
Review Article
J Stem Cell Res Transplant. 2015;2(1): 1015.
Endothelial Progenitor Cells: Biology and Application in Ischemic Tissue Vascularization
Venkata Naga Srikanth Garikipati1, Suresh Kumar Verma1 and Raj Kishore1, 2*
1Center for Translational Medicine, Temple University School of Medicine, Philadelphia, PA 19140
2Department of Pharmacology, Temple University School of Medicine, Philadelphia, PA 19140
*Corresponding author: Raj Kishore, PhD, FAHA, Center for Translational Medicine, Temple University School of Medicine, MERB-9533500, N Broad Street, Philadelphia, PA 19140
Received: January 06, 2015; Accepted: February 19, 2015; Published: February 21, 2015
Abstract
Endothelial progenitor cells (EPC) have rapidly emerged as a potential novel therapeutic approach in ischemic diseases. After the initial characterization of putative bone marrow-derived endothelial progenitor cells and their potential to promote neovascularization and to attenuate ischemic injury, a decade of intense preclinical research has led to the EPC-based clinical trials that have suggested their safety but with modest results. This review focuses on the role of endogenous and exogenous EPC therapy in promoting neovascularization in the ischemic settings to illustrate the future directions of EPC in therapeutic applications. We also reviewed clinical applications of EPC and potential strategies in improving EPC based therapeutics for ischemic tissue repair.
Keywords: Endothelial progenitor cells; Cell therapy; Ischemic diseases; Neovascularization
Introduction
For more than a decade now, biology and therapeutic efficacy of Endothelial progenitor cells (EPCs) were largely driven by the first observations of Asahara et al. in identifying EPCs in adult peripheral blood (PB[1] and were shown to derive from bone marrow (BM) further migrate and incorporate into foci of physiological or pathological neovascularization [2,3]. As a matter of fact, postnatal neovascularization was believed to be established by the mechanism of "angiogenesis," by in situ proliferation and migration of preexisting endothelial cells (ECs) [4]. However, the finding that EPCs can home to sites of neovascularization and differentiate into ECs in situ is consistent with "vasculogenesis," a critical paradigm has been demonstrated in embryonic neovascularization [5] and also shown recently for the adult organism in which a pool of progenitor cells contributes to postnatal neovascular formation [6]. The discovery of EPCs has therefore considerably changed our understanding of adult blood vessel formation. Furthermore, we and other groups envisage the potential of EPC to improve the clinical applicability in the fight against ischemic diseases.
This review focuses on the potential value of EPCs (both endogenous and exogenous therapy) as a therapeutic regimen for the treatment of ischemic diseases.
Characterizationof EPC
EPCs in circulation can be broadly sub-divided mainly into two categories, hematopoietic lineage EPCs and non-hematopoietic lineage EPCs (Figure 1). The hematopoietic EPCs originate from BM and represent a pro-vasculogenic subpopulation of hematopoietic stem cells (HSCs) ([7,9]. The non-hematopoietic EPCs are blood or tissue derived entities, exhibiting EC like phenotype [10] or ability to differentiate into EC likecells[11].
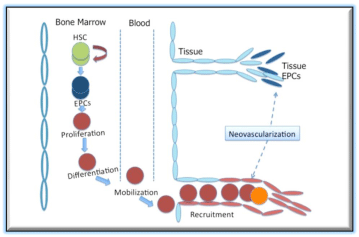
Figure 1: Mechanism of EPC mediated postnatal neovascularization. Circulating EPCs mobilized from BM are recruited into foci of neovascularization and contribute to new blood vessel formation.
EPCs and HSCs have been shown to express variety of cell surface markers, including membrane receptors like CD31, CD133, (Fetal liver kinase-1) Flk- 1, CXC chemokine receptor-4 (CXCR-4) , CD105 (Endoglin), c-Kit for human samples[12-15]and receptors like c-Kit (CD117), stem cell antigen (Sca-1), and CD34 in combination with Flk-1 (vascular endothelial growth factor receptor-2 (VEGFR-2) in case of mouse samples [16-18]. Nevertheless, identifying a particular combination of markers for isolation of these cells has been a big challenge for the researchers in this field.
