
Research Article
Austin Pharmacol Pharm. 2023; 6(1): 1014.
Prevalence of Bovine Tuberculosis Using Comparative Intradermal Tuberculin Test and Associated Risk Factors in Dairy Farms in Central and Southern Ethiopia
Abdi Ahmed Umer*; Getachew Kinfe; Chala Dima; Abebe Olani; Ebisa Mezgebu
Microbiology Research Laboratory, Animal Health Institute (AHI), Ethiopia
*Corresponding author: Abdi Ahmed Umer Microbiology Research Laboratory, Animal Health Institute (AHI), PO Box 04, Sebeta, Ethiopia. Email: abdivet2014@gmail.com
Received: October 09, 2023 Accepted: November 14, 2023 Published: November 21, 2023
Abstract
Bovine Tuberculosis (BTB) Infect cattle and also other domestic animals, and some wildlife is highly contagious, chronic, and debilitating disease caused by the bacterium Mycobacterium bovis, and is considered a zoonotic disease. BTB is prevalent in many regions worldwide, including most of Africa, parts of Asia, and the Americas. In Africa, as well as in Ethiopia, BTB is an endemic disease, and there are currently no effective control measures available. This disease leads to significant economic losses, especially in the dairy industry, due to its impact on animal production. To prevent or eliminate bovine tuberculosis in livestock industry, many advanced countries use the test-and-slaughter method. However, in developing nations like Ethiopia, where resources and expertise in testing and control measures are limited, they often rely on comparative intradermal tuberculin skin tests.
A cross-sectional study was carried out in selected areas of central and southern Ethiopia from December 2022 to September 2023 aimed to determine the prevalence and associated risk factors of Bovine Tuberculosis (BTB) in dairy cattle. Comparative intradermal tuberculin skin tests were conducted on 202 dairy cattle from four dairy farms, namely Hawasa, Wondo Genet, Sululta, and Mojo. Factors such as age, breed, sex, and body condition score were taken into account to identify the potential risk factors and 20.29% of BTB occurrence was obtained. In this study statistically significant factors (P<0.05) age showing (χ2=12.118; P=0.0165), body condition score (χ2=61.289, P=0.0024), and animal farms (χ2=88.918, P=0.0022). Sex and breed analysis were not statistically significant (P>0.05). These results indicate that bovine tuberculosis is highly prevalent, and control measures are necessary to reduce its spread.
Keywords: Bovine tuberculosis; CIDT test; Mycobacterium bovis; Tuberculin test; Risk factors; Ethiopia
Introduction
Bovine Tuberculosis (BTB) caused by Mycobacterium bovis, a gram positive, acid-fast bacterium in the Mycobacterium tuberculosis complex of the family Mycobacteriaceae. It is a zoonotic disease, and remains a cause of concern for livestock, wildlife and human health. This disease can affect practically all mammals, causing a general state of illness, coughing and eventual death. The name TB comes from the nodules, called ‘tubercles’, which form in the lymph nodes of affected animals [19]. Until the 1920s when control measures began in developed nations, it was one of the major diseases of domestic animals throughout the world. In Africa, the disease has a wide distribution with a high prevalence in both wild and domestic animals. Cattle serve as the main host for Mycobacterium bovis, which is genetically related and clinically indistinguishable from TB caused by Mycobacterium tuberculosis, while other domestic animals such as pigs, cats, dogs, horses and sheep are considered to be spill-over hosts [6]. The role of M. bovis in human TB is well established in developed world, though it is not well known in developing countries [20]. In Africa to which 82% of human and 85% of animal population live in area where BTB is either partly or not controlled at all, it was a signicant zoonotic human pathogen that aggravate the ‘triple trouble’ of HIV/AIDS, TB infection and malnutrition [7].
The transmission of BTB between animals is mainly aerogenic, close contact between animals or sharing of feed and water between infected and non-infected animals are main risk factors for transmission. BTB outbreaks can trigger large economic costs to society since it can affect international trade of animals and animal products, create productivity losses on milk yields, meat production, and fertility, animal market restriction, trigger large control and eradication programs, and increase human health costs [8]. Diagnosis of M.bovis infection in cattle is often based on history, clinical, necropsy findings and tuberculin test. For confirmatory diagnosis, culture and biochemical property, MALDI TOF identification. To further detection of M. bovis from other members of M. tuberculosis complex, Polymerase Chain Reaction (PCR) and DNA genome sequence. Several new laboratory procedures like lymphocyte proliferation and γ-interferon assays have been introduced as aid in the diagnosis of mycobacterium infections [17].
