploidy embryos as an experimental model to study vitrifcation practices. The objectives of our study were to: (1) assess whether LN2 vapor sealing of straws is a safe, secure and reliable practice; (2) determine if ambient loading & sealing of flexipettes (μSVTF; Control) for VTF is equally effective to ultra-rapid cooling and the LN2 vapor loading/sealing (UR-TRT) of a hybrid device system; and (3) to reveal a validation-verification quality control practices which effectively tests the competency of straw seals.
Materials and Methods
Experimental design
Using a rVTF model on embryos consented by patients for discard research, 41 blastocysts were randomly assigned to either Control (n=19) or UR-TRT (n=22) following standard μS-VTF warming [13]. Randomly applying an apriori arrangement of rVTF treatments, 1 min post-warming each blastocyst-flexipette underwent rVTF by either: 1) Control μS-VTF; or 2) UR-TRT. Survival was assessed at 0 hr and 24 hr post-secondary warming, standard sucrose elution, isotonic equilibration and in vitro culture. Differences in blastocyst survival and formation (blastocyst0hr survival ÷ blastocyst24hr reformation) were assessed by a χ2 test (*p<0.05).
Additionally, we performed a secondary sealing quality control test to validate and exam the potential inadequacy of heat sealing super cooled/LN2 vapor exposed straws (n=20) using a water submersion procedure.
Embryo culture
Research embryos were derived from 2PN zygotes fertilized by intra cytoplasmic sperm injection (ICSI) and group cultured in 25uL droplets of Global™ medium (LG; Life Global, USA) supplemented with 7.5% synthetic protein supplement under Ovoil™ (Vitrolife, USA). Embryos were cultured in MCO-5M mini Sanyo/Panasonic tri-gas incubators (5% 02/5.3-6.0% CO2) under humidified air at 37°C. Only good to excellent quality blastocysts (=3BA/AB or AA) vitrified on either Day 5 or Day 6 was selected for research treatment. Following rVTF, LN2 storage of at least 30 min and then warming/ sucrose dilutions, all embryos were evaluated and returned to LG micro-droplet culture in fresh research dishes for 24 h before reevaluation and discard.
Micro secure vitrification (μS-VTF) and warming
All blastocysts were vitrified in a hyaluronate-enriched, non- DMSO BL-VTF solution (Innovative Cryo Enterprises, USA). Aseptic μS-VTF was performed using: a 3-step dilution exposure to equilibrium, intermediate and final vitrification solutions for 5 min, 5 min and 1min, respectively. Individual blastocysts were loaded into shortened (i.e., 3 mm cut from the base end) 300 μm ID flexipettes (Cook Medical, USA; 3 μl volume); the flexipettes removed from the pipettor, dried repeatedly on sterile gauze and the flexipettes inserted tip first into internally prelabeled 0.3 ml CBS™ embryo straws [13]. Each straw was weld sealed at room temperature (Control treatment); and plunged directly into LN2. The cooling rate was ≈1500°C/ min, while rapid warming (≈6000°C/min) was achieved by direct placement of each vitrified flexipettes into a 37°C 0.5M sucrose bath [13]. Under standard elution treatment, within 10 sec each blastocyst was pipette directly from the flexipette into an open 200 μl droplet of 1.0 M sucrose solution and then transferred to a 100 μl droplet under oil for 3min. Embryos were then serially diluted in declining sucrose solutions (T2-T4, 3 min each), before isotonic equilibration in Hepes- LG medium. Warmed blastocysts were then cultured in LG medium + protein for 24 hr prior to final evaluation of continued blastocyst development and re-expansion typically characterized by hatching and hatched blastocysts.