The origin of non-hematopoietic EPCs (NHEPCs) remains to be clarified, but they are generally thought to be derived from nonhematopoietic tissue resident lineage stem cells or organ blood vessels but not likely from HSC [19].
NHEPCs have been shown to be derived from non-hematopoietic tissues. A novel cell type endothelial out growth cell (EOC) has been shown to be a type of NHEPC, these EOCs are the derived from endothelial colony formation assay system developed and reported by Yoder's group and others [20,21]. Unlike hematopoietic EPC, EOC readily form tubes in vitro. However, origin and role of EOC is still a debatable but appears to be a potential therapeutic regimen for neovascularization [22,23]. Recently, Aicher et al.[24], has shown circulating cells from BM and non-BM help in vasculature, demonstrated by the mobilization of c-kit+/CD45- progenitor by a parabiosis model from liver and small intestine. In spite of the above findings, it is difficult to define EPCs because of the ambiguity in the phenotype and characteristics that are fundamental for EPC identity.
EPCs contribute to neovascularization
In the perspective of EPC biology, vasculogenesis mainly comprises the de novo blood vessel formation via in situ migration, proliferation, differentiation, and/or incorporation of bone marrow (BM)-derived EPCs into regenerating vasculature [3]. This fact of BM-derived EPCs incorporation into site of physiological or pathological neovascularization has been thoroughly validated in various animal models. Nevertheless, one well-established model, allowing the detection of BM-derived EPCs, uses transplantation of BM cells from transgenic mice in which LacZ is expressed under the regulation of flk-1 or Tie-2(Flk-1/LacZ/BMT, Tie-2/LacZ/BMT) lineage-specific promoter, into wild-type control mice, using the same in various ischemic injury models. It has been shown by using the above model that BM-derived Flk-1- and/or Tie-2- expressing endothelial lineage cells can localize to vascular structures wound healing [25,3]and cardiac ischemia [16,17], Regardless of the origin of EPCs, they undoubtedly play a significant role contributing to neovascularization via vasculogenesis in ischemic tissues.
The tissue resident EPCs secrete a variety of pro-angiogenic cytokines and growth factors in a paracrine fashion there by actively supporting proliferation and migration of pre-existing ECs thus promoting angiogenesis and contributing in an indirect mode to neovascularization to restore tissue homeostasis[4].
In fact observations of the preclinical studies remind us the evidence of enhanced intrinsic recipient angiogenesis by extrinsic factors derived from transplanted EPCs in myocardial ischemia models [26,27].
This paracrine factor secretion of EPC activity show their indirect contribution to neovascularization was established by our and several othergroups, identifying EPC secretome with presence of various cytokines and other proangiogenic factors such as viz., vascular endothelial growth factor (VEGF), hepatic growth factor (HGF), angiopoetin (Ang-1, stroma derived factor (SDF)-1a, insulinlike growth factor (IGF)-1, and endothelial nitric oxide synthase (eNOS[28-30].Therefore, EPCs can also mediate tissue-protective effects and contribute to neovascularization in ischemic tissues via production of several important indirect support factors such as exosomes [31].
EPC Therapy:Pre-clinical animal models
Since EPCs were first described, more than a decade ago, our group focused especially on the regenerative potential of these enigmatic cells trying to understand the biology and functions with the ultimate goal to translate to bedside. As this cell type has got enormous potential to treat ischemic diseases [5,11,32,33]. The preclinical transplantation of blood/BM-derived EPCs, opened new avenue for the treatment of ischemic diseases.
It was shown that therapeutic efficacy of EPCs to be safe and enhanced neovascularization and regeneration of ischemic tissues [5,11,32,33]. Coronary artery disease can be attenuated by the collateral circulation development; after recognizing the role of EPCs in neovascularization, investigators started evaluatingpotential therapeutic efficacy of EPCs. This was validated by recovery of blood flow s in mice with hindlimb ischemia treated with EPCs compared to control mice that received mature ECs [34]. It was also suggested a similar benefits with administration of cord blood derived EPCs isolated in a nude rat model of hind limb ischemia [35]. Lately, human CD34+ cells transplanted into a nude rat model of myocardial ischemia and observed preservation of (Left ventricular) LV function and inhibition of cardiac apoptosis. These findings were in corroboration with the observed preservation of LV function and a reduction in infarction size [17,27,36]. Our study demonstrates that EPC therapy after diabetic myocardial infarction (MI) effectively reduces cardiac fibrosis and preserves cardiac function through release of HGF, which effectively inhibits cardiac microRNA-155-mediated pro-fibrosis signaling and attenuates left ventricular dysfunction in diabetic MI mice [37].
