
Research Article
J Stem Cell Res Transplant. 2015; 2(1): 1017.
Neuropilin-2 Identifies Cardiovascular Precursor Cells and is Required for Vascular Differentiation in Murine Embryonic Stem Cells System
Ding S1,2, Ma M¹, Negro A¹, Berry C1,3, Kovacic JC1,4, Cimato TR5 and Boehm M¹*
¹Center for Molecular Medicine, National Heart Lung and Blood Institute, National Institutes of Health, USA
²Institute of Stem Cell, H&B Tech. Inc. Being, China
³Institute of Cardiovascular and Medical Sciences, University of Glasgow, Glasgow, UK
4Wiener Cardiovascular Institute, Mount Sinai School of Medicine, USA
5Clinical and Translational Research Center, SUNY at Buffalo School of Medicine and Biomedical Sciences, USA
*Corresponding author: Boehm M, Center for Molecular Medicine, National Heart Lung and BloodInstitute, National Institutes of Health, Bethesda,Maryland, USA
Received: November 26, 2014; Accepted: February 19, 2015; Published: February 24, 2015
Abstract
Neuropilins are cell surface receptors that bind Class III semaphorins and Vascular Endothelial Growth Factor (VEGF). While Neuropilin-1 is essential for vascular development, the role of Neuropilin-2 is less well defined. The objective of this study was to determine the role of Neuropilin-2 in early cardiac and vascular differentiation in embryonic stem cells. We report that expression of Neuropilin-2 coincides temporally with Brachyury expression in murine embryonic stem cells and that Neuropilin-2 expression occurs in the majority of Brachyury positive cells. In contrast to Neuropilin-1, we found very few Neuropilin-2 positive cells that co-express the hemangioblast marker Flk-1. Both Neuropilin-2 positive and Brachyury positive cells express mesoderm markers that can differentiate into cardiomyocyte, endothelial and smooth muscle cell types with equivalent efficiency. However, inhibition of Neuropilin-2 expression impairs the differentiation of cardiovascular precursors to endothelial cells and smooth muscle cells, but not cardiomyocytes. The onset of Neuropilin-2 expression identifies a sub-population of Brachyury positive cardiovascular precursors that can differentiate to endothelial, smooth muscle and cardiomyocyte cell types. Furthermore, Neuropilin-2 is functionally required for differentiation of vascular cell types but not cardiomyocytes.
Keywords: Neuropilins; Cardiovascular differentiation, Vascular cells
Introduction
Neuropilins (NRP) -1 and -2 are transmembrane receptors for class III semaphorin and Vascular Endothelial Growth Factors (VEGFs) [1,2]. NRPs are implicated in embryonic neurogenesis and vasculogenesis [3-5], with NRP-1 gene deletion leading to embryonic lethality due to cardiovascular malformations between E10.5 and E13.5 [4]. Embryos lacking NRP-1 display abnormal yolk sac vascular development and subsequent formation of the cardiac outflow tract and aortic arches is also disturbed [2,6]. In contrast, NRP-2 is a receptor for semaphorin IV and VEGF-C, and is required for embryonic vessel development [6-8]. While NRP-2 is expressed in embryonic blood islands in a pattern distinct from NRP-1, NRP-2 is preferentially expressed in the lymphatics and veins of more mature embryos [9]. NRP-2 knockout embryos exhibit defective vascular development with disordered small vessel formation [10], but does not result in early embryonic lethality, as found in VEGF and VEGFR-2 knockout embryos. Knockout of both NRP-1 and NRP-2 results in an even more pronounced phenotype, with severe impairment of yolk sac vascular development and early embryonic lethality [4,6]. These observations suggest a role for NRP-2 in embryonic vascular differentiation although the mechanisms involved underlying this process remain to be defined.
Brachyury (Bry), one of the most important mesoderm inducing factors (25) is a member of the T-box transcription factor gene family and is required for posterior mesoderm and notochord formation during vertebrate development [11]. In murine embryonic stem cells, Bry expression is an important tool for tracking mesendodermal precursors [12]. To facilitate the use of Bry as a mesendodermal marker, the GFP gene was targeted to the Bry locus (Bry-GFP) in Murine Embryonic Stem Cells (mESC), with GFP expression correlating with endogenous Bry transcription. Consequently, developing mesendodermal progenitors can be identified and separated based on GFP expression [12].
Recently, cardiovascular progenitors were shown to differentiate from Bry+/Flk-1- cells acquired from EBs of Bry-GFP mESCs [13]. We previously reported that NRP-1 expression identifies Bry+ and Flk-1+ vascular precursors in both murine and human embryonic stem cells [14]. However, the relationship between Bry and NRP- 2, if any, are unknown. Since NRP-2 and Bry are involved in early aspects of cardiovascular development, we hypothesized that NRP-2 expression may temporally correspond to Bry expression, and may have an important role in the formation of cardiovascular tissues including cardiomyocytes, endothelial cells and smooth muscle cells. In this study, using Bry-GFP mESCs, we determined that the timing of NRP-2 expression coincides with Bry expression during mesoderm differentiation, and that NRP-2 is functionally required for the differentiation of mesodermal precursors into vascular cell types but not cardiac precursors.