For rVTF treatment, following the 10 sec rapid warming, the pipettes were dried and either randomly assigned to control or UR-TRT rVTF. The flexipettes containing Control blastocysts were directly reinserted into a new, treatment labeled CBS embryo straw and weld sealed using a Syms 1 automated sealer at room temperature (20-22°C). In the UR-TRT group, the flexipette was secured and placed directly into LN2 (i.e., within a 0.5 L dewar flask), while the new; labeled straw was supported in LN2, with its open end in the vapor layer (approximately 3 cm above liquid). The super cooled flexipette was then carefully lifted into the LN2 vapor phase and inserted into the lumen of the straw. Each UR-TRT straw was then lifted up (halfway submerged in LN2; 5-6 cm) and sealed with a hand sealer at least 2-3 times (with 180° rotations) until an overt, adherent flattening was observed. To validate the competency of straw seals, it is an effective quality control practice to use a water submersion test. Therefore, using 20 empty “air-filled” straws exposed to LN2 vapor sealing conditions, each straw with an apparent intact seal was placed underwater in a water bath. Each straw was then assessed as having either a complete (normal, 180° intact), nearly incomplete (sealed at upper edge but overt air present on one side) or incomplete seal (air bubbling detected).
Results
The first 3 UR-TRT straws exploded upon ambient air extraction and cutting, forcing us to modify our warming process to account for possible LN2 accumulation inside straws due to incomplete seals. By allowing for 15 sec of N2 out gassing, the remaining 19 straws warmed without incident, yet 6 did reveal evidence of LN2 seepage (41% incomplete seals). No problems were experienced in the warming of control straws, and no difference in 0 h blastocyst survival being evident between treatments (Table 1). However, survival at 24 h tended to be lower and sustained blastocyst development was reduced in the UR-TRT group compared to the control group (Table 1).
Observations
μS-VTF Control
Ultra-Rapid TRT
P-value
# BL rVTF/warmed
19
19ψ
N.S.
# Survived (0hr)
15 (78.9%)
16 (84.2%)
N.S.
# Survived (+24hr)
13 (68.4%)
10 (52.6%)
p<0.10
BL Reformation (+24hr)
13/15 (86.7%)*
10/16 (62.5%)
p<0.05*
Table 1: UR-TRT group compared to the control group.
In a secondary “sealing” quality control examination, all the seals appeared visually complete. However only 70% actually were complete (Figure 1A), of which 30% of those were nearly incomplete (i.e., partial) exhibiting a distinct air bubble progressing up the sidewall of the seal (Figure 1B). The remaining 30% of the straws displayed incomplete seals, evidenced by overt air bubbling underwater (Figure 1C) which dissipated to a slow release of bubbles (Figure 1D).
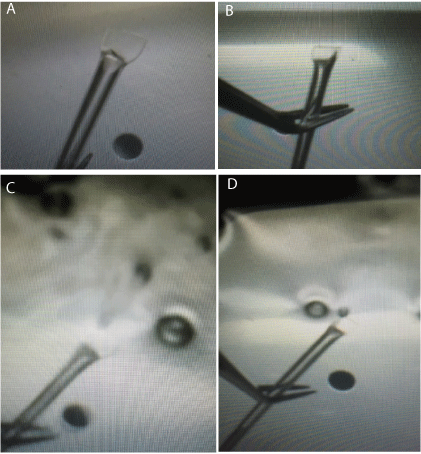
Figure 1: A post-study quality control validation test was performed on the
patency of straw seals (n=20) created under the experimental LN2 vapor
conditions. The straws were not submerged in LN2, but instead the straws
were directly placed underwater to inspect the seals. Air in a straw with a
complete seal (Figure 1A) can be seen as a straight backline, whereas the
partial-complete seal (Figure 1B) reveals air seeping up the right-side edge
of the seal. Incomplete seals are overtly apparent by the rapid bubbling
of escaping air from the straw (Figure 1C), which tapered down to a slow
release (Figure 1D). Note, no explosive events occurred because ambient air
was allowed to freely escape, as opposed to LN2 being converted to a rapidly
expanding N2 gas, resulting in over-expansion of the inner straw lumen.
