
Research Article
Annals Thyroid Res. 2022; 8(1): 373-378.
Predictors of Serum TSH in a Healthy Adult Sample of the Lebanese Population
Achkar AA1#, Mourad D2#, Naous E2, Sleilaty G3, Gannagé-Yared MH1,2*
¹Department of Laboratory Medicine and Endocrinology, Saint-Joseph University, Lebanon
²Department of Endocrinology, Saint-Joseph University, Lebanon
³Department of Biostatistics and Clinical Research Center, Saint-Joseph University, Lebanon
#These authors have contributed equally to this article
*Corresponding author: Marie-Hélène Gannagé-Yared, Division of Endocrinology, Hotel-Dieu de France Hospital, Beirut, Lebanon
Received: June 10, 2022; Accepted: July 11, 2022; Published: July 18, 2022
Abstract
Purpose: Measuring thyroid-stimulating hormone (TSH) is essential for diagnosing and monitoring thyroid diseases. The aim of this study is to determine the factors predicting TSH variability.
Materials and Methods: Plasma TSH, free T3 (FT3), total T3 (TT3), free T4 (FT4), anti-peroxidase antibodies (TPOAb) and anti-thyroglobulin antibodies (TgAb) were measured in 301 healthy Lebanese adults (198 women and 103 men) aged 18 to 65 years. Measurements were performed on the Cobas Roche automate. Age, sex, Body Mass Index (BMI), arterial blood pressure and the presence of dyslipidemia were collected from the population.
Results: The mean age of the population was 38.98 ± 13.28 years and the mean BMI 25.36 ± kg/m². There was no correlation between TSH and FT4, while a positive correlation was found between TSH and TT3, FT3, TPOAb and TgAb (respectively p <0.001, p=0.002, p<0.0001and p< 0.0001). TSH was not associated with age or gender but was positively correlated with BMI (p=0.053) and systolic blood pressure (p=0.03).In a multiple linear regression analysis, the independent predictors of TSH were FT4, FT3 and TPOAb. In addition, FT4, TPOAb, and dyslipidemia were independently associated with the TSH 97.5th percentile.
Conclusion: Our study showed that the main predictors of TSH are FT4, FT3 and TPOAb. This finding supports the role of TSH in enhancing T3 production as well as the main role of TPOAb in predicting the rise in TSH.
Keywords: TSH; Predictors; Lebanese; Healthy; Adults
Introduction
TSH (Thyroid stimulating hormone) is the most sensitive marker for assessing the thyroid status and is considered by the American Thyroid Association (ATA) as the most helpful test for screening thyroid dysfunction [1].
Serum TSH secretion is pulsatile [2] and exhibits a diurnal variation with a nocturnal peak leading to intraindividual TSH variations depending on the time of blood withdrawal during the day [3,4] Serum TSH is also affected by acute stress [5] and varies according to age [3,6,7], sex [3,7], BMI [8] and iodine status [9]. TSH increases with BMI [10,11], is higher in females [12] and elderly people [12]. Some studies have also reported an increased prevalence of hypertension [13] and dyslipidemia [14] with the increase in TSH levels. Finally, the presence of antithyroid antibodies, either thyroid peroxidase antibodies (TPOAb) [15,16] or thyroglobulin antibodies (TgAb) [16], is associated with higher TSH values, with a stronger relationship with the former [15].
No previous studies were conducted in the Middle East to establish factors that can influence the variability of TSH. The aim of this study was to identify predictive factors of TSH variation in a healthy adult sample of the Lebanese population.
Materials and Methods
Population
This is a cross-sectional study conducted on a sample of Lebanese subjects aged between 18 and 65 years. Recruitment was done between November 2020 and January 2021 based on volunteering among healthy hospital employees and visitors. Were excluded from the study: subjects with a personal history of thyroid disease or clinical goiter, or with any recent acute stress (hospitalization or acute infection within the last month) or chronic diseases (such as hepatic or renal disease), as well as subjects on medication that can affect thyroid tests (such as levothyroxine, oral contraceptives, estrogen replacement therapy, glucocorticoids or biotin) or pregnancy.