The Tuberculin Skin Tests (TST), which are based on delayed hypersensitivity reactions, are now the best methods for worldwide field diagnosis of bovine TB in live animals and World Organization for Animal Health (WOAH) recommended difference between the increases in skin thickness for the test after 72 hours [4]. In contrast to the Single Intradermal Tuberculin (SIT) test, which employs only BT, the Single Intradermal Comparative Cervical Tuberculin (SICCT) test involves the intradermal injection of Bovine Tuberculin (BT) and Avian Tuberculin (AT) at different places in the skin of the neck [2]. M. bovis infection in cattle can be successfully detected by TST, which also enables quick evacuation of sick animals, minimal transmission, and quick eradication of bovine TB, reduce the risk of Zoonosis [22].
The tuberculin skin test may demand physical exertion in the field but, it is also simple and relatively inexpensive and offers reliable means of screening cattle populations in an entire region [2]. The effectiveness of the TST may be impacted by ambient conditions, host characteristics state of immunity, genetics, and the type of tuberculin being utilized [5]. Ethiopia is one of the African countries where BTB is considered as a major disease burden in animals. Major causes of BTB in developing countries such as demographic factors, eating habits, living and socio-economic status of families, illiteracy, culture and customs, the existence of HIV/AIDS, and close proximity with animals. There are no national data on the prevalence of BTB in Ethiopia, it is believed that the disease is becoming more common due to the growth of the dairy industry. Using tuberculin skin testing, a few studies have been conducted in Ethiopia (Id et al., 2021). Consequently, nationwide study will be helpful for establishing disease control and prevention at the national level. The goal of the current study is to determine the prevalence and risk factors of BTB in a dairy farm in central and southern Ethiopia.
Materials and Methods
Study Area
The study was conducted in Sidama and Oromia Regional state of four selected towns. In Sidama region (Hawasa and Wondo genet) and from oromia Sululta and Mojo, respectively. Hawassa city is located at 273 km south of Addis Ababa via Bishoftu, 130 km east of Sodo, and 75 km north of Dilla. The town serves as the capital of the Sidama Region. It lies a latitude and longitude of 7°3′N 38°28′E and an elevation of 1,708 meters above sea level. Wondo Genet town is a located southeast of Shashemene in the Sidama Region with latitude and longitude 7.08N, 38.61E an and elevation of 1723 meters (Mofed et al., 2020). Sululta is a town in found shagger city located at 35 Km south of Addis Ababa. It is located at an elevation of 2,567 meters and 9°10'60" N and 38°45'0" E latitude and longitude respectively. Mojo is a town located in the East Shewa Zone of the Oromia Region, it has a latitude and longitude of 8°39′N 39°5′E with an elevation between 1788 and 1825 meters above sea level. It is the administrative center of Lome district [16].
Study Animals
The study was conducted on Holstein, Jersey and cross breeds of Borena kept in the intensive dairy farms.
Study Design, Sample size and Sampling Method
The cross-sectional study was design by using Random sampling method in order to test animals in the farms. The comparative intra dermal tuberculin test determines the prevalence of BTB in the study area. In this study 202 cattle (10 from Hawasa, 100 Wondo Genet, 32 Sululta and 60 Mojo) with different breed, sex, age and origin to be included.
The Comparative Intra Dermal Tuberculin Test Methods
Two sites were used, one for avian PPD (upper site) and the other for bovine PPD (lower site). The upper site was 10 cm below the crest and the lower site was 12.5 cm from the upper site, on a line drawn parallel with the line of the shoulder. The selected site was shaved and skin thickness was measured using a caliper before injection of the Purified Protein Derivative (PPD) and recorded as A1 for avian PPD site and B1 for bovine. Then 0.1 ml of Bovine Tuberculin PPD and 0.1ml of Avian Tuberculin PPD was injected intradermally in the lower and upper site, respectively. A correct injection was checked by palpating papula (a small pea-like swelling) at each injection site. The two injection sites were remeasured after 72 hours by the same person who measured the skin thickness before the injection and recorded as A2 for avian PPD and B2 for bovine PPD [15].