Further, Schatteman et al. [38] reported transplantation of human CD34+ cells into diabetic nude mice with hind limb ischemia resulted in significant blood flow recovery in ischemic limbs. Interestingly, a recent study in porcine models of MI, EPC administration along with AKT inhibitor (LY294002) significantly reduced their neovascularization ability suggesting EPC mediated neovascularization and preserved cardiac function were Aktdependent[ 39]. All these above studies indicate that EPC to be a potential stem cell type for the treatment of ischemic diseases in the pre-clinical settings and encouraged to move on to EPC based clinical trials.
The functional benefits observed with EPC-based therapy preclinical studies might have been from several aspects. At the ischemic zone, both transplanted as well as endogenous EPCs could protect ECs and ischemic tissue resident cells from ischemia-induced cell death by secreting various growth factors such as VEGF, SDF-1 and IGF-1. Thus EPCs secreted factorsenhance neovascularization and functionally and structurally rebuild the injured tissue contributing to the recovery.
Novel therapeutic strategies for improving EPC based therapeutics for ischemic tissue repair
A central recognized challenge that precludes full functional benefits of EPC based therapies is the poor survival and retention of transplanted cells thereby greatly compromising their therapeutic efficacy. Emerging evidence from preclinical studies suggests that hostile microenvironment in the infarcted myocardium, including inflammation and oxidative damage, has adverse effects on transplanted stem cell survival and function [40,41].
Previous reports from our and other labs has established a significant cardio-protective role of anti-inflammatory cytokine, interleukin-10 (IL-10) therapy in mice models of AMI and pressureoverload, which in turn is mediated by IL-10-mediated modulation in a number of signal transduction pathways including p38 mitogenactivated protein kinases (MAPK), nuclear factor kappa-lightchain- enhancer of activated B cells (NF-kB) and signal transducer and activator of transcription-3 (STAT-3) pathway [42-45]. Our recently published work [17] demonstrated that combined therapy with EPC+IL-10 is significantly more effective in the improvement of post-acute myocardial infarction (AMI) left ventricular function and ischemic angiogenesis than EPC alone, and that this effect of IL-10 is mediated in part by its effect on enhanced EPC survival and function in the ischemic myocardium.
Our recent report suggests that small molecules such as valproic acid and/or 5-azacytidine based epigenetic reprogramming of EPCs, both murine and human, results in an open epigenome conducive to enhanced global gene expression including the induction of cardiomyocyte specific gene expression. We have shown that, modifying human CD34+ EPCs with small molecules induce cardiomyogenic differentiation and histological fibrosis area, and echocardiographic functional parameters were better persevered in immune deficient mice model of MI [16]. Further, intramyocardial transplantation of reprogrammed EPCs improved paracrine capacity of reprogrammed cells. Our approach to use small molecules is an innovative way to induce multipotency and cardiomyogenic differentiation potential in EPCs, without the undesired complications involved with induced pluripotent cells, is novel.
We recently also reported Arg-Gly-Asp-Ser peptide (RGDS-PA) nanofibers presenting integrin-binding epitope facilitate enhanced retention of bone marrow derived angiogenic progenitor cells and improved efficacy upon their therapy in a murine model of hind limb ischemia. This represents a novel application of PA nanofiber technology to augment cell retention after cell-based therapy [46].
EPC Therapy: Clinical trials
Numerous clinical trials have been initiated and are currently in progress to understand the safety issues and therapeutic efficacy of EPCs observed in animal models on ischemic diseases [47](Table 1).