Discussion
The application of standard μS-VTF warming practices to UR-TRT straws proved unreliable. The fatal rupturing of the first 3 UR-TRT straws was caused by the rapid vaporization of LN°(at 21°C) which had seeped into the straws due to incomplete seals. We performed a secondary sealing test to exam the inadequacy of heat sealing super cooled/LN° vapor exposed straws (Figure 1) using a water submersion test. Although all seals appeared normal, only 40% actually were complete, while another 30% were intact but nearly incomplete. Finally, a third of the straw seals were inadequate and at risk of an explosive outcome under standard rapid warming conditions. Fortunately, the explosive pressure build-up was avoided in our primary study, following the former initial incidents, by allowing the N° gas to escape the leaky seal by holding the straw gradually above the dewar flask vapor phase for 15 sec before cutting the straw. By allowing for N° out gassing before cutting the straw near the inner plug, and the flexipettes were safely released by tilting the straws downward over the warm sucrose bath. With a slight tap to the straw, all 38 flexipettes were released, rapidly warmed and all the embryos were recovered. The reliable recovery (100%) of vitrified embryos has been a superior characteristic of the μS-VTF system [13].
Once recovered, we did not observe any advantage to the ultrarapid cooling of flexipettes, in contrast to the slower cooling achieved in the insulated environment of a CBS embryo straw. Some decline in embryo survival was observed in both groups (16-21%) following rVTF. The reduced development of the UR-TRT blastocysts at +24 h was likely an artifact of the rVTF model used. The slower cooling rate of an aseptic closed VTF device tolerates the slower warming rates inherent to the system applying safe lab practices to effectively retrieve and warm the device. Based on recent clinical experience, a more optimal developmental outcome may have been achieved by cutting the straws in LN² and somehow retrieving the flexipette before ambient exposure and rapid warming in a warm bath. Hybridvitrified cut straw devices were recently transferred from another ART Lab in southern California to our laboratory. Upon inspection, ID confirmation and secured storage, it was noticed that LN² was bubbling inside each of 10+ straws from 3 separate shipments (Figure 2). Using the current warming practices of the commercial Cryotopclosed VTF hybrid system, we successfully warmed the former flawed straw devices by retrieving the device inside LN². Even then, this successful warming practice would be device dependent to a system capable of achieving ultra-rapid warming to prevent recrystallizing ice formation upon devitrification. As shown by Mazur and Seki [6- 8], and discussed in a slow warming model by Wowk [17], the latter rate dependent event outcome is further dependent on the stability of the VTF solution used (i.e., concentration of the cryoprotective agents used). It has been proposed that commercial 30-32% (v/v; 4.8- 5.2M) VTF solutions are relatively unstable in a slow warming model, which would put most hybrid-VTF systems at increased risk of user variation in warming practices having an adverse effect. In this study, our own metastable VTF solutions (>7.9M) displayed the risk of reduced blastocyst post-warming viability due to warming variation.
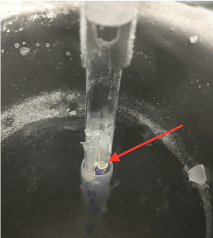
Figure 2: Upon inspecting a group of hybrid –vitrified straws transferred to
our lab in the spring of 2016, we observed LN2 present in the inner lumen
of all seals (see arrow), which appeared to possess closed seals. The
originating lab failed to provide accurate procedural paper work mentioning
the latter potential flaw in their ‘cut-straw, rapid plunge, vapor sealed-closed
straw system’, and how to safely warm incompletely sealed straws.
The handling of VTF devices and open straws in LN2, and LN2 vapor, is performed at increased risks to the safety of the Embryologist. Furthermore, the embryos themselves are susceptible to unexpected destruction if a hybridized VTF straw is warmed to quickly before extracting the device. This study has proven that UR-closed VTF may be a potentially unreliable, unsafe and less effective procedure. The inability to guarantee complete seals of super-cooled straws or the possible entry of N2 vapors inside a straw upon sealing create significant risks which are unnecessary quality control variables absent in standard aseptic, closed VTF methods. As standard aseptic, closed VTF systems (e.g., HSV, μS-VTF, Vitrisafe) have proven to be highly effective for the cryopreservation of oocytes and embryos; it is time that the ART industry begins recognizing quality control variables and avoidable risks associated with flawed device concepts.
Acknowledgement
Our Summer Student Science Training Program was established to assist 1 or 2 outstanding high school seniors and/or underdivision university students with an introduction to embryology research, experimental design and scientific writing. We wish to thank Dr. Robert E. Anderson, MD for supporting and fostering the establishment of this program at the SCIRS/Ovation Fertility Laboratory.
