
Special Article – Surgery Case Reports
Austin J Surg. 2019; 6(13): 1195.
Trousseau Syndrome Secondary to a Gallbladder Adenocarcinoma
Hall MA, Berenson AM and Wang YZ*
Department of Surgery, Baylor University Medical Center, USA
*Corresponding author: Wang YZ, Department of Surgery, Louisiana State University School of Medicine, Baylor University Medical Center, USA
Received: April 30, 2019; Accepted: May 24, 2019; Published: May 31, 2019
Abstract
Trousseau syndrome is a phenomena described as the presence of subacute hypercoagability (i.e. migratory thrombophlebitis, microangiopathy, arterial emboli, chronic disseminated intravascular coagulopathy) in association with, oftentimes occult, malignant disease. Firm associations with pulmonary, pancreatic, and other visceral malignancies have been reported but not with a gallbladder adenocarcinoma. We present here the first reported case, to our knowledge, of Trousseau syndrome as the presenting symptom of an invasive papillary adenocarcinoma of the gallbladder, which was initially misdiagnosed as a diabetic foot ulcer. An en bloc resection of the gallbladder, segment IVb and V liver resection and portal lymph nodes dissection were performed. The patient remained disease free after nine years of follow-up without any postoperative adjuvant chemotherapy or radiation therapy. The presentation of Trousseau syndrome can be variable and subtle such that it may be misinterpreted as a more common disease entity. With high indices of suspicion, careful differential diagnosis and diligent work up it may ultimately reveal an occult malignancy that would not commonly be attributed to the phenomena. In turn, this finding can lead to a curative resection and a long-term cancer-free survival for a very malignant disease entity.
Keywords: Trousseau Syndrome; Gallbladder Adenocarcinoma; Hypercoagulability
Background
Trousseau syndrome was defined by Armand Trousseau in 1865. He first described, and ultimately self-diagnosed, the phenomenon in which an occult visceral malignancy is the underlying cause of an unexpected thrombotic event. Initially, the most frequent description involved migratory thrombophlebitis [1-4]. Almost a century later, the definition was expanded to include chronic disseminated intravascular coagulopathy, micro angioplasty, verrucous endocarditis, and arterial emboli. Trousseau syndrome has a well-established association with advanced primary pancreatic and pulmonary malignancies, approximately 20% and 24% respectively [5-10]. Associations with prostate, stomach, colon, and hematologic malignancies have also been reported. Seemingly, cancer-free patients with symptomatic idiopathic venous thromboembolism have an approximate 10% incidence of a subsequent cancer diagnosis. The risk factors for venous thromboembolism (VTE) in patients with a known malignancy have been evaluated in a number of large populationbased, case-control studies [11-17]. A Danish cohort study of cancer subjects that only excluded non-melanoma skin cancers compared to a general population as control was undertaken, and the incidence of VTE was noted to be higher among the cancer patients (IR 8.0 versus IR 4.7, respectively) [13]. Incidence rates were highest in patients with pancreatic cancer (IR 41) and those with advanced-stage cancer.
Gallbladder carcinoma is relatively rare, and the incidence in the United States has been reported to range from 1.2-2.5 cases per 100,000 [18,19]. It most often develops in elderly patients in their sixth or seventh decade of life, and affects females three to four times more often than males. The exact etiology of gallbladder carcinoma is not known. Cholelithiasis has been reported to occur in 75% to 92% of cases [18]. Patients with gallstones greater than 3 cm in diameter have as much as a ten times greater risk of developing cancer than those patients with stones of 1 cm or less. Other factors linked to gallbladder carcinoma include: cholecystoenteric fistulas, anomalous pancreaticobiliary junction, exposure to chemical carcinogens, inflammatory bowel disease, familial predisposition, chronic salmonella, and Mirizzi syndrome.