Trail Name
Disease type
Patients
(T/C)
Outcome
Reference
G-CSF-PB-CD34+
Fujita et al.
2014
CLI
11
Rest pain scale
Physiological parameters é
[ 55 ]
G-CSF-PB-CD34+
Povsic et al, 2013
AP
200/100
Improvement of functional capacity in patients with refractory angina.
[ 56 ]
CD133+
Forcillo et al 2013
CICM
LVEF increased
[ 57 ]
G-CSF-PB-CD34+/
Poglajen et al, 2014
ICM
33
LVEF?
( 58)
CD34+
Vrtovec et al, 2013
NICM
20/20
LVEF é
Transendocardial injections associated with better clinical outcome
[ 59 ]
G-CSF-PB-CD34+/CD133+
Burt et al.
2010
CLI
9/0
Physical component score é
Leg amputation ê
[ 60 ]
PB/BM Derived EPCs
TOPCARE-AMI.2009
AMI
30/29
LVEF é
Perfusion é
Infarct size ê
[ 61 ]
G-CSF-PB-CD34+
Losordo et al.
2007
AP
18/6
LVEF é
CCS class é
[ 48 ]
CD133+
Li et al.
2007
AMI
35/35
LVEF é
[ 62 ]
CD133+
Stamm et al
2007
CICM
20/20
LVEF é
[ 63 ]
G-CSF-PB-CD34+
Boyle et al.
2006
OMI
5/0
Myocardial neovascularization é
[ 64 ]
G-CSF-PB-CD34+
Bartunek et al.
2005
AMI
19/16
LVEF é
Perfusionê
[ 65 ]
G-CSF-PB-CD34+
Stamm et al.
2003
RMI
46/9
LVEF é
Perfusionê
[ 66 ]
Table 1: Abbreviations: AMI, acute myocardial infarction; AP, angina pectoris; BM, bone marrow; CLI, critical limb ischemia; EPCs, endothelial progenitor cells; G-CSF (granulocyte colony-stimulating factor; NUF, nonunion fracture; OMI, old myocardial infarction; PB, peripheral blood; RMI, recent myocardial infarction; RT, randomized trial; T/C, treatment/control, ICM- ischemic cardiomyopathy, NICM, non-ischemic cardiomyopathy, CICM, chronic ischemic cardiomyopathy. ⌈- Increased, ⌈- decreased.<
Our group performed a phase I/II, randomized, placebocontrolled, dose-ranging, clinical trial to evaluate the intramyocardial transplantation of G-CSF-mobilized CD34+ cells in 24 patients with intractable angina pectoris [48]. Favorable trends in angina frequency, exercise tolerance, and perfusion defect were observed in patients administered CD34+ cells compared with patients who received placebo.
As for the evaluation of safety issues, neither death nor lifethreatening adverse events were observed in therapy group, the results from this phase II study support the safety and efficacy of intramyocardially injected autologous CD34+ cells for symptom reduction and improved exercise capacity in "no-option" patients with refractory angina. Larger-scale studies are warranted to verify these effects and to refine the methods for collecting and administering CD34+ cells to patients with disabling angina symptoms.
Clinical EPC Therapies: Challenges and Limitations
In most patients undergoing EPC therapy for ischemic diseases have background diseases such as aging [49],diabetes[50] hypercholesterolemia [51], hypertension [52] and smoking[53,54] which affect the number and function of both circulating and BM EPCs. Which might be a potential cause for the modest clinical outcome of EPC transplantation for ischemic diseases.
Therefore, considering autologous EPC therapy, resolving certain issues may help to overcome the shortcomings of EPCs should include: [1] EPCs delivery at the site of injury [2], enhancing strategies to mobilize endogenous EPC (appropriate cytokine/growth factor therapy) [3], EPC enrichment techniques (such as apheresis or BM aspiration) [4], enhancement of EPC functions/ salvage of EPC dysfunction by gene therapy or small molecule treatment [5] Improving strategies to scale-up EPCs culture and expansion for clinical use[6]. Enhancing transplanted EPC retention and survival in ischemic tissues by anti-inflammatory cytokine IL-10 or biomaterials.