On the day of sampling, height in meter (m) and weight in kilogram (kg) of all participants were measured. Height was measured without shoes using a wall mounted tape. Weight was measured without shoes and with light clothes on using a calibrated scale (Soehnle Professionals).
Body mass index (BMI) was calculated as weight (in kilograms) divided by the squared value of height (in meters) and expressed in kg/m². BMI was divided into 3 subclasses: underweight for a BMI<18 kg/m², normal weight for a BMI between 18 and 25 kg/m², overweight for a BMI between 25 and 30 kg/m², and obese for a BMI superior to 30 kg/m². Systolic and diastolic arterial pressures were taken in a sitting position after 5 minutes of rest.
All participants signed an informed written consent, and the study was approved by the Ethics Committee at Hôtel-Dieu de France university hospital, Beirut, Lebanon (CEHDF1524).
Biological Analysis
For all the subjects enrolled in the study, fasting blood samples were collected at the laboratory of the authors’ institution between 8 and 10 am, then were centrifuged on the day of collection, and the resulting serum frozen at -20°C for <2 months before biochemical measurements: TSH, free T4 (FT4), free T3 (FT3), and total T3 (TT3), Thyroid antibodies (TPOAb and TgAb). Measurements were performed on the fully automated Cobas Core electrochemiluminescence (ECL) technology immunoassay system (Roche Cobas e411). The respective reference ranges defined by the manufacturer for TSH, FT4, FT3, TT3, TPOAb, and TgAb are defined as 0.27- 4.2 μIU/mL, 12-22 pmol/L, 3.1 - 6.8 pmol/L, 1.3 - 3.1 nmol/L, < 34 IU/mLand < 115 IU/mL. For all the biological parameters the coefficient of variation is less than 8%.
Statistical Analysis
The distribution of the biological values (TSH, FT4, FT3, TPOAb and TgAb) was checked using Shapiro-Wilk (SW) tests and visual inspection of the quartile-quartile (Q-Q) graphics. Native variables with a skewed distribution were expressed as a median value with an interquartile interval (quartile 1 - quartile 3) and the 95% distribution interval (percentile 2.5 - percentile 97.5). Correlations between the quantitative variables were estimated by the Spearman correlation coefficient for variables departing from normality, and by the Pearson correlation coefficient otherwise, including log-transformed variables. 95% Confidence intervals (95% CI) for Spearman’s rho were derived by bootstrapping based on 10,000 samples. The Student test, the Mann-Whitney test, the Chi-square test were used as appropriate. TPOAb and TgAb antibodies’ levels, reported initially as <5 and <10 respectively, were considered to be equal to 5 and 9 respectively. In the multivariate analyses, a primary model of multiple linear regression was done to study the correlation between Ln (TSH) and the different independent variables. Model calibration used Cox-Snell R2, Cook distances and the studentized residuals. An additional quantile regression model was used to study the variations of percentile 2.5, percentile 50 (median) and percentile 97.5 with the independent factors. All statistical analyses were performed using IBM SPSS (IBM Corp; SPSS Statistics for Windows v26.1, Armonk, NY, USA).
Results
Baseline Anthropometric and Socioeconomic Characteristics of the Sample
The sample consisted of 301 subjects, 103 men and 198 women (respectively 34.2% and 65.8%). Most of the participants were recruited from Beirut and Mount Lebanon areas (34.9% and 51.8% respectively). Demographic, anthropometric, and clinical characteristics of the sample, and according to sex are shown in (Table 1). The mean age was 39.0 ± 13.4 years, comparable in males and females (p=0.95). Men had a higher BMI (27.1 ± 4.7 vs 24.5 ± 4.6 Kg/m², p<0.001), had more frequently hypertension and dyslipidemia (p=0.07 and p=0.037 respectively). 29 subjects reported to have dyslipidemia of which 14 were treated by hypolipidemic agents and 26 reported to be hypertensive of which 21 treated by antihypertensive drugs.