Materials and Methods
mESC Culture
Bry/GFP knock-in murine embryonic stem cells (Bry- GFP mESCs) [12] were initially grown on mitomycin-c-treated primary murine embryonic fibroblasts in ES Cell Qualified DMEM (EmbryoMax®, CHEMICON) supplemented with 1.5 x 10-4 M monothioglycerol (Sigma, St. Louis, MO), 15% Fetal Calf Serum (FCS) (Hyclone, Thermo Fisher Scientific, Waltham, MA), 10 mg/ ml leukemia inhibitory factor (# L5158-5UG, Sigma), 10 mg/ml recombinant human Bone Morphogenetic Protein (BMP) 4 (#314- BP, R&D Systems, Minneapolis, MN), and transferred to feeder-free, serum-free conditions in N2B27 medium (Invitrogen, Carlsbad, CA) supplemented with 2 mM L-glutamine (Sigma), 0.1 mM β-mercaptoethanol (BDH), 10 mg/ml LIF and 10 mg/ml BMP4 as previously showed [15].
Mesodermal differentiation
Bry-GFP mESCs were differentiated as EBs by trypsinization of mESCs and plating at a density of 1.5 x 103 cells/cm2 in 150 x 25 mm suspension culture dishes in N2B27 medium supplemented with BMP4 and basic fibroblast growth factor (bFGF), both at 5 mg/ml (# 233FB, R&D Systems).
Cardiovascular Differentiation: Day 3.5-EBs derived from Bry- GFP mESCs were dissociated to single cells by treatment with 0.25% trypsin (Invitrogen) and stained with antibodies for flow cytometry analysis as described below. NRP-2+/Flk-1- cells and Bry+/Flk-1- cells were sorted using a BD FACSAria and reaggregated for 20 hours at a density of 4 x 105/ml in ultra low attachment 24-well plates (Costar, Thermo Fisher Scientific) containing N2B27 media supplemented with 5 mg/m rhVEGF (# 293VE, R&D Systems) and 30 mg/ml bFGF. Reaggregated cells were then trypsinized and plated in 24-well cell culture plates (Costar) at density of 2 x 104 /cm2 , and cultured for an additional 5 - 7 days in N2B27 medium. For cardiomyocyte differentiation, medium was supplemented with 10 mg/ml rhBMP4 and 10 mg/ml rhVEGF; for endothelial cell differentiation, the medium was supplemented with 10 mg/ml rhVEGF; for smooth muscle differentiation, the medium was supplemented with 10 mg/ ml rhPDGF-BB (# 220BB, R&D Systems).
Flow cytometry analysis
Day 3.5-EBs derived from Bry-GFP mESCs were dissociated to single cells by treatment with 0.25% trypsin. Cells were incubated with the following antibodies: APC conjugated anti-mouse Flk-1 IgG (# 17-5821-81, eBioscience, San Diego, CA) or co-incubated with goat polyclonal anti-NRP-2 IgG (# AF2215, R&D Systems) and Flk- 1-APC for 40 minutes at 4°C. Cells were washed with 0.2 mM EDTA-, 1% FCS-PBS, centrifuged at 500g for 5 minutes then incubated with swine anti-goat IgG-phycoerythrin (# MBS674933, MYBIOSOURCE, San Diego, CA) for 30 minutes at 4°C. The quantity of Bry-GFP, Flk- 1 and NRP-2 expressing cells was determined by flow cytometry analysis using a BD FACSCalibur.
Immunofluorescence microscopy
Reaggregated NRP-2+/Flk-1- and Bry+/Flk-1- cells were washed with 1X PBS and fixed in 4% paraformaldehyde (Electron Microscopy Sciences) for 10 minutes at room temperature. The following primary antibodies were applied to cells overnight at 4°C: cardiac lineage differentiation, Nkx2.5 (#sc-74692, Santa Cruz biotechnology), Islet-1 (#AF1837, R&D Systems), Troponin I (#sc-8118, Santa Cruz biotechnology) and Connexin 43 (#35-5000, Invitrogen); endothelial and smooth muscle cell differentiation, VE-Cadherin (#550548, BD Pharmingen, San Diego, CA), CD31 (#14-0311-82, eBioscience) and SM-22 (#ab14106 Abcam, San Francisco, CA), α-smooth muscle actin (#A2547, Sigma). Cells were washed with 0.5% Tween 20 in PBS, and detected with donkey anti-goat, anti-rabbit, and antirat IgG conjugated Alexa fluor 488 or 555 (Invitrogen). Cells were imaged with an Olympus IX71 fluorescence microscope.