The above strategies might compensate the current disadvantages of transplanting dysfunctional EPCs for autologous therapy in ischemic diseases by increasing the number and also the quality of transplanted EPCs.
Conclusions
Taken together, EPCs serve as a highly promising and novel therapeutic option for ischemic tissue repair. It is important to clarify if EPC-based therapy is superior over other types of stem/ progenitor cells. Thus additional investigations related to optimize techniques for EPC isolation, expansion, mobilization, recruitment, and EPC retention and survival strategies post transplantation in a ischemic tissue are needed to continue the advancement of this novel therapeutic modality (Figure 2). Thus resolving the above issues will achieve full-benefits of EPC based therapies for ischemic tissue repair representing a future direction.
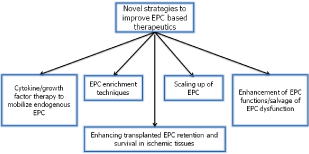
Figure 2: Diagram representing novel strategies to improve EPC based therapeutics for ischemic tissue repair.
Acknowledgements
This article was in part supported by American Heart Association Fellowship 15POST22720022 (V.N.S.G) and Scientist Development Grant 14DG20480104 (S.K.V) and National Institutes of Health Grants HL091983, HL105597, HL053354, HL108795 and HL126186 (R.K).
References
- sahara T, Murohara T, Sullivan A, Silver M, van der Zee R, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997; 275:964-967.
- Shi Q, Rafii S, Wu MH, Wijelath ES, Yu C, et al. Evidence for circulating bone marrow-derived endothelial cells. Blood 1998; 92:362-367.
- Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circulation research 1999; 85:221-228.
- Folkman J, Shing Y. Angiogenesis. JBio Chem 1992; 267:10931-10934.
- Risau W, Sariola H, Zerwes HG, Sasse J, Ekblom P, et al. Vasculogenesis and angiogenesis in embryonic-stem-cell-derived embryoid bodies. Development 1988; 102:471-478.
- Isner JM, Asahara T. Angiogenesis and vasculogenesis as therapeutic strategies for postnatal neovascularization. J Clin Invest 1999; 103:1231-1236.
- Bailey AS, Jiang S, Afentoulis M, Baumann CI, Schroeder DA, et al. Transplanted adult hematopoietic stems cells differentiate into functional endothelial cells. Blood 2004; 103:13-19.
- Pelosi E, Valtieri M, Coppola S, Botta R, Gabbianelli M, et al. Identification of the hemangioblast in postnatal life. Blood 2002; 100:3203-3208.
- Grant MB, May WS, Caballero S, Brown GA, Guthrie SM, et al. Adult hematopoietic stem cells provide functional hemangioblast activity during retinal neovascularization. Nat Med 2002; 8:607-612.
- Ingram DA, Caplice NM, Yoder MC. Unresolved questions, changing definitions, and novel paradigms for defining endothelial progenitor cells. Blood 2005; 106: 1525-15231.
- Aicher A, Brenner W, Zuhayra M, Badorff C, Massoudi S, et al. Assessment of the tissue distribution of transplanted human endothelial progenitor cells by radioactive labeling. Circulation 2003; 107:2134-2139.
- Lamirault G, Susen S, Forest V, Hemont C, Parini A, et al. Difference in mobilization of progenitor cells after myocardial infarction in smoking versus non-smoking patients: insights from the BONAMI trial. Stem Cell ResTher 2013; 4:152.
- Malecki M, Sabo C, Putzer E, Stampe C, Foorohar A, et al. Recruitment and retention of human autologous CD34+ CD117+ CD133+ bone marrow stem cells to infarcted myocardium followed by directed vasculogenesis: Novel strategy for cardiac regeneration. Mol Cell Ther 2013; 1.
- Igreja C, Fragoso R, Caiado F, Clode N, Henriques A, et al. Detailed molecular characterization of cord blood-derived endothelial progenitors. Exp Hematol 2008; 36:193-203.
- Asahara T, Kawamoto A, Masuda H. Concise review: Circulating endothelial progenitor cells for vascular medicine. Stem Cells. 2011; 29:1650-1655.