Total Sample n=301
Men
n=103Women
n= 198P value
Age, years (SD)
38.98 (13.28)
39.06 (13.60)
38.94 (13.14)
0.95
BMI, Kg/m2 (SD)
Underweight + Normal
Overweight
Obesity25.36 (4.80)
52.8%
30.6%
16.6%27.09 (4.74)
36.9%
43.7%
19.4%24.46 (4.59)
61.1%
23.7%
15.2%<0.001
<0.001SBP, mmHg (SD)
11.76 (1.36)
12.21 (1.34)
11.52 (1.31)
<0.001
DBP, mmHg (SD)
7.51 (1.07)
7.86 (1.13)
7.33 (0.99)
<0.001
Hypertension (%)
8.6
12.6
6.6
0.07
Dyslipidemia (%)
9.6
14.6
7.1
0.037
SBP and DBP are systolic and diastolic blood pressure, respectively
Continuous variables are expressed as mean +/- standard deviation (SD), categorical variables are expressed as frequency.
Table 1: Demographic, anthropometric and clinical characteristics of the total sample, and according to sex.
Biological Parameters (TSH, FT4, FT3, and TT3)
The median and interquartile range (Q1–Q3) of the biological variables in the total sample and according to sex are shown in (Table 2). There was no significant difference in TSH and FT4 values between men and women (p= 0.494 and p=0.308 respectively). However, men had significant higher FT3 and TT3 values than women (p<0.001 and p=0.043 respectively).
Total Sample
Men
Women
P value
TSH, μIU/mL
1.42 (0.99; 2.11)
1.42 (1.03; 2.25)
1.42 (0.94; 2.09)
0.494
FT4, pmol/L
14.8 (13.72; 16.53)
14.82 (13.79; 16.75)
14.79 (13.7; 16.46)
0.308
TT3, nmol/L
1.71 (1.56; 1.93)
1.8 (1.6; 1.96)
1.69 (1.55; 1.91)
0.043
FT3, pmol/L
4.53 (4.16; 5.01)
4.77 (4.36; 5.22)
4.46 (4.05; 4.88)
<0.001
TPOAb, IU/mL
6.93 (4; 10.19)
6.57 (4; 10.36)
7.16 (4; 10.11)
0.64
TgAb, IU/mL
10.18 (9; 14.95)
9 (9; 12.3)
10.31 (9; 17.8)
0.043
Data are expressed as median and its interquartile range (Q1–Q3)
Table 2: Biological values in the total sample and according to sex.
Relationship between TSH and Other Variables
TSH was positively correlated with BMI (Spearman’s rho 0.112, 95% CI -0.002; 0.222, p=0.053) but not with age (Spearman’s rho -0.021, 95% CI -0.132; 0.095, p=0.723). Median TSH values were not influenced by gender (1.42 (IQR 1.03-2.25) for men vs 1.42 (IQR 0.94-2.08) for women, p=0.494). Figure 1 depicts TSH values across BMI categories, with medians (IQR) of 1.33 (0.95; 2.02), 1.47 (0.98; 2.1) and 1.74 (1.1; 2.4)μIU/mL respectively in normal/ underweight, overweight, and obese BMI subjects. There was no correlation between TSH and FT4 (Spearman’s rho -0.053, 95% CI -0.171; 0.066, p=0.357) whereas a positive correlation was observed between TSH and TT3 (Spearman’s rho 0.215, 95% CI 0.099; 0.324, p<0.001), FT3 (Spearman’s rho 0.182, 95% CI 0.068; 0.295, p=0.002), TPOAb (Spearman’s rho 0.205, 95% CI 0.087; 0.317, p<0.001), and TgAb (Spearman’s rho 0.211, 95% CI 0.094; 0.321, p<0.001). TSH was higher in subjects with positive thyroid antibodies (median 2.16 (1.38-3.2) versus 1.37 (0.94-1.91) μIU/mL respectively, p<0.001). Finally, TSH was correlated with systolic blood pressure (Spearman’s rho 0.125, 95% CI 0.013; 0.231, p=0.031) but not with diastolic blood pressure (Spearman’s rho 0.075, 95% CI -0.040; 0.184, p=0.197). TSH was not significantly different in subjects with dyslipidemia compared to those without (median 1.42 (1.00-2.10) versus 1.34 (0.99-E2.54) μIU/mL respectively, p=0.902).