Reverse transcription-polymerase chain reaction (RTPCR)
Total RNA was extracted from sorted NRP-2+ cells and Bry+ cells using RN easy Mini Kit (#74104, QIAGEN, Valencia, CA). Firststrand cDNA was synthesized using M-MLV Reverse Transcriptase (#9PIM170, Promega, Madison, WI), followed by PCR using PuReTaq Ready-To Go PCR Beads (#27955701, GE Healthcare, Pittsburgh, PA). PCR cycle conditions were: 1 cycle of 94°C for 5 minutes; 35 cycles of 94°C for 20 seconds, 60°C for 20 seconds, 72°C for 40 seconds; and 1 cycle of 72°C for 7 minutes. GAPDH was used as an internal control. The sequences of primers used were (forward and reverse): PDGFR-β, GTCTGGTCTTTTGGGATCCT and AAGGCTGGTTACAGTTTGGC;
Cadherin 11, CCGACTTGTGAATGGGACTC and CTCCGCAGTCAGCTTCTTCT;
Flk-1, TCTGTGGTTCTGCGTGGAGA and GTATCATTTCCAACCACCCT;
PDGFR-α, AATCCTGCAGACGAGAGCAC and GCCACCAAGGGAAAAGATTT;
GAPDH, TTGCCATCAATGACCCCTTCA and CGCCCCACTTGATTTTGGA.
shRNA Knockdown of NRP-2 in Bry-GFP mESCs: Murine NRP- 2 shRNA plasmid vectors (#sc-36041-SH, Santa Cruz biotechnology) were isolated from E.Coli using Endo Free DNA Maxi Kit (#12362, QIAGEN). NRP-2 shRNA vectors were then transfected into HEK293 cells plated at a density of 2.5 x 105 cells/well on 6-well plates. Transfection was performed using DNA/Arrest-In reagent according to the manufacturer’s instructions. Lent viral particles were collected from the cell supernatant and used for transfection of Bry- GFP mESCs. Bry-GFP mESCs were cultured at density of 1.5 x 104 / cm2 in N2B27 medium for 24 hours. The medium was then replaced with 1 ml of the supernatant medium obtained from the transfected 293 cell supernatant medium, and after 48 hr another 1 ml N2B27 medium was added with culture at 37°C, 20% Oxygen, 5% CO2. Transfected cells were selected by culture for 6 additional passages in N2B27 medium μg/ml containing puromycin1.
Statistical analysis
Experimental data were analyzed by unpaired 2-tailed t-test. Results are expressed as mean ± SEM. Differences were deemed significant when P < 0.05. Statistical analyses were performed using Prism, Version 4.00 (GraphPad Software, La Jolla, CA, USA).
Results
NRP-2 identifies Bry+ mesodermal precursor cells
Bry is an important regulator of early mesodermal cell commitment during development and in the differentiation of murine ESCs [16]. Consistent with previous findings [12,15], flow cytometry analysis of GFP expression in EBs derived from Bry-GFP mESCs showed that Bry expression was up-regulated at 2.5 days, peaked at 3.5 days, decreased dramatically at 4.5 days, and was undetectable at 5.5 days (Figure 1A). To determine if NRP-2 was co-expressed in Bry+ cells, we performed flow cytometry analysis of dissociated EBs 3.5 days after differentiation in mesoderm-inducing conditions. The majority of Bry+ cells co-expressed NRP-2 (> 80%) at day 3.5 (Figure 1B). Immunofluorescence staining of NRP-2 of day 3.5 EBs derived from Bry-GFP mESCs confirmed that NRP-2 positive cells co-expressed Bry+ cells at that stage of differentiation (Figure 1C). Collectively, these results indicate that NRP-2 identifies the majority of Bry+ differentiated mESCs under mesoderm-inducing conditions.
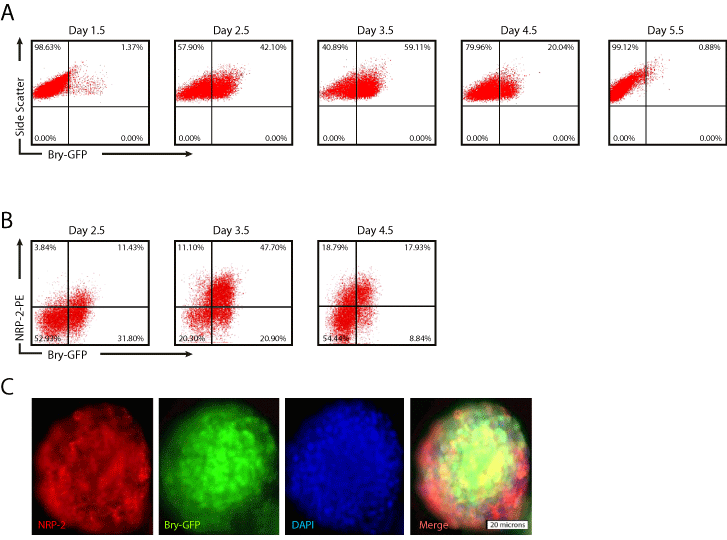
Figure 1: NRP-2 expression coincides with Bry in differentiating mESCs. EBs was dissociated at the indicated time points after differentiation and the number
of Bry-GFP and NRP-2+ cells determined by flow cytometry. Panel A) Representative flow cytometry plots of Bry-GFP time course of expression. Panel B)
Representative flow cytometry plots of NRP-2 and Bry-GFP expression over time. Panel C) Immunofluorescent photomicrographs of EBs stained with antibody
against NRP-2, and endogenous Bry-GFP expression. Results shown are representative of at least five independent experiments.