- Thal MA, Krishnamurthy P, Mackie AR, Hoxha E, Lambers E, et al. Enhanced angiogenic and cardiomyocyte differentiation capacity of epigenetically reprogrammed mouse and human endothelial progenitor cells augments their efficacy for ischemic myocardial repair. Circ Res 2012; 111:180-190.
- Krishnamurthy P, Thal M, Verma S, Hoxha E, Lambers E, et al. Interleukin-10 deficiency impairs bone marrow-derived endothelial progenitor cell survival and function in ischemic myocardium. Circ Res 2011; 109:1280-1289.
- Timmermans F, Plum J, Yoder MC, Ingram DA, Vandekerckhove B, et al. Endothelial progenitor cells: identity defined? J Cell Mol Med 2009; 13:87-102.
- Timmermans F, Van Hauwermeiren F, De Smedt M, Raedt R, Plasschaert F, et al. Endothelial outgrowth cells are not derived from CD133+ cells or CD45+ hematopoietic precursors. ArteriosclerThromb vas Biol 2007; 27:1572-1579.
- Lin Y, Weisdorf DJ, Solovey A, Hebbel RP. Origins of circulating endothelial cells and endothelial outgrowth from blood. The J Clin Invest 2000; 105:71-77.
- Ingram DA, Mead LE, Tanaka H, Meade V, Fenoglio A, et al. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood 2004; 104:2752-2760.
- Hur J, Yoon CH, Kim HS, Choi JH, Kang HJ, et al. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol. 2004; 24:288-2893.
- Sieveking DP, Buckle A, Celermajer DS, Ng MK. Strikingly different angiogenic properties of endothelial progenitor cell subpopulations: insights from a novel human angiogenesis assay. J Am Coll Cardiol 2008; 51:660-668.
- Aicher A, Rentsch M, Sasaki K, Ellwart JW, Fandrich F, et al. Nonbone marrow-derived circulating progenitor cells contribute to postnatal neovascularization following tissue ischemia. Circ Res 2007; 100:581-589.
- Bauer SM, Goldstein LJ, Bauer RJ, Chen H, Putt M, et al. The bone marrow-derived endothelial progenitor cell response is impaired in delayed wound healing from ischemia. J Vasc Surg 2006; 43:134-141.
- Urbich C, Aicher A, Heeschen C, Dernbach E, Hofmann WK, et al. Soluble factors released by endothelial progenitor cells promote migration of endothelial cells and cardiac resident progenitor cells. J Mol Cell Cardiol 2005; 39:733-742.
- Kawamoto A, Gwon HC, Iwaguro H, Yamaguchi JI, Uchida S, et al. Therapeutic potential of ex vivo expanded endothelial progenitor cells for myocardial ischemia. Circulation 2001; 103:634-637.
- Jujo K, Ii M, Losordo DW. Endothelial progenitor cells in neovascularization of infarcted myocardium. J Mol Cell Cardiol 2008; 45:530-544.
- Miyamoto Y, Suyama T, Yashita T, Akimaru H, Kurata H. Bone marrow subpopulations contain distinct types of endothelial progenitor cells and angiogenic cytokine-producing cells. J Mol Cell Cardiol 2007; 43:627-635.
- Hynes B, Kumar AH, O'Sullivan J, Klein Buneker C, Leblond AL, et al. Potent endothelial progenitor cell-conditioned media-related anti-apoptotic, cardiotrophic, and pro-angiogenic effects post-myocardial infarction are mediated by insulin-like growth factor-1. Eur Heart J 2013; 34:782-789.
- Mackie AR, Klyachko E, Thorne T, Schultz KM, Millay M, et al. Sonic hedgehog-modified human CD34+ cells preserve cardiac function after acute myocardial infarction. Circ Res 2012; 111:312-321.
- Kawamoto A, Asahara T, Losordo DW. Transplantation of endothelial progenitor cells for therapeutic neovascularization. Cardiovascular radiation medicine 2002; 3:221-225.
- Kocher AA, Schuster MD, Szabolcs MJ, Takuma S, Burkhoff D, et al. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med 2001; 7:430-436.