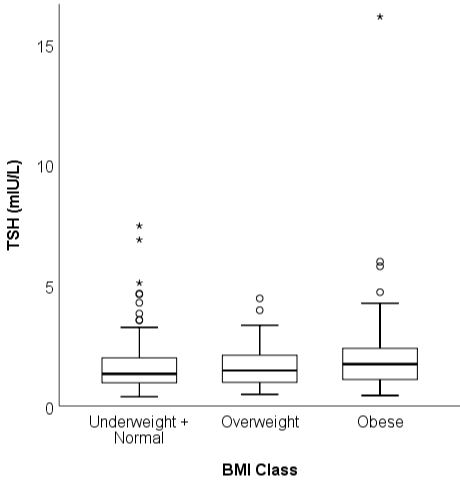
Figure 1: TSH variation according to BMI categories.
Multiple Linear Regression and Quantile Regression
A multiple linear regression analysis using Ln (TSH) as a dependent variable is shown in (Table 3). Only TPOAb, FT4, and FT3 were found to be independently associated with TSH (p=0.001, p=0.001 and p=0.04 respectively). A quantile regression analysis with TSH as a dependent variable was performed, taking 2 thresholds, the first one for the 2.5th percentile, and the second one for the 97.5th percentile (Table 4). For the 2.5th percentile cut-off, none of the predictors influenced the variation of TSH 2.5th percentile. For the 97.5th percentile cut-off, FT4, TPOAb, and the presence of dyslipidemia were independent predictors associated with TSH 97.5th percentile (p<0.0001 for the 3 variables), while age, BMI and TgAb values tended but did not reach statistical significance (p=0.06, p=0.07, p=0.08 respectively).
Unstandardized Coefficients
p-value
95.0% Confidence Interval
B
Std. Error
Lower Bound
Upper Bound
constant
1.053
0.677
0.121
-0.280
2.385
Ln(FT4)
-0.778
0.243
0.001
-1.256
-0.301
TT3
0.163
0.115
0.158
-0.064
0.391
FT3
0.127
0.061
0.040
0.006
0.247
Ln(ATG)
0.052
0.034
0.134
-0.016
0.119
Ln(ATPO)
0.130
0.038
0.001
0.055
0.205
Age
-0.001
0.003
0.741
-0.006
0.004
Sex
-0.027
0.068
0.690
-0.160
0.106
Hypertension
-0.086
0.112
0.444
-0.307
0.135
Dyslipidemia
-0.005
0.104
0.962
-0.209
0.199
BMI
0.010
0.007
0.146
-0.003
0.023
Variables whose distribution deviates significantly from statistical normality (Figures QQ, Shapiro-Wilk tests) were log-transformed to normalize their distribution, and incorporated into the regression in a logarithmic form
Table 3: Multiple linear regression with TSH as a dependent variable.
Parameter
Coefficient
Std. Error
p-value
95% Confidence Interval
Lower bound
Upper bound
(Intercept)
12.398
2.0333
0.000
8.396
16.400
FT4
-0.415
0.0878
0.000
-0.587
-0.242
TT3
-0.951
0.6335
0.134
-2.198
0.296
FT3
0.394
0.3384
0.245
-0.272
1.060
ATPO
0.079
0.0032
0.000
0.073
0.085
ATG
-0.003
0.0017
0.076
-0.006
0.000
Age
-0.026
0.0138
0.059
-0.054
0.001
BMI
0.067
0.0364
0.066
-0.005
0.139
Sex
0.391
0.3692
0.290
-0.335
1.118
HTA
-0.984
0.6129
0.109
-2.191
0.222
Dyslipidemia
-2.582
0.5693
0.000
-3.703
-1.462
Sex: 0 for males, 1 for females
Hypertension: 0 for no hypertension, 1 for the presence of hypertension
Dyslipidemia: 0 for absence of dyslipidemia, 1 for the presence of dyslipidemia
Table 4: 97.5th quantile regression analysis with TSH as a dependent variable.