BMP4 signaling is required for NRP-2 and Bry expression during mesodermal differentiation
Our prior studies indicated that Bry+ cells are readily obtained under serum free conditions in the presence of BMP4 and bFGF [15]. However, the growth factor requirement for NRP-2 expression in differentiating mESCs is not known. To determine the growth factor requirements for NRP-2 and Bry expression we differentiated Bry- GFP mESCs as EBs under serum free conditions in the presence of the following growth factor conditions: no treatment, BMP4 and bFGF, bFGF, Activin A, or 15% FCS. The quantity of Bry-GFP+ and NRP-2+ cells was determined by flow cytometry analysis. We found that the combination of BMP4 and bFGF was as effective as 15% FCS for induction of Bry and NRP-2 expression, and that these growth factors induce NRP-2 expression in the majority of Bry+ cells (Figure 2A). To verify the requirement for BMP4 signaling, we antagonized BMP4 with Noggin. Noggin effectively blocked the differentiation of Bry-GFP mESCs, resulting in a significant decrease in the quantity of Bry+ or NRP-2+ cells (Figure 2A, B). Collectively, our findings indicate that the induction of both Bry and NRP-2 expression requires BMP4 signaling.
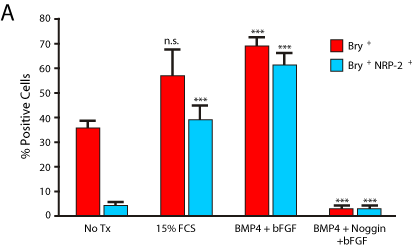
Figure 2a: NRP-2 identifies Bry-GFP+ cells in differentiating mESCs, and
requires BMP4 for induction of NRP-2 expression. Panel A) The quantity of
Bry-GFP+ and NRP-2+ cells in EBs grown in either 15% FCS, serum free
conditions with BMP4 and bFGF, or with antagonism of BMP4 with Noggin in
the presence of BMP4 and bFGF, as determined by flow cytometry analysis.
Results shown are the means +/- SEM of five independent experiments.
(Bry+: No Tx vs. 15% FCS: 35.15 ± 3.0%, P = n.s. No Tx vs. BMP4 + bFGF:
71.75 ± 2.9%, P = 0.001. BMP4 + bFGF vs. Noggin + BMP4 + bFGF: 7.25 ±
1.5%, P = 0.001. Bry+/NRP-2+: No Tx vs. 15% FCS: 5.0 ± 1.1%, P = 0.0009.
No Tx vs. BMP4 + bFGF: 66.75 ± 3.6%, P = 0.001. BMP4 + bFGF vs. Noggin
+ BMP4 + bFGF: 7.3 ± 1.7%, P = 0.001).
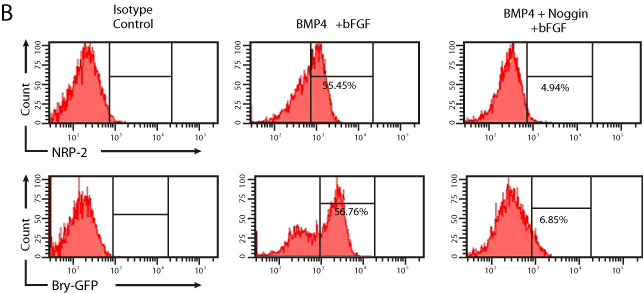
Figure 2b: Representative flow cytometry plots showing Bry-GFP and NRP-
2 expression following culture with BMP4 and bFGF, or with Noggin, BMP4
and bFGF.
Both NRP-2+ and Bry+ cells express mesoderm-specific transcripts and the majority of NRP-2+ cells are Flk-1 negative
Our flow cytometry data indicated that NRP-2 is co-expressed with Bry in differentiating mESC, suggesting these cells were mesoderm precursors. To provide additional evidence that both NRP-2+ and Bry+ cells were equivalent mesoderm precursors, we used PCR analysis to determine if both cell types expressed mRNAs found in mesodermal cells. Transcripts encoding PDGFR-β, PDGFR-α, Cadherin 11 and Flk-1 mRNA were found in both Bry+ and NRP-2+ cells, but not in undifferentiated mESCs (Figure 3A).
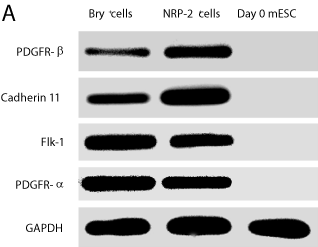
Figure 3a: Bry-GFP+ and NRP-2+ cells express overlapping mesodermal
markers. Bry-GFP+ and NRP-2+ cells were sorted from EBs and mRNA
obtained. RT-PCR was performed to detect mesodermal transcripts for
PDGFR-β, Cadherin-11, FLK-1; PDGFR- α. GAPDH was included as an
internal control. Results shown are representative of three independent
experiments.
Flk-1 expression in mesodermal progenitors identifies vascular and hematopoietic progenitor cells [12,15,17]. However, Flk-1 expression is typically observed in only 15 - 20% of cells differentiated under the conditions used in our studies [12,15,17]. To determine the proportion of NRP-2+ cells that express Flk-1, flow cytometry analysis was performed on dissociated single cells from EBs after differentiation in mesoderm-inducing conditions. We identified that the majority of NRP-2+ cells were negative for Flk-1. (Figure 3B). These data indicate that Bry+ and NRP-2+ cells carry similar mesodermal transcript markers, and that NRP-2+ cells are Flk-1 negative. Collectively, these data also suggest that these cells may be cardiovascular progenitors.