Interpretation of the reaction was considered to be positive if the difference in skin fold thickness at the bovine site of injection was 4 mm or more higher than the reaction shown at the site of the avian injection. When the difference in the skin fold thickness at the bovine site of inoculation was greater than 2 mm but lower than 4 mm, it was considered as doubtful, but if lower than or equal to 2 mm it was taken as negative [4].
Data Analysis
During the study, individual animal identification number, breeds, sex, age, data were entered MS Excel sheets. Then, coded and analyzed using SPSS version 20 statistical software. The associated risk factors with M. bovis infection were calculated by using Chi-square (χ2) [18].
Results
Demographic Features
The Comparative Intradermal Tuberculin test (CIDT) in the current study showed that from a total of 202 cattle tested, 20.29 %( 41) were found to be positive, 7.4 % (15) doubtful and the rest 72.3 % (146) animals were negative for BTB test (Table 1).
Characteristics
Frequency
Percent (%)
Age
Adult
147
72.77
Young
55
27.22
Body conditions
Good
147
72.77
Medium
23
11.38
Poor
32
15.84
Breed
Holstein
190
94.05
Jersey
7
3.46
Borena
5
2.47
Result
Positive
41
20.29
Doubtful
15
7.4
Negative
146
72.3
Table 1: Overall status of cattle level parameters in study animals.
Comparative Intradermal Tuberculin (CIT) test implicates the intradermal injection site of bovine tuberculin (BT) and Avian Tuberculin (AT) on the neck of tested animals (Figure 1).
Figure 1: Map of study area.
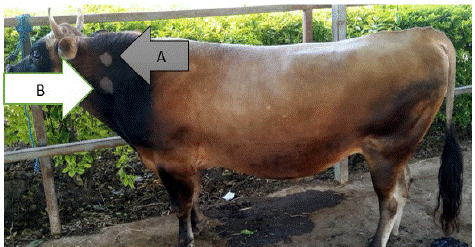
Figure 2: Bovine tuberculosis injection site (A) Avian Tuberculin (AT) and (B) Bovine Tuberculin (BT) and its reactions.
Risk Factors
From the total of 202 cattle 19.8 % (40) Holstein, 0.49% (1) Borena and 0 % (0) Jersey breed are tested for the presence of bovine tuberculosis by the comparative intradermal tuberculin test (Table 2). There were statistically significant differences (P<0.05) in proportions of positive reactor animals among body condition score (χ2=61.289, P=0.0024), Age (χ2=12.118, P=0.0165) and animal farms (χ2=88.918, P=0.0022). Conversely, Sex and breed analysis were not statically significant (P>0.05) with the association of bovine tuberculosis reactions.
Variable
Category
No of
Positive
animals% of Positive
X-squared
P-value
Age
Young
9
4.45
12.118
0.0165
Adult
32
15.84
BCS
Good
34
16.83
61.289
0.0024
Medium
7
3.46
Poor
0
0
Sex
Female
41
91.08
5.1268
0.07704
Male
0
0
Farms
Hawasa
0
0
88.918
0.0022
Wondo Genet
2
0.99
Sululta
5
2.47
Mojo
34
16.83
Breed
Holstein
40
19.80
2.5191
0.6412
Jersey
0
0
Borena
1
0.49
Table 2: Summary of risk factors for Bovine tuberculosis analysis (n=202).
Discussion
BTB caused by Mycobacterium bovis is considered one of the most important diseases facing farming industry, cattle owners, government, abattoir workers and veterinary profession in Ethiopia. In urban intensive dairy farms, BTB transmission high with prevalence [14]. In this study, BTB prevalence assessed in four intensive dairy farms and identified risk factors in cattle. The study was carried out in Hawasa, Wondo Genet, Sululta and Mojo to diagnosis BTB using comparative intradermal Tuberculin Test (CIDT) and its risk factors. The overall prevalence of BTB in all four towns was 20.29% (41) in dairy farms. This finding is moderately in line with [14], who found 20.3% BTB prevalence in dairy cattle from eastern Ethiopia. Another important finding was the reported of [9], who found 30% high Prevalence of BTB in Dairy Cattle in Central Ethiopia. This variation may be brought by the various study methods, research designs, and farming systems. Dairy cattle kept under intensive management are reportedly more likely to contract the disease than other cattle because of their closer confinement, longer life spans, and higher levels of productivity stress [21]. Sex: All bovine tuberculosis positive animals were female due to large proportion female cattle in the study. Female cows are kept for longer period by farmers for breeding purpose than male cattle, and since tuberculosis is a chronic disease, its prevalence had been reported to be higher among adult cattle than the younger ones. The finding was similar to previous reports (Article et al., 2020), that state tuberculosis was more common among female and adult cattle. BCS: animal body conditions analyses also 16.83% (34) good, 3.46% (7) medium and 0% (0) Poor respectively. The finding of this study showed that the prevalence of tuberculosis was higher among cattle with poor body condition. In contrast to report of (Article et al., 2020) all poor body condition score are negative.