Discussion
The current study found an independent relationship between TSH and both FT3 and TPOAb while a negative independent correlation was noted between TSH and FT4. The negative association between TSH and FT4 is expected and explained by the negative feedback exerted by FT4 on the pituitary gland. In fact, TSH increases when FT4 decreases and vice versa in order to maintain a state of euthyroidism [17]. FT4 is then converted by type 1 and 2 deiodinases into FT3, which is the most active hormone at the receptor level [17]. The positive correlation we observed between TSH and FT3 was only described in a previous study performed on adults and was shown to be age dependent since when TSH levels increase, the FT3/FT4 ratio increases until age 40, but not in the older age groups [18]. Another pediatric study [19] demonstrated that both obese and non-obese children had elevated TSH and FT3, without an increase in FT4. This positive association between TSH and FT3 could be explained by the preferential effects of TSH on deiodinase stimulation, secretion and FT3 metabolism [19], a finding that is attenuated with the aging process. In addition, and similarly to previous studies [15,16,20], a positive relationship was found in the current study between TSH and the prevalence of TPOAb and TgAb, even if this independent association was only noted for TPOAb. Tipu et al, [20] found in an adult Pakistani population that TSH is higher in subjects who had positive antibodies compared to those who had negative ones. Also, a Saudi study [21] showed higher TSH levels when both antibodies are positive whereas in two other studies [15,22], this association was only found for TPOAb. Finally, Roos et al, [15] and Li [16] et al, showed that subjects with positive thyroid antibodies at baseline developed with time thyroid dysfunction more commonly than seronegative subjects.
We found a positive correlation between TSH and BMI, as well as a significant increase in TSH values among the 3 BMI classes (normal, overweight, and obese). BMI was also an independent predictor of the 97.5th percentile of TSH even if this association was at the limit of significance. A positive correlation between TSH and BMI was described mainly in obese subjects [23]. In addition, the loss of more than 10% of weight in 98 women over a duration of 6 months was followed by a significant decrease in TSH [24]. Finally, data analysis of 14 cohorts (from Europe, US, Australia, and South America) with 55,412 individuals with TSH levels within the normal range demonstrated that BMI did not differ between the lower (0.45–1.49 mIU/L) and the higher TSH quartile (3.50–4.49 mIU/L) [25] suggesting that at least in non-obese subjects TSH did not differ within the reference intervals. The link between TSH and BMI is explained by the effect of leptin on TSH [23]. Leptin is shown to regulate the expression and secretion of Thyroid releasing hormone (TRH) via the stimulation of paraventricular nuclei and arcuate nucleus [10]. In addition, leptin increases the conversion of pro-TRH to TRH through an action on the activity of pro-hormones convertases (PC) 1/3 and 2 [10]. The subsequent increase in TSH secretion leads to an increase in FT4 level, which in turn induces thermogenesis and basal metabolism limiting further weight gain [10]. TSH is also shown to have a role in adipocyte differentiation and adipose tissue expansion, in vitro and in vivo, by binding to its receptors on adipocytes [23].
The current study did not show a significant relationship between age and TSH. However, age was an independent predictor of the 97.5th percentile of TSH even if this relationship was only at the limit of significance. A physiological increase in TSH levels with age has been reported in several studies worldwide [3,12,26]. This increase was demonstrated in the NHANES III study [12] and has been shown to occur regardless of the presence of thyroid diseases, or to drug intakes that might affect TSH and/or FT4 levels [27]. The absence of a positive correlation between age and TSH in our study can be explained by the age limit of 65 of our participants or to the small size of our population.