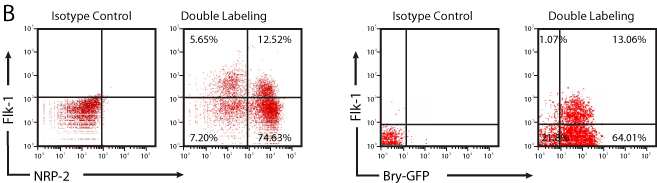
Figure 3b: Overall survival, autologous stem cell transplant (ASCT) versus no ASCT (p=0.12).
NRP-2+/Flk-1- cells are cardiac and vascular progenitors
Our findings indicated that NRP-2 expression overlaps with that of Bry in mESCs differentiated under mesoderm-inducing conditions. However, the differentiation potential of either cell type remained to be determined. Given that NRP-2+ cells were largely Flk-1- and, as prior work indicated, that cardiovascular precursors may be derived from Bry+/Flk-1- cells differentiated under similar conditions to those used here [12,15,17], we hypothesized that the Bry+/Flk-1- or NRP-2+/ Flk-1- cells may be cardiovascular precursors. To test this hypothesis, we performed flow cytometry-based cell sorting of Bry+/Flk-1- and NRP-2+/Flk-1-cells from day 3.5 EBs. We then differentiated these cells under reaggregation conditions that promote cardiovascular differentiation and determined the proportion of cells expressing markers of cardiac, endothelial and smooth muscle cell types (Figure 4A). We identified that Nkx2.5+/islet-1+/troponin-I+/connexin-43+ cardiomyocyte progenitors could be derived from both NRP-2+/Flk- 1- (Figure 4A, C) and Bry+/FLK-1- cells (data not shown) in presence of VEGF and BMP4. Moreover, VE-Cadherin+/CD31+endothelial progenitors were induced by VEGF alone, while SM22+/α-actin+ smooth muscle progenitor cells were induced by PDGF alone from both the NRP-2+/Flk-1- (Figure 4B, C) and Bry+/Flk-1- populations (data not shown). Collectively, our findings indicate that Bry+/ Flk-1- and NRP-2+/Flk-1- cells are cardiovascular precursors with differentiation potential to cardiomyocyte, endothelial and smooth muscle cell types.
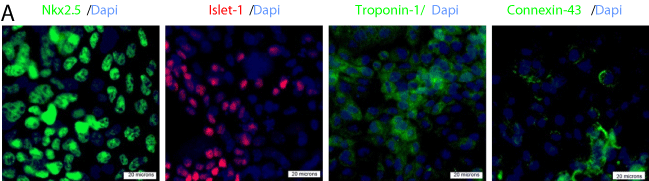
Figure 4a: NRP-2+/FLK-1- and Bry-GFP+/FLK-1- cells differentiate into
cardiomyocytes, endothelial cells and smooth muscle cells with equivalent
efficiency. Panel A) Representative immunofluorescence photomicrographs
showing cardiomyocyte differentiation of NRP-2+/FLK-1- cells following
treatment with BMP4 and VEGF. Staining was with antibodies against
Nkx2.5, Islet-1, Troponin-I, and Connexin-43.
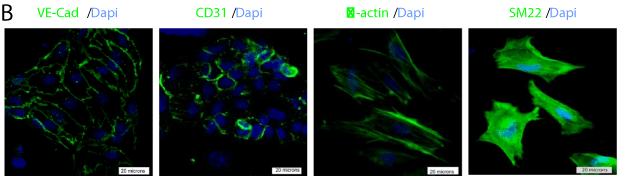
Figure 4b: NRP-2+/FLK-1- cells differentiated into endothelial cells following
treatment with VEGF and smooth muscle cells after treatment with PDGF-BB.
Staining is shown with antibodies against endothelial specific VE-Cadherin
and CD31, and smooth muscle cell specific α-actin and SM22.
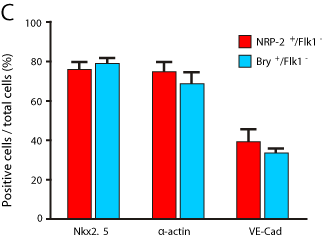
Figure 4c: Quantification of differentiation of NRP-2+/FLK-1-and Bry-GFP+/
FLK-1- cells into cardiomyocytes (Nkx2.5), smooth muscle cells (α -actin),
and endothelial cells (VE-Cadherin). Results shown are means +/- SEM, and
representative of five independent experiments (Nkx2.5; NRP-2+/FLK-1-: 77.2
± 2.3% and Bry-GFP+/FLK-1-: 78.3 ± 1.5%, P = n.s. α-actin; NRP-2+/FLK-1-:
77.6 ± 2.5% and
Bry-GFP+/FLK-1-: 74.6 ± 2.3%, P = n.s. VE-Cad; NRP-2+/FLK-1-: 38.2 ± 2.7%
and Bry-GFP+/FLK-1-: 36.9 ± 1.8%, P = n.s.).