Patients most commonly present with abdominal pain, although nausea, vomiting, weight loss, and jaundice are other frequent symptoms. Right upper quadrant tenderness to palpation with associated hepatomegaly or a palpable distended gallbladder is rarely present on physical examination. There have been no reported cases of migratory thrombophlebitis, microangiopathy, or arterial emboli within this patient population. Laboratory analysis is usually unremarkable, unless obstructive jaundice has occurred. The paucity of clinical signs and symptoms makes for a difficult preoperative diagnosis. The disease is usually diagnosed either incidentally after cholecystectomy, or at an advanced stage, when presentation is accompanied by a mass, jaundice, ascites, or carcinomatosis. Ultrasonography may demonstrate an abnormally thickened gallbladder wall or the presence of a mass. Usually, additional imaging by contrast-enhanced Computed Tomography (CT) or Magnetic Resonance Imaging (MRI) is needed to determine respectability and to provide information about the local extent of disease, including portal vascular invasion, the presence of lymphadenopathy, and distant metastases. With the recent improvement in MRI, magnetic resonance cholangiopancreatography (MRCP) has evolved into a single noninvasive imaging modality that allows for complete assessment of loco regional involvement.
Gallbladder carcinoma in situ and carcinoma limited to the mucosa (T1) can be adequately treated with a cholecystectomy alone, if the cystic duct margin is negative for disease [18]. This approach is associated with a 5-year survival rate as high as 100%. For T2 lesions, the minimal recommendation is an extended cholecystectomy that includes resection of the gallbladder en bloc along with the portal lymph nodes. Addition of a wedge resection of segments IVb and V of the liver is controversial, but is indicated in many cases [20-23]. Locally advanced tumors (T3 and T4) have been reported to present with lymph node metastasis in approximately 75% of cases and are associated with metastatic peritoneal disease in 79% of patients. T3 and T4 disease is often associated with 5-year survival rates that range from 0% to 5%. The most significant negative prognostic factor in gallbladder carcinoma is lymph node involvement. Vascular invasion has also been reported as indicative of poor prognosis.
Case Presentation
A 61-year-old African-American female with a past medical history of insulin-dependent diabetes mellitus and hypertension presented to the University Medical Center in New Orleans in 2004 with a five-day history positive for bluish-black discoloration of the left first and second toes. The patient denied trauma, pain or paresthesia of the area, but did note associated bullae formation. The patient was admitted afebrile, but hypertensive, tachycardic, and with poor glycemic control. The patient was noted, on examination, to have a dark reddish-brown discoloration of the plantar surface of the left first and second distal phalanges. There was no evidence of tenderness to palpation, purulence, bloody discharge, or cellulites, but epidermal peeling was present. Although clear signs of distal ischemic changes were noted in the first and second toes, appropriate capillary refill was noted in the remaining phalanges on that side. The patient was noted to have 2+ femoral, popliteal, dorsalis pedis, and 1+ posterior tibial pulses on the affected side. The patient showed no signs of venous insufficiency on further examination, and had no evidence of osteomyelytic changes or soft tissue air on distal lower extremity roentgenography. The source of the ischemic changes did not seem to be arterial or venous in nature, therefore, a working diagnosis of embolic disease was entertained. The patient was placed initially on intravenous antibiotics and appropriate glycemic control was obtained. A CT angiogram of the abdomen, pelvis, and bilateral lower extremities demonstrated the common femoral, profunda, and popliteal arteries were of normal caliber and patency, bilaterally. There was evidence of scattered atheromatous changes throughout the distal vasculature bilaterally, but still had evidence of contiguous blood flow to the distal extremity. The most interesting finding on CT Angiogram was an exophytic lesion arising from a focally thickened and irregular medial wall of the gallbladder body with areas of wall disruption. The mass was noted to contain small foci of calcium and measured approximately 2.0 x 1.8cm. Localized peri-portal and aortic lymph node enlargement was noted within the region. The liver parenchyma and other intra-abdominal organs were noted to be without evidence of intra-peritoneal metastatic disease. There was no evidence of distal lower extremity embolization (Figure 1).
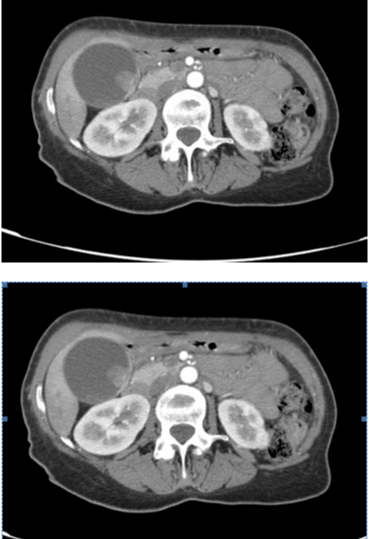
Figure 1: Computed Tomography Angiography showing an exophytic lesion
arising from a focally thickened and irregular medial wall of the gallbladder
body with areas of wall disruption. The mass measures approximately 2.0
x 1.8cm. Localized peri-portal and aortic lymph node enlargement is noted
within the region.