Also, a difference in TSH values according to sex was not demonstrated in our study even though the prevalence of TPOAb and TgAb was higher in women than in men (16.2% in women vs 6.8% in men). The relationship between TSH and sex is controversial in the literature. Hollowell et al, [12] found that TSH concentrations greater than 4.5 mUI/L were more commonly observed in women than in men and this was attributed to a higher prevalence of thyroid antibodies in women than in men [20,28], whereas at the opposite the Scottish TEARS study [6] reported that males had significantly higher median TSH compared to females. A third study did not find an association between TSH and sex [29]. Finally, the WHICKHAM survey showed that TSH levels did not vary with age in males but increased markedly in females after the age of 45 years, a rise that was abolished when persons with thyroid antibodies were excluded from the sample [30]. This discrepancy between our data and the literature could be explained by the small size of the current sample, the younger age of the participants since the prevalence of autoimmune disease increases after the 4th decade [31] or to ethnic differences.
There was a positive correlation between TSH and systolic blood pressure in univariate analysis that faded out after adjustment for other covariates. An association between clinical thyroid dysfunction and hypertension has been previously reported in both hyperthyroidism [32,33] and hypothyroidism [32] but also in subclinical hypothyroidism [34]. In adults [13] as well as in children and adolescents [35], the prevalence of hypertension increases with the linear increase in TSH values within the normal reference range. Hyperthyroidism has been shown to increase pulse pressure [33], while hypothyroidism primarily increases diastolic blood pressure [36]. This relationship could be explained by a decrease in vascular compliance, or by an increase in renal vascular resistance secondary to hormonal changes. The reason for our results could be ethnic differences between populations.
A relationship between mean log (TSH) and dyslipidemia was not observed in the study. However, dyslipidemia was independently associated with TSH 97.5th percentile. A positive correlation between TSH and lipid parameters was described in several studies [14,37,38] This finding is explained by the fact that FT4 increases lipolysis, and facilitates cholesterol hepatic clearance [17]. In addition, TSH within the reference normal range is associated with central obesity, hyperglycemia, insulin resistance and high blood pressure [14], and therefore is considered as a new marker in the evaluation of the cardiovascular risk.
The strength of the study lies in the fact that all the biological parameters of the thyroid workup were measured in the same laboratory and using the same measurement method. In addition, samples were collected in the morning, avoiding nychthemeral cycle fluctuations. However, the main limitations of the study are the sample size that is relatively small, and the cross-sectional nature of the study.
Conclusion
In a healthy Lebanese population, an independent positive association between TSH, TT3, and TPOAb was noted, while a negative relationship was observed with FT4. No significant independent relationship was found between TSH and age, gender, BMI, SBP, DBP, and dyslipidemia. TPOAb and the presence of dyslipidemia are also associated with the TSH 97.5th percentile, confirming the fact that TSH may be a helpful test in the diagnosis and follow-up of auto-immune thyroid diseases. Additional studies should be performed to clarify more precisely the role of each of these factors in TSH variations.
Ethics and Consent to Participate
All procedures followed were in accordance with the ethical standards of the ethics committee of Hôtel-Dieude France on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. The study had the approval of the ethics committee of Hôtel- Dieu de France hospital (CEHDF1524). All participants signed a written informed consent to participate in the study.
Availability of Data and Material
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing Interests
The authors have nothing to disclose.
Funding
This work was supported by a grant of the “Conseil de recherche de l’Université Saint-Joseph” FM406, Beirut.
Author Contributions Statement
DM and AA have performed most of the volunteers’recruitment and have contributed to the redaction of the manuscript, EN has contributed to the redaction of the manuscript, GS has performed the statistical analysis and participated in the redaction of the manuscript, and MHGY has conceived and designed the study, and has wrote the manuscript. All the authors have read and approved the final version of the paper.
References
- Garber JR, Cobin RH, Gharib H, Hennessey JV, Klein I, Mechanick JI, et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Thyroid: official journal of the American Thyroid Association. 2012; 23: 251-251.
- Roelfsema F, Veldhuis JD. Thyrotropin secretion patterns in health and disease. Endocrine reviews. 2013; 34: 619-657.