NRP-2 is functionally required for differentiation of endothelial and smooth muscle cells, but not cardiomyocytes
To determine the function of NRP-2 in the differentiation of cardiovascular precursors to cardiomyocytes, endothelial and smooth muscle cells, we transfected Bry-GFP mESCs with NRP-2 shRNA vectors to suppress NRP-2 expression. We found that NRP-2 shRNA had no effect on the expression of Bry-GFP, but efficiently suppressed NRP-2 mRNA and protein expression (Figure 5A, B). We then isolated Bry+/FLK-1-cardiovascular precursors from NRP-2 shRNAtransfected Bry-GFP mESCs, and determined their differentiation efficiency to endothelial, smooth muscle and cardiomyocyte cell types. We found that transfection with NRP-2 shRNA resulted in a significant decrease in the number of endothelial and smooth muscle cells, but had no effect on the quantity of cardiomyocytes (Figure 5C, D). These data indicate a requirement for NRP-2 in the differentiation of vascular cell types, but not for cardiomyocyte differentiation from Bry+/Flk-1- cardiovascular precursors.
Discussion
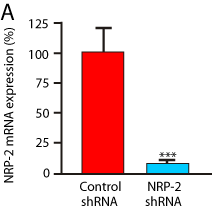
Figure 5a: NRP-2 is functionally required for differentiation of NRP-2+/FLK-
1- cells into endothelium and smooth muscle cells, but not cardiomyocytes.
Panel A) shRNA knockdown of NRP-2 mRNA in differentiating mESCs
detected by RT-PCR analysis. Results are representative of five experiments
(Reduction: 96.5 + 0.3% p<0.0001).
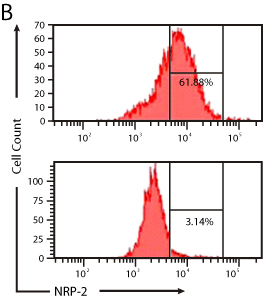
Figure 5b: Representative flow cytometry plots showing the effect of NRP-2
shRNA on NRP-2 protein expression in differentiating mESCs.
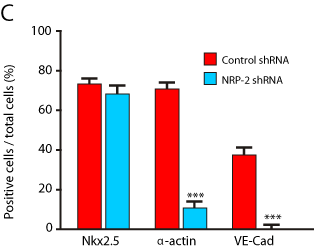
Figure 5c: Effect of NRP-2 shRNA on cardiomyocyte, smooth muscle and
endothelial cell differentiation in mESCs. Results are representative of three
independent experiments (Nkx2.5; Control shRNA: 72.9+1.6% and NRP-2
shRNA: 70.4 + 1.6%. p = n.s. α-actin; Control shRNA: 70.6 + 1.7% and NRP-
2 shRNA: 11.1 + 1.5%. p <0.0001. VE-Cad; Control shRNA: 37.9 + 1.5% and
NRP-2 shRNA: 3.7 + 1.1%. p=0.0002.
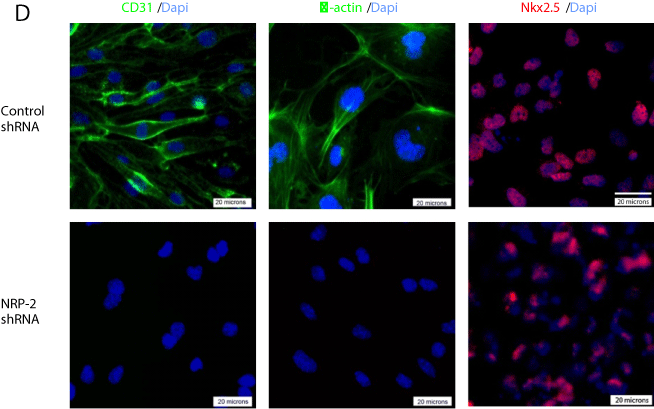
Figure 5d: Representative immunofluorescence photomicrographs of
differentiated mESCs transfected with control or NRP-2 shRNA and stained
with antibodies for αCD31, -actin, and Nkx2.5.
Previously we reported that the onset of NRP-1 expression is an early event in mesoderm formation and corresponds with Bry and Flk-1 expression in murine and human embryonic stem cells, and that NRP-1 is functionally required for the differentiation of mesodermal precursors into endothelial cells [14]. The goals of the current study were to determine if NRP-2 expression displayed a similar temporal expression pattern during mesoderm formation, determine the differentiation potential of NRP-2+/Flk-1- cells, and determine the function of NRP-2 in the murine ESC differentiation model.
Our time course studies in differentiating mESCs indicate that NRP-2 expression is dependent on BMP4 stimulation and coincides with Bry. Surprisingly, under these differentiation conditions NRP-2 expression is found in the majority of Bry+ cells, but does not appear to be functionally required for Bry expression as transfection with NRP-2 shRNA had no effect on the differentiation capacity of Bry- GFP mESCs. These findings provide novel information on the timing and distribution of NRP-2 expression in mesoderm precursors. At slightly later stages of embryonic development, NRP-2 expression is found predominantly in veins, lymphatic’s and in the heart of murine embryos [9,18-20]. Very few prior studies have assessed the onset of expression of the neuropilins near the time of mesoderm formation in early development. In the chick embryo, NRP-2 expression is found in the blood islands, as well as the primitive streak and lateral plate mesoderm, and provides an independent verification that our findings in this ESC model accurately replicate in vivo events [9].