The patient was taken to the operative suite where she received an open cholecystectomy with segment IVb and V liver resection and associated portal triad lymphcarc node dissection. One palpable node was excised intra-operatively, and was sent for frozen section, returning as benign granulomatous disease. The gallbladder was excised and frozen section of the 2.0cm polyp revealed moderately differentiated invasive papillary adenocarcinoma of the fundus. The tumor was noted to invade through the muscularis into the perimuscular connective tissue. Liver segments IVb and V were resected and a nodal dissection was undertaken, extending from the liver edge to the proper hepatic artery. Both the liver and the 10 lymph nodes contained within the porta-hepatis were free of localized metastatic disease upon final pathological review (T2N0M0, Stage IB). Additional staining of the granulomatous nodes obtained with Grocott’s methenamine silver and periodic acid-Schiff, as well as those for acid-fast bacilli were negative. Postoperatively, she received no adjuvant chemotherapy, given the extent of her resection and clinical stage. Approximately nine years from the original date of diagnosis, the patient remained free of local or metastatic recurrence (Figure 2).
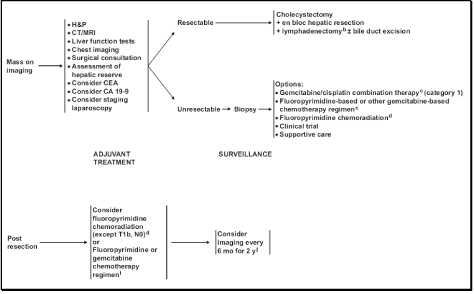
Figure 2: Guidelines on management of Gallbladder Carcinoma as adapted from the NCCN Clinical Practice Guidelines in Oncology - Hepatobiliary Cancers.
Conclusion
To date, there has never been a reported case of Trousseau Syndrome as the presenting symptoms of gallbladder adenocarcinoma. The question remains, as to whether the presence of Trousseau Syndrome correlates into a poorer prognosis in those patients with primary biliary malignancies. This cannot be answered at this time, given the sparseness of this phenomenon in this patient population. The presentation of our patient with Trousseau syndrome ultimately yielded asymptomatic malignant disease. The subsequent curative en bloc resection of the gallbladder and portal lymph nodes, with the addition of a of segment IVb and V liver wedge resection, may have greatly decreased the risk of mortality in this patient, and as to date, has kept her free of recurrence.
References
- van Doormaal FF, Atalay S, Brouwer HJ, van der Velde EF, Buller HR, van Weert HC. Idiopathic superficial thrombophlebitis and the incidence of cancer in primary care patients. Ann Fam Med. 2010; 8: 47-50.
- Kawaguchi S, Ishiguro A, Suzuki K, Hanada N, Takahata T, Itoh J, et al. A case of unrespectable advanced gastric cancer with Trousseau syndrome. Gan to Kagaku Ryoho. 2009; 36: 317-320.
- dos Santos VM, Rodrigues DB, Castro EC, Saldanha JC, Soares S, Teixeira VP, et al. Widespread hematogenous metastases and Trousseau’s syndrome in gastric adenocarcinoma. Rev Hosp Clin Fac Med Sao Paulo. 2001; 56: 91- 96.
- Varki A. Trousseau’s syndrome: multiple definitions and multiple mechanisms. Blood. 2007; 110: 1723-1729.
- Mazer LM, Losada HF, Chaudhry RM, Velazquez-Ramirez GA, Donohue JH, Kooby DA, et al. Tumor characteristics and survival analysis of incidental versus suspected gallbladder carcinoma. Department of Surgery, Emory University, 1364 Clifton Road, NE, Suite H-124-C, Atlanta, GA, 30322, USA. J Gastrointest Surg. 2012; 16:1311-1317.
- NCCN Clinical Practice Guidelines in Oncology – Hepatobiliary Cancers.