- Wang D, Li D, Guo X, Yu S, Qiu L, Cheng X, et al. Effects of sex, age, sampling time, and season on thyroid-stimulating hormone concentrations: A retrospective study. Biochemical and biophysical research communications. 2018; 506: 450-454.
- Spoel EVD, Roelfsema F, Heemst DV. Within-Person Variation in Serum Thyrotropin Concentrations: Main Sources, Potential Underlying Biological Mechanisms, and Clinical Implications. Frontiers in Endocrinology. 2021; 12.
- Wong ET, Bradley SG, Schultz AL. Elevations of thyroid-stimulating hormone during acute nonthyroidal illness. Archives of internal medicine. 1981; 141: 873.
- Vadiveloo T, Donnan PT, Murphy MJ, Leese GP. Age- and gender-specific TSH reference intervals in people with no obvious thyroid disease in Tayside, Scotland: the Thyroid Epidemiology, Audit, and Research Study (TEARS). The Journal of clinical endocrinology and metabolism. 2013; 98: 1147-1153.
- Razvi S, Bhana S, and Mrabeti S. Challenges in Interpreting Thyroid Stimulating Hormone Results in the Diagnosis of Thyroid Dysfunction. J. Thyroid Res. 2019; 2019: 4106816.
- Nyrnes A, Jorde R, and Sundsfjord J. Serum TSH is positively associated with BMI. Int J Obes. 2006; 30: 100-105.
- Gérard AC , Poncin S, Caetano B, Sonveaux P, Audinot JN, Feron O, et al. Iodine deficiency induces a thyroid stimulating hormone-independent early phase of microvascular reshaping in the thyroid. Am J Pathol. 2008; 172: 748–760.
- C Bétry C, Challan-Belval MA , A Bernard A , Charrié A , Drai J, Laville M, et al. Increased TSH in obesity: Evidence for a BMI-independent association with leptin. Diabetes Metab. 2015; 41: 248–251.
- Biondi B. Thyroid and obesity: an intriguing relationship. The Journal of clinical endocrinology and metabolism. 2010; 95: 3614-3617.
- Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). The Journal of clinical endocrinology and metabolism. 2002; 87: 489-499.
- Asvold BO, Bjøro T, Nilsen TIL, Vatten LJ. Association between blood pressure and serum thyroid-stimulating hormone concentration within the reference range: a population-based study. The Journal of clinical endocrinology and metabolism. 2007; 92: 841-845.
- Chang Y, Hua S, Chang C, Kao W, Lee H, Chuang L, et al. High TSH Level within Normal Range Is Associated with Obesity, Dyslipidemia, Hypertension, Inflammation, Hypercoagulability, and the Metabolic Syndrome: A Novel Cardiometabolic Marker. Journal of Clinical Medicine. 2019; 8: 817.
- Roos A, Links TP, Berg LTWDJD, Gans ROB, Wolffenbuttel BHR, Bakker SJL. Thyroid peroxidase antibodies, levels of thyroid stimulating hormone and development of hypothyroidism in euthyroid subjects. European journal of internal medicine. 2010; 21: 555-559.
- Li Y, Teng D, Shan Z, Teng X, Guan H, Yu X, et al. Antithyroperoxidase and Antithyroglobulin antibodies in a five-year follow-up survey of populations with different iodine intakes. J Clin Endocrinol Metab. 2008; 93: 1751–1757.
- Mullur R, Liu Y, Brent GA. Thyroid hormone regulation of metabolism. Physiological reviews. 2014; 94: 355-382.
- trich D, Karavani G, Edri S, and Gillis D. TSH enhancement of FT4 to FT3 conversion is age dependent. Eur J Endocrinol. 2016; 175: 49–54.
- Karavani G, Strich D, Edri S, Gillis D. Increases in thyrotropin within the nearnormal range are associated with increased triiodothyronine but not increased thyroxine in the pediatric age group. The Journal of clinical endocrinology and metabolism. 2014; 99: E1471-E1475.
- Tipu HN, Ahmed D, Bashir MM, and Asif N. Significance of testing anti-thyroid autoantibodies in patients with deranged thyroid profile. J Thyroid Res. 2018; 2018: 1–5.