We found that the cellular potential of the NRP-2+/Flk-1- cells was consistent with a population of cardiovascular precursors with similar differentiation capacity to Bry+/Flk-1- cells [13]. We were able to obtain differentiation to endothelial, smooth muscle and cardiomyocyte cell types from NRP-2+/Flk-1-mesodermal precursors, illustrating the overlapping differentiation potential of these two cell types (Figure 6). While NRP-2 expression marked a population of cells with differentiation capacity to cardiac and vascular cell types, NRP-2 was only functionally important for differentiation towards endothelial and smooth muscle cell fates, but not to cardiomyocytes. These data are also consistent with findings in murine knockout models where the NRPs are functionally required during the early stages of vascular development [6,21].
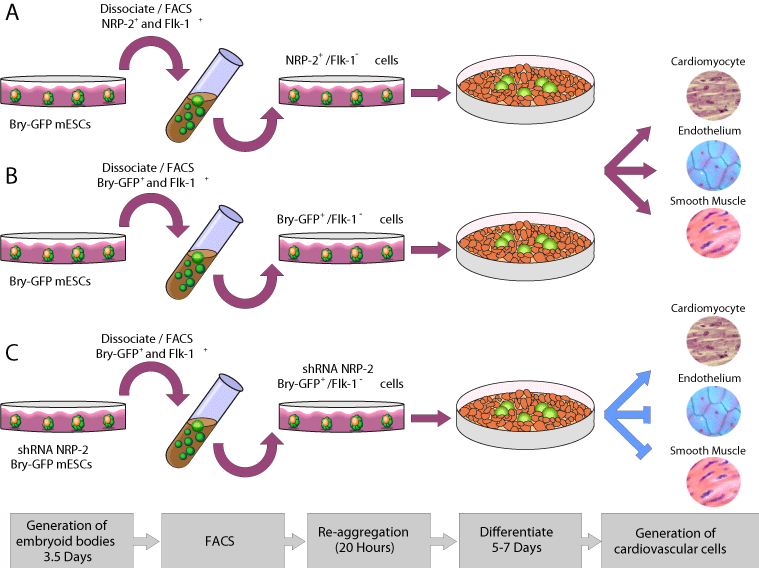
Figure 6: Model of differentiation of mESCs to cardiomyocyte, endothelial
and smooth muscle cell types via equivalent NRP-2+/FLK-1- (A) Bry-GFP+/
FLK-1-, precursors and also NRP-2 shRNA-treated Bry-GFP+/FLK-1- cells.
The fact that NRP-2 is expressed in the majority of Bry+ cells under mesoderm-inducing differentiation conditions and is not functionally required for cardiomyocyte formation implies a role for NRP-2 during primitive streak formation and potentially in the migration and patterning of mesodermal cells [17]. Potential candidate molecules to direct primitive streak migration may include the Semaphorins. Semaphorins are major regulations of morphogenesis in the nervous system and in malignant cellular transformation [22,23]. Semaphorin expression in early mammalian development is not well characterized, but in Xenopus embryos Semaphorins 3A, 3F and 4B are localized to somatic mesoderm, making it plausible that Semaphorins may play a role in the guidance and migration of NRP-2+ mesoderm progenitors during gastrulation [20,24].
The similar expression profiles of NRP-2 and Bry provides a convenient means to identify mesoderm precursors based on the cell surface expression of NRP-2. This correlation in marker expression may be of importance for two clinically relevant applications: tissue biomarkers and tissue engineering of stem cells. Thus, the correlation of Bry and NRP-2 expression may facilitate the identification of cells with cardiovascular differentiation potential from ESCs, induced pluripotent stem cells and other cell sources, including those from non-genetically modified cell lines. The further extension of these findings using these cells will facilitate the translation of cardiovascular precursors as a therapy for cardiovascular disease.
Acknowledgement
This work was funded by the Intramural Program of the National Institutes of Health. CB was funded a Kelvin Smith Fellowship from the University of Glasgow. TC is funded by an American Heart Association Scientist Development Grant (10SDG3990004). JCK is supported by National Institutes of Health Grant K08HL111330 and has received research support from AstraZeneca. We thank Gordon Keller for providing Bry-GFP mESC.
References
- Neufeld G, Cohen T, Shraga N, Lange T, Kessler O, Herzog Y, et al. The neuropilins: multifunctional semaphorin and VEGF receptors that modulate axon guidance and angiogenesis. Trends Cardiovascular Med. 2002; 12: 13-19.
- Pellet-Many C, Frankel P, Jia H, Zachary I. Neuropilins: structure, function and role in disease. Biochem J. 2008; 411: 211-226.
- Behar O, Golden JA, Mashimo H, Schoen FJ, Fishman MC. Semaphorin III is needed for normal patterning and growth of nerves, bones and heart. Nature. 1996; 383: 525-528.