- Kalka C, Masuda H, Takahashi T, Kalka-Moll WM, Silver M, et al. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proceedings of the National Academy of Sciences of the United States of America 2000; 97:3422-3427.
- Murohara T, Ikeda H, Duan J, Shintani S, Sasaki K, et al. Transplanted cord blood-derived endothelial precursor cells augment postnatal neovascularization. J Clin Invest 2000; 105:1527-1536.
- Kawamoto A, Iwasaki H, Kusano K, Murayama T, Oyamada A, et al. CD34-positive cells exhibit increased potency and safety for therapeutic neovascularization after myocardial infarction compared with total mononuclear cells. Circulation 2006; 114:2163-2169.
- Kishore R, Verma SK, Mackie AR, Vaughan EE, Abramova TV, et al. Bone marrow progenitor cell therapy-mediated paracrine regulation of cardiac miRNA-155 modulates fibrotic response in diabetic hearts. PloS one 2013;8:e60161.
- Schatteman GC, Hanlon HD, Jiao C, Dodds SG, Christy BA. Blood-derived angioblasts accelerate blood-flow restoration in diabetic mice. J Clin Invest 2000; 106:571-578.
- Zhang S, Zhao L, Shen L, Xu D, Huang B, et al. Comparison of various niches for endothelial progenitor cell therapy on ischemic myocardial repair: coexistence of host collateralization and Akt-mediated angiogenesis produces a superior microenvironment. Arterioscler Thromb Vasc Biol 2012; 32:910-923
- Grisar J, Aletaha D, Steiner CW, Kapral T, Steiner S, et al. Depletion of endothelial progenitor cells in the peripheral blood of patients with rheumatoid arthritis. Circulation 2005; 111:204-211.
- Werner N, Nickenig G. Influence of cardiovascular risk factors on endothelial progenitor cells: limitations for therapy? Arterioscler Thromb Vasc Biol 2006; 26:257-266.
- Krishnamurthy P, Lambers E, Verma S, Thorne T, Qin G, et al. Myocardial knockdown of mRNA-stabilizing protein HuR attenuates post-MI inflammatory response and left ventricular dysfunction in IL-10-null mice. FASEB 2010; 24:2484-2494.
- Krishnamurthy P, Rajasingh J, Lambers E, Qin G, Losordo DW, et al. IL-10 inhibits inflammation and attenuates left ventricular remodeling after myocardial infarction via activation of STAT3 and suppression of HuR. Circ Res 2009; 104:e9-18.
- Verma SK, Krishnamurthy P, Barefield D, Singh N, Gupta R, et al. Interleukin-10 treatment attenuates pressure overload-induced hypertrophic remodeling and improves heart function via signal transducers and activators of transcription 3-dependent inhibition of nuclear factor-kappaB. Circulation 2012; 126:418-429.
- Jones SP, Trocha SD, Lefer DJ. Cardioprotective actions of endogenous IL-10 are independent of iNOS. Cardioprotective actions of endogenous IL-10 are independent of iNOS 2001; 281:H48-52.
- Tongers J, Webber MJ, Vaughan EE, Sleep E, Renault MA, et al. Enhanced potency of cell-based therapy for ischemic tissue repair using an injectable bioactive epitope presenting nanofiber support matrix. J Mol Cell Cardiol 2014; 74:231-239.
- Dai Y, Ashraf M, Zuo S, Uemura R, Dai YS, et al. Mobilized bone marrow progenitor cells serve as donors of cytoprotective genes for cardiac repair. J Mol Cell Cardiol 2008; 44:607-617.
- Losordo DW, Schatz RA, White CJ, Udelson JE, Veereshwarayya V, et al. Intramyocardial transplantation of autologous CD34+ stem cells for intractable angina: a phase I/IIa double-blind, randomized controlled trial. Circulation 2007; 115:3165-3172.
- Heiss C, Kleinbongard P, Dejam A, Perre S, Schroeter H, et al. Acute consumption of flavanol-rich cocoa and the reversal of endothelial dysfunction in smokers. J Am Coll Cardiol 2005; 46:1276-1283.