- Al-Rabia MW. Correlation of thyroid antibodies with TSH, T3 and T4 hormones in patients diagnosed with autoimmune thyroid disorders. Pak J Pharm Sci. 2017; 6.
- Brown SJ, Bremner AP, Hadlow NC, Feddema P, Leedman PJ, O’Leary PC, et al. The log TSH–free T4 relationship in a community-based cohort is nonlinear and is influenced by age, smoking and thyroid peroxidase antibody status. Clinical Endocrinology. 2016; 85: 789-796.
- Bastemir M, Akin F, Alkis E, Kaptanoglu B. Obesity is associated with increased serum TSH level, independent of thyroid function. Swiss medical weekly. 2007; 137: 431-4.
- Sari R, Balci MK, Altunbas H, Karayalcin U. The effect of body weight and weight loss on thyroid volume and function in obese women. Clinical Endocrinology. 2003; 59: 258-262.
- Åsvold BO, Vatten LJ, Bjøro T, Bauer DC, Bremner A, Cappola AR, et al. Thyroid function within the normal range and risk of coronary heart disease: an individual participant data analysis of 14 cohorts. JAMA internal medicine. 2015; 175: 1037.
- Surks MI, Hollowell JG. Age-specific distribution of serum thyrotropin and antithyroid antibodies in the US population: implications for the prevalence of subclinical hypothyroidism. The Journal of clinical endocrinology and metabolism. 2007; 92: 4575-4582.
- Dong BJ. How medications affect thyroid function. The Western journal of medicine. 2000; 172: 102-106.
- Karimi F, Kalantarhormozi MR, Dabbaghmanesh MH, Omrani GR. Thyroid disorders and the prevalence of antithyroid antibodies in Shiraz population. Archives of Iranian medicine. 2014; 17: 347-51.
- Kahapola-Arachchige KM, Hadlow N, Wardrop R, Lim EM, Walsh JP. Agespecific TSH reference ranges have minimal impact on the diagnosis of thyroid dysfunction. Clinical Endocrinology. 2012; 77: 773-779.
- TUNBRIDGE WMG, EVERED DC, HALL R, APPLETON D, BREWIS M, CLARK F, et al. THE SPECTRUM OF THYROID DISEASE IN A COMMUNITY: THE WHICKHAM SURVEY. Clinical Endocrinology. 1977; 7: 481-493.
- Vanderpump MPJ. The epidemiology of thyroid disease. Br Med Bull. 2011; 99: 39–51.
- Danzi S, Klein I. Thyroid hormone and blood pressure regulation. Current Hypertension Reports. 2003; 5: 513-520.
- Prisant LM, Gujral JS, Mulloy AL. Hyperthyroidism: A Secondary Cause of Isolated Systolic Hypertension. The Journal of Clinical Hypertension. 2006; 8: 596-599.
- Faber J, Petersen L, Wiinberg N, Schifter S, and Mehlsen J. Hemodynamic changes after Levothyroxine treatment in subclinical hypothyroidism. Thyroid. 2002; 12: 319–324.
- Ittermann T, Thamm M, Wallaschofski H, Rettig R, Völzke H. Serum thyroidstimulating hormone levels are associated with blood pressure in children and adolescents. The Journal of clinical endocrinology and metabolism. 2012; 97: 828-834
- Klein I, Ojamaa K. Thyroid hormone and the cardiovascular system. Current Opinion in Endocrinology and Diabetes. 2012; 96: 257-268.
- Roos A, Bakker SJL, Links TP, Gans ROB, Wolffenbuttel BHR. Thyroid function is associated with components of the metabolic syndrome in euthyroid subjects. The Journal of clinical endocrinology and metabolism. 2007; 92: 491-496.
- Ruhla S, Weickert MO, Arafat AM, Osterhoff M, Isken F, Spranger J, et al. A high normal TSH is associated with the metabolic syndrome. Clinical Endocrinology. 2010; 72: 696-701.