- Bakoyiannis CN, Georgopoulos SE, Tsekouras NS, Klonaris CN, Xiromeritis KN, Bastounis EA. Simultaneous venous gangrene in both lower and upper limbs. A case report. First Department of Surgery, University of Athens, Medical School, Laiko Hospital, Athens, Greece. Int Angiol. 2006; 25: 90-92.
- Veras E, Srodon M, Neijstrom ES, Ronnett BM. Metastatic HPV-related cervical adenocarcinomas presenting with thromboembolic events (Trousseau Syndrome): clinicopathologic characteristics of 2 cases. Departments of Pathology, The Johns Hopkins University School of Medicine and Hospital, Baltimore, Maryland 21231, USA. Int J Gynecol Pathol. 2009; 28: 134-139.
- Donati MB. Thrombosis and cancer: Trousseau syndrome revisited. “RE ARTU” Research Laboratories, Centre for High Technology and Education in Biomedical Sciences, Catholic University, L. Agostino Gemelli, 1, 86100 Campobasso, Italy. Best Pract Res Clin Haematol. 2009; 22: 3-8.
- Donati MB, Lorenzet R. Thrombosis and cancer: 40 years of research. Division of Immunohematology and Transfusion Medicine, Ospedali Riuniti di Bergamo, Bergamo, Italy. Thromb Res. 2012; 129: 348-352.
- Sack GH, Levin J, Bell WR. Trousseau’s syndrome and other manifestations of chronic disseminated coagulopathy in patients with neoplasms: clinical, pathophysiologic, and therapeutic features. Medicine (Baltimore). 1977; 56: 1-37.
- Sack GH Jr, Levin J, Bell WR. Trousseau’s syndrome and other manifestations of chronic disseminated coagulopathy in patients with neoplasms: clinical, pathophysiologic, and therapeutic features. Medicine (Baltimore). 1977; 56: 1-37.
- Cronin-Fenton DP, Søndergaard F, Pedersen LA, Fryzek JP, Cetin K, Acquavella J, et al. Hospitalisation for venous thromboembolism in cancer patients and the general population: a population-based cohort study in Denmark, 1997-2006. Br J Cancer. 2010; 103: 947-953.
- Piccioli A, Lensing AW, Prins MH, Falanga A, Scannapieco GL, Ieran M, et al. Extensive screening for occult malignant disease in idiopathic venous thromboembolism: a prospective randomized clinical trial; SOMIT Investigators Group. J Thromb Haemost. 2004; 2: 884-889.
- Bura A, Cailleux N, Bienvenu B, Léger P, Bissery A, Boccalon H, et al. Incidence and prognosis of cancer associated with bilateral venous thrombosis: a prospective study of 103 patients. J Thromb Haemost. 2004; 2: 441-444.
- Sørensen HT, Mellemkjaer L, Olsen JH, Baron JA. Prognosis of cancers associated with venous thromboembolism. N Engl J Med. 2000; 343: 1846- 1850.
- Blom JW, Doggen CJ, Osanto S, Rosendaal FR. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA. 2005; 293: 715.
- The Anderson M Surgical Oncology Handbook, 4th edition. By Barry W Feig, MD, David H. Berger, M.D., George M. Fuhrman, M.D. 356-358.
- Grobmyer SR, Lieberman MD, Daly JM. Gallbladder cancer in the twentieth century: single institution’s experience. World J Surg. 2004; 28: 47-49.
- Brunicardi FC, Andersen DK, Billiar TR, Dunn DL, Hunter JG, Matthews JB, et al. Schwartz’s Principles of Surgery, 9th edition.
- Shih SP, Schulick RD, Cameron JL, Lillemoe KD, Pitt HA, Choti MA, et al. Gallbladder cancer: the role of laparoscopy and radical resection. Ann Surg. 2007; 245: 893-901.
- Cavallaro A, Piccolo G, Panebianco V, Menzo E, Berretta M, Zanghì A, et al. Incidental gallbladder cancer during laparoscopic cholecystectomy: Managing an unexpected finding. World J Gastroenterol. 2012; 18: 4019-4027.
- Bartlett DL, Fong Y, Fortner JG, Brennan MF, Blumgart LH. Long-term results after resection for gallbladder cancer. Implications for staging and management. Ann Surg. 1996; 224: 639.