Age: The highest prevalence BTB was adult age animals 15.84 % (32) and 4.45% (9) young. This finding showed the age are significant associated with BTB infection. The results of this study reveals in consisted with previous report of [8], BTB prevalence increased with the age of the animals, probably because of the longer exposure to the agent over time of older animals [12]. Another important finding of [13] reflect adult animals were more likely to present with BTB than young animals. It is suggested that the risk of BTB infection positively correlates with the age of an animal, with older animals being at higher risk due to prolonged exposure to the pathogen in the environment, reactivation of latent infections, and physiological decline of immunity. Farm: In the present study, the most dominant positive prevalence was record in Mojo 16.83% (34), when compared with sululta 2.47% (5), Wondo Genet 0.99% (2) and Hawasa 0% (0) respectively. This is due to farm not test for long time from its establishments. This finding in agreement with previously reported by [10] the intensive farming facilitates transmission of BTB among cattle as individual animals are kept in close proximity to one another, overcrowding itself was claimed to stress cattle and compromise their ability to thrive with the disease.
Conclusions
BTB causes impact on human and animal health, particularly in developing countries. The absence of a gold standard single test to detect all cases of BTB, the absence of a realistic vaccine against the disease, and the zoonotic impact are the main challenges. The prevalence reported in the current study only using CIDT test revealed that BTB is present and is well established in dairy farms. The farm owners and community have the habit of consuming raw meat and milk and share the same microenvironment with their livestock. This further disseminates the causative agent, both through inhalation and ingestion resulting in high economic loss and public health effect.
Bovine tuberculosis can be controlled by test-and-slaughter or test-and-segregation methods. Affected herds are re-tested periodically to eliminate cattle that may shed the organism; the tuberculin test is generally used. Infected herds are usually quarantined, and animals that have been in contact with reactors are traced. Only test-and-slaughter techniques are guaranteed to eradicate tuberculosis from domesticated animals. However, some countries use test- and-segregation programs during the early stages of eradication, and switch to test-and-slaughter methods in the final stage. Once eradication is nearly complete, slaughter surveillance, with tracing of infected animals, may be a more efficient use of resources.
Based On the Above Conclusion the Following Recommendation Were Forwarded
Introduction of new animal should be tested before mixing to the herd. After test isolation of animals in to category of positive, doubt full and negative in separate room. Hygiene and avoidance of contamination should be implemented to prevent herd infection. Awareness creation on zoonotic importance of BTB to should be given on safety precaution for animal’s attendant and milker. Pasteurized or Boiling milk before drinking. The relevance of screening test for BTB before purchasing the animals and after. Moreover, further detailed agent characterization and Public awareness should be done to investigate bovine tuberculosis in the nationwide in order to design appropriate strategic prevention and control measures.
Author Statements
Acknowledgments
Great acknowledgement goes to Animal Health Institute and dairy farm owners for their cooperation and facilities this work.
Conflict of Interest
There is no conflict of interest regarding the publication of this manuscript
References
- Iliyasu D, Rwuaan JS, Sani D, AI Nwannenna, CO Njoku, et al. Evaluation of Moringa oleifera (L) Aqueous Seed Extracts on Aphrodisiac, Gonadal and Epididymal Sperm Reserves of Wistar Rats. Sahel J Vet Sci. 2020; 17: 26-32.
- Awah-Ndukum J, Kudi AC, Bah GS, Bradley G, Tebug SF, Dickmu PL, et al. Bovine tuberculosis in cattle in the highlands of Cameroon: seroprevalence estimates and rates of tuberculin skin test reactors at modified cut-offs. Vet Med Int. 2012; 2012: 798502.
- Awah-Ndukum J, Kudi AC, Bradley G, Ane-Anyangwe I, Titanji VPK, Fon-Tebug S, et al. Prevalence of bovine tuberculosis in cattle in the highlands of Cameroon based on the detection of lesions in slaughtered cattle and tuberculin skin tests of live cattle. Vet Med. 2012; 57: 59-76.