References
- Rall WF, Wood MJ, Kirby C, Whittingham DG. Development of mouse embryos cryopreserved by vitrification. J Reprod Fertil. 1987; 80: 499-504.
- Schiewe MC, Rall WF, Stuart LD, Wildt DE. Analysis of cryoprotectant, cooling rate and in situ straw dilution using conventional freezing or vitrification for cryopreserving sheep embryos. Theriogenol. 1991; 36: 279-293.
- Mukaida T, Nakamura S, Tomiyama T, Wada S, Oka C, Kasai M, et al. Vitrification of human blastocysts using cryoloops: Clinical outcome of 223 cycles. Hum Reprod. 2003; 18: 384-391.
- Mukaida T, Oka C, Goto T, Takahashi K. Artificial shrinkage of blastocoeles using either a micro-needle or a laser pulse prior to the cooling steps of vitrification improves survival rate and pregnancy outcome of vitrified human blastocysts. Hum Reprod. 2006; 21: 3246-3252.
- Kuwayama M. Highly efficient vitrification for the cryopreservation of human oocytes and embryos: The Cryotop method. Theriogenology. 2007; 67: 73- 80.
- Seki S, Mazur P. The dominance of warming rate over cooling rate in the survival of mouse oocytes subjected to a vitrification procedure. Cryobiology. 2009; 59: 79-82.
- Mazur P, Seki S. Survival of mouse oocytes after being cooled in a vitrification solution to -196°C at 95°C to 70,000°C/min and warmed at 610°C to 118,000°C/ min: a new paradigm for cryopreservation by vitrification. Cryobiology. 2011; 62: 1-7.
- Seki S, Mazur P. Ultra-rapid warming yields high survival of mouse oocytes cooled to -196°C in dilutions of a standard vitrification solution. PLoS One. 2012; 7: e36058.
- Isachenko V, Katkov I, Yakovenko S, Lulat A, Ulug, M, Arvas A, et al. Vitrification of human laser treated blastocysts within cut standard straws (CSS): Novel aseptic packaging and reduced concentrations of cryoprotectants. Cryobiology. 2007; 54: 305-309.
- Lopes AS, Frederick V, Van Kerkhoven G, Campo R, Puttemans P, Gordts S. Survival, re-expansion and cell survival of human blastocysts following vitrification and warming using two vitrification systems. J Asst Reprod Genet. 2015; 32: 83-90.
- Panagiotidis Y, Vanderzwalmen P, Prapas Y, Kasapi E, Goudakou M, Papatheodorou A, et al. Open versus closed vitrification of blastocysts from an oocyte-donation programme: a prospective randomized study. Reprod Biomed Online. 2013; 26: 470-476.
- Papatheodorou A, Vanderzwalmen P, Panagiotidis Y, Prapas N, Zikopoulos K, Georgiou I, et al. Open versus closed oocyte vitrification system: a prospective randomized study. Reprod Biomed Online. 2013; 26: 595-602.
- Schiewe MC, Zozula S, Anderson RE, Fahy GM. Validation of microSecure vitrification (μS-VTF) for the effective cryopreservation of human embryos and oocytes. Cryobiology. 2015; 71: 264-272.
- Pomeroy KO, Harris S, Conaghan J, Papadakis M, Centola G, Basuray R, et al. Storage of cryopreserved reproductive tissues: evidence that crosscontamination of infectious agents is a negligible risk. Fertil Steril. 2010; 94: 1181-1188.
- Stachecki JJ, Garrisi J, Sabin S, Caetano JP, Wiemer K, Cohen J. A new safe, simple, and successful vitrification method for bovine and human blastocysts. Reprod Biomed Online. 2008; 17: 360-367.
- Parmegiani L, Cognigni GE, Bernardi S, Cuomoc S, Ciampaglia W, Infante FE, et al. Efficiency of aseptic open vitrification and hermetical cryostorage of human oocytes. Reprod Biomed Online. 2011; 23: 505-512.
- Wowk B. Thermodynamic aspects of vitrification. Cryobiology. 2010; 60: 11- 22.