- Kitsukawa T, Shimizu M, Sanbo M, Hirata T, Taniguchi M, Bekku Y, et al. Neuropilin-semaphorin III/D-mediated chemo repulsive signals play a crucial role in peripheral nerve projection in mice. Neuron. 1997; 19: 995-1005.
- Schwarz Q, Maden CH, Davidson K, Ruhrberg C. Neuropilin-mediated neural crest cell guidance is essential to organize sensory neurons into segmented dorsal root ganglia. Development. 2009; 136: 1785-1789.
- Takashima S, Kitakaze M, Asakura M, Asanuma H, Sanada S, Niwa H, et al. Targeting of both mouse neuropilin-1 and neuropilin-2 genes severely impairs developmental yolk sac and embryonic angiogenesis. Proc Natl Acad Sci USA. 2002; 99: 3657-3662.
- Goishi K, Klagsbrun M. Vascular endothelial growth factor and its receptors in embryonic zebra fish blood vessel development. Curr Top Dev Biol. 2004; 62: 127-152.
- Schwarz Q, Vieira JM, Howard B, Eickholt BJ, Ruhrberg C. Neuropilin 1 and 2 control cranial gangliogenesis and axon guidance through neural crest cells. 2008; 135: 1605-1613.
- Herzog Y, Kalcheim C, Kahane N, Reshef R, Neufeld G. Differential expression of neuropilin-1 and neuropilin-2 in arteries and veins. Mech Dev. 2001; 109: 115-119.
- Lin FJ, Chen X, Qin J, Hong YK, Tsai MJ, Tsai SY, et al. Direct transcriptional regulation of neuropilin-2 by COUP-TFII modulates multiple steps in murine lymphatic vessel development. J Clin Invest. 2010; 120: 1694-1707.
- Cunliffe V, Smith JC. Ectopic mesoderm formation in Xenopus embryos caused by widespread expression of a Brachyury homologue. 1992; 358: 427-430.
- Fehling HJ, Lacaud G, Kubo A, Kennedy M, Robertson S, Keller G, et al. Tracking mesoderm induction and its specification to the hemangioblast during embryonic stem cell differentiation. Development. 2003; 130: 4217-4227.
- Kattman SJ, Huber TL, Keller GM. Multipotent flk-1+ cardiovascular progenitor cells give rise to the cardiomyocyte, endothelial, and vascular smooth muscle lineages. Dev Cell. 2006; 11: 723-732.
- Cimato T, Beers J, Ding S, Ma M, McCoy JP, Boehm M, et al. Neuropilin-1 identifies endothelial precursors in human and murine embryonic stem cells before CD34 expression. Circulation. 2009; 119: 2170-2178.
- Ma M, Ding S, Lundqvist A, San H, Fang F, Konoplyannikov M, et al. Major histocompatibility complex-I expression on embryonic stem cell-derived vascular progenitor cells is critical for syngeneic transplant survival. Stem Cells. 2010; 28: 1465-1475.
- Martin BL, Kimelman D. Regulation of canonical Want signaling by Brachyury is essential for posterior mesoderm formation. Dev Cell. 2008; 15: 121-133.
- Mazzarella L, Jorgensen HF, Soza-Ried J, Terry AV, Pearson S, Kouskoff V, et al. Embryonic stem cell-derived hemangioblasts remain epigenetically plastic and require PRC1 to prevent neural gene expression. Blood. 2011; 117: 83-87.
- Bovenkamp DE, Goishi K, Bahary N, Davidson AJ, Zhou Y, Becker T, et al. Expression and mapping of duplicate neuropilin-1 and neuropilin-2 genes in developing zebra fish. Gene Expr Patterns. 2004; 4: 361-370.
- Chilton JK, Guthrie S. Cranial expression of class 3 secreted semaphorins and their neuropilins receptors. Dev Dyn. 2003; 228: 726-733.
- Yuan L, Moyon D, Pardanaud L, Breant C, Karkkainen MJ, Alitalo K, et al. Abnormal lymphatic vessel development in neuropilins 2 mutant mice. Development. 2002; 129: 4797-4806.
- Shen J, Samul R, Zimmer J, Liu H, Liang X, Hackett S, et al. Deficiency of Neuropilin 2 suppresses VEGF-induced retinal neovascularization. Mol Med. 2004; 10: 12-18.
- Guttmann-Raviv N, Kessler O, Shraga-Heled N, Lange T, Herzog Y, Neufeld G, et al. The neuropilins and their role in tumor genesis and tumor progression. Cancer Lett. 2006; 231: 1-11.
- Yu HH, Moens CB. Semaphorin signaling guides cranial neural crest cell migration in zebra fish. Dev Biol. 2005; 280: 373-385.
- Koestner U, Shnitsar I, Linnemannstons K, Hufton AL, Borchers A. Semaphorin and Neuropilin expression during early morphogenesis of Xenopus laevis. Dev Dyn. 2008; 237: 3853-3863.
- Vidricaire G, Jardine K, McBurney MW. Expression of the Brachyury gene during mesoderm development in differentiating embryonic carcinoma cell cultures. Development. 1994; 120: 115-122.