- Ii M, Takenaka H, Asai J, Ibusuki K, Mizukami Y, et al. Endothelial progenitor thrombospondin-1 mediates diabetes-induced delay in reendothelialization following arterial injury. CircRes2006; 98:697-704.
- Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, et al. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res 2001; 89:E1-7.
- Imanishi T, Moriwaki C, Hano T, Nishio I. Endothelial progenitor cell senescence is accelerated in both experimental hypertensive rats and patients with essential hypertension. Journal of hypertension 2005; 23:1831-1837.
- Kondo T, Hayashi M, Takeshita K, Numaguchi Y, Kobayashi K, et al. Smoking cessation rapidly increases circulating progenitor cells in peripheral blood in chronic smokers. Arterioscler Thromb Vasc Biol 2004; 24:1442-1447.
- Michaud SE, Dussault S, Haddad P, Groleau J, Rivard A. Circulating endothelial progenitor cells from healthy smokers exhibit impaired functional activities. Atherosclerosis 2006; 187:423-432.
- Fujita Y, Kinoshita M, Furukawa Y, Nagano T, Hashimoto H, et al. Phase II Clinical Trial of CD34+ Cell Therapy to Explore Endpoint Selection and Timing in Patients With Critical Limb Ischemia. Circ J 2014; 78:490-501.
- Povsic TJ, Junge C, Nada A, Schatz RA, Harrington RA, et al. A phase 3, randomized, double-blinded, active-controlled, unblinded standard of care study assessing the efficacy and safety of intramyocardial autologous CD34+ cell administration in patients with refractory angina: design of the RENEW study. Am Heart J. 2013; 165:854-861 e2.
- Forcillo J, Stevens LM, Mansour S, Prieto I, Salem R, et al. Implantation of CD133+ stem cells in patients undergoing coronary bypass surgery: IMPACT-CABG pilot trial. Can J Cardiol 2013; 29:441-447.
- Poglajen G, Sever M, Cukjati M, Cernelc P, Knezevic I, et al. Effects of transendocardial CD34+ cell transplantation in patients with ischemic cardiomyopathy. Circ Cardiovasc Interv 2014; 7:552-559.
- Vrtovec B, Poglajen G, Lezaic L, Sever M, Socan A, et al. Comparison of transendocardial and intracoronary CD34+ cell transplantation in patients with nonischemic dilated cardiomyopathy. Circulation 2013;128:S42-49.
- Burt RK, Testori A, Oyama Y, Rodriguez HE, Yaung K, et al. Autologous peripheral blood CD133+ cell implantation for limb salvage in patients with critical limb ischemia. Bone marrow transplantat 2010; 45:111-116.
- Schachinger V, Assmus B, Britten MB, Honold J, Lehmann R, et al. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction: final one-year results of the TOPCARE-AMI Trial. J Am Coll Cardiol 2004; 44:1690-1699.
- Li ZQ, Zhang M, Jing YZ, Zhang WW, Liu Y, et al. The clinical study of autologous peripheral blood stem cell transplantation by intracoronary infusion in patients with acute myocardial infarction (AMI). Int J Cardiol 2007; 115:52-56.
- Stamm C, Kleine HD, Choi YH, Dunkelmann S, Lauffs JA, et al. Intramyocardial delivery of CD133+ bone marrow cells and coronary artery bypass grafting for chronic ischemic heart disease: safety and efficacy studies. J Thorac Cardiovasc Surg 2007; 133:717-725.
- Boyle AJ, Whitbourn R, Schlicht S, Krum H, Kocher A, et al. Intra-coronary high-dose CD34+ stem cells in patients with chronic ischemic heart disease: a 12-month follow-up. Int J Cardiol. 2006; 109:21-27.
- Bartunek J, Vanderheyden M, Vandekerckhove B, Mansour S, De Bruyne B, et al. Intracoronary injection of CD133-positive enriched bone marrow progenitor cells promotes cardiac recovery after recent myocardial infarction: feasibility and safety. Circulation 2005; 112:I178-1183.
- Stamm C, Westphal B, Kleine HD, Petzsch M, Kittner C, et al. Autologous bone-marrow stem-cell transplantation for myocardial regeneration. Lancet 2003; 361:45-46.