- Awah-Ndukum J, Temwa J, Ngwa VN, Mouiche MM, Iyawa D, Zoli PA. Interpretation criteria for comparative intradermal tuberculin test for diagnosis of bovine tuberculosis in cattle in Maroua Area of Cameroon. Vet Med Int. 2016; 2016: 4834851.
- Carranza C, Pedraza-Sanchez S, de Oyarzabal-Mendez E, Torres M. Diagnosis for latent tuberculosis infection: new alternatives. Front Immunol. 2020; 11: 2006.
- Davey S. Challenges to the control of Mycobacterium bovis in livestock and wildlife populations in the South African context. Ir Vet J. 2023; 76: 14.
- Defar D, Gashaw A, Webete A. Prevalence of bovine tuberculosis and its zoonotic implication, Holeta, central Ethiopia. Ethiop J Health Sci. 2009; 19: 179–185.
- Dejene SW, Heitkönig IMA, Prins HHT, Lemma FA, Mekonnen DA, Alemu ZE, et al. Risk factors for bovine tuberculosis (bTB) in cattle in Ethiopia. PLOS ONE. 2016; 11: e0159083.
- Firdessa R, Tschopp R, Wubete A, Sombo M, Hailu E, Erenso G, et al. High prevalence of bovine tuberculosis in dairy cattle in central Ethiopia: implications for the dairy industry and public health. PLOS ONE. 2012; 7: e52851.
- Frey-Law LA Bohr, Nicole L Sluka, KA Herr, K Noiseux, Rakel BA. HHS Public Access. Pain. 2016; 159: 1-26.
- Almaw GA, Conlan AJK, Ameni G, Gumi B, Alemu A, Guta S, et al. The variable prevalence of bovine tuberculosis among dairy herds in Central Ethiopia provides opportunities for targeted intervention. PLoS One. 2021; 16: e0254091.
- Islam SKS, Rumi TB, Kabir SML, van der Zanden AGM, Kapur V, Rahman AKMA, et al. Bovine tuberculosis prevalence and risk factors in selected districts of Bangladesh. PLOS ONE. 2020; 15: e0241717.
- Kapalamula TF, Kawonga F, Shawa M, Chizimu J, Thapa J, Nyenje ME, et al. Prevalence and risk factors of bovine tuberculosis in slaughtered cattle, Malawi. Heliyon. 2023; 9: e13647.
- Kemal J, Sibhat B, Abraham A, Terefe Y, Tulu KT, Welay K, et al. Bovine tuberculosis in eastern Ethiopia: prevalence, risk factors and its public health importance. BMC Infect Dis. 2019; 19: 39.
- Mekonnen GA, Conlan AJK, Berg S, Ayele BT, Alemu A, Guta S, et al. Prevalence of bovine tuberculosis and its associated risk factors in the emerging dairy belts of regional cities in Ethiopia. Prev Vet Med. 2019; 168: 81-9.
- NMA. Seasonal magnitude study on seasonal magnitude and variation of fine particlet matter pollution (PM2.5) over Addis Ababa, Ethiopia in meteorology perspective. Glob Sci J. 2020; 8: 1020-36.
- Ramos DF, Silva PEA, Dellagostin OA. Diagnosis of bovine tuberculosis: review of main techniques. Braz J Biol. 2015; 75: 830-7.
- Saville WJ, Wittum TE. Veterinary epidemiology. In: Equine internal medicine. 2nd ed; 2004: 1513-1528.
- Getahun TK, Gutema F, Wodajo F. Status and public health significance of Mycobacterium bovis in Ethiopia. Vet Med Open J. 2023; 8: 18-28.
- Tschopp R, Schelling E, Hattendorf J, Aseffa A, Zinsstag J. Risk factors of bovine tuberculosis in cattle in rural livestock production systems of Ethiopia. Prev Vet Med. 2009; 89: 205-11.
- Tulu B, Zewede A, Belay M, Zeleke M, Girma M, Tegegn M, et al. Epidemiology of bovine tuberculosis and its zoonotic implication in Addis Ababa milkshed, central Ethiopia. Front Vet Sci. 2021; 8: 595511.
- Xu F, Tian L, Li Y, Zhang X, Qi Y, Jing Z, et al. High prevalence of extrapulmonary tuberculosis in dairy farms: evidence for possible gastrointestinal transmission. PLOS ONE. 2021; 16: e0249341.