Case Report
Austin J Womens Health. 2014;1(1): 3.
An Unusual Presentation of Pelvic Inflammatory Disease: Case Report and Discussion
Patricia Rekawek1, Frederick Friedman1*
1Department of Obstetrics, Gynecology and Reproductive Science, Icahn School of Medicine at Mount Sinai, New York, New York
*Corresponding author: Frederick Friedman, Department of Obstetrics, Gynecology and Reproductive Science, Icahn School of Medicine at Mount Sinai, 1 Gustave L Levy Place, Box 1170, New York, NY 10029
Received: October 09, 2014; Accepted: October 23, 2014; Published: October 27, 2014
Abstract
Pelvic Inflammatory Disease (PID) generally results from an infectious process that involves the entire upper reproductive tract. It is generally felt that the inciting event is contraction of a sexually transmitted cervicitis, with ascension from the cervix leading to peritonitis. Classical teaching states that sterilization via tubal ligation is protective. Laparoscopy has been employed to establish or confirm a diagnosis when there is uncertainty or failure to respond to standard treatment. We present a case of PID occurring in a patient who had undergone a tubal ligation 20 years prior to presentation. Her low risk behavior and atypical complaints and physical exam findings necessitated laparoscopy to establish the diagnosis.
Keywords: Post-Sterilization Pelvic Inflammatory Disease; Post- Sterilization Salpingitis; Laparoscopy
Introduction
Pelvic Inflammatory Disease (PID) encompasses a spectrum of infectious disorders of the upper reproductive tract, but is often used synonymously with salinities. Excluding the rare hematogenously spread pathogens such as Mycobacterium tuberculosis, which is a causetypically limited to developing countries, most cases arise from ascending infections from the cervico-vaginal tract. The sexually transmitted diseases gonorrhea and chlamydia are responsible for about two-thirds of cases; the remaining inciting pathogens remain cryptic [1,2]. It is generally believed that sexual activity is the sine qua non for PID. Women who are abstinent or in a mutually monogamous relationship have almost no risk, while those with multiple partners are at greater risk. Barrier methods, especially condoms, are considered to be protective. Tubal ligation is usually felt to lower one’s susceptibility to salpingitis as well. In fact, PID following tubal ligation is a rare occurrence. Due to the nonspecific signs and symptoms of PID, diagnosis is often difficult. Some have advocated the liberal use of laparoscopy to establish the diagnosis [3]; more recently, this option has been found to have low sensitivity, and thus may be more suitable for confirmation [4]. Despite improvements in imaging technologies, the use of non-invasive diagnostic modalities has yielded variable results. We report an unusual case of PID which appears to have been precipitated by a retained tampon. This patient had a previous tubal ligation, and due to her atypical presentation and findings, laparoscopy was necessary for diagnosis.
Case Presentation
A 44 year old para 2012 presented to a local emergency room with a three day history of progressive lower abdominal pain and nausea. Twenty years prior, the patient underwent a bilateral tubal ligation following delivery of her second child. She had normal cyclic menses and no other prior surgeries. She was sexually active with one male partner for the past several years, although had no coitus for the antecedent 4 weeks.
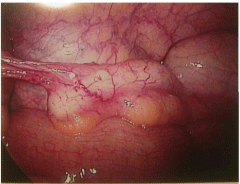
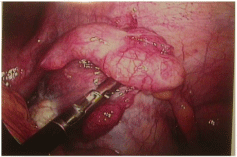
The patient’s pain began gradually and increased in severity over a few days.She had no fever, nausea, diarrhea, constipation or other changes in bowel or bladder habits. The pain was exacerbated on motion but did not improve with resting. Upon presentation to a local Emergency Room, a retained tampon was removed. An abdominopelvic CT scan showed no abdominal or pelvic masses; pelvic sonography revealed normal ovaries and no free fluid. Electrolytes, white blood cell count and liver enzymes were all within normal limits; a urinalysis was negative. She was given oral doxycycline and metronidazole, which she vomited, and was discharged. The patient then presented to her gynecologist the next day for further evaluation. At that time, examination revealed a well-appearing woman who appeared uncomfortable, but in no acute distress. She was afebrile with normal vital signs; she had no rash. Her abdomen was soft, non-distended and without masses or organomegaly. She had focal bilateral lower quadrant tenderness but no rebound. Pelvic exam revealed lichen sclerosis of the vulva, and slight staining per os without malodorous discharge. There was no cervical motion tenderness or overt cervicitis. The uterus was midposition, minimally enlarged, and mobile without tenderness. The adnexae were without masses and palpation elicited minimal tenderness. Rectovaginal exam was unremarkable. Swabs were sent for cervical culture and gonorrhea and chlamydia testing. The patient was then admitted for further observation and management. A serum pregnancy test was negative. All routine admission laboratory values were within normal limits, including liver enzymes, electrolytes and a WBC of 9.0 x 103/ μl. surgical consultation was obtained, and the patient was started on empiric ampicillin/sulbactam. Repeat pelvic sonography showed a normal uterus with an 8mm endometrial echo, a hyperechoic area in her anterior lower uterine segment (likely representing her prior cesarean section scar,) normal ovaries and a small amount of free pelvic fluid. Abdominal sonography revealed a 1cm round echogenic lesion, presumably a hemangioma, in the right lobe of her liver; the gall bladder, pancreas, spleen and both kidneys were unremarkable. The aorta was normal without para-aortic adenopathy. The patient remained afebrile with increasing pain overnight; pelvic exam was unchanged. An abdominopelvic CT scan revealed a normal size liver with mild fatty infiltration and a possible small hemangioma; thegall bladder and ductal system were unremarkable. The stomach and bowel were incompletely visualized, but appeared normal. The spleen, pancreas, adrenal glands, and kidneys were also normal. The aorta was normal without retroperitoneal adenopathy; scattered small lymph nodes were seen in the pelvic and inguinal regions. The uterus was unremarkable, but clusters of small cystic structures were seen in the pelvis on both sides of the uterine fundus. The cluster on the left measures overall 4.1 cm x 2.7 cm and on the right measures 3.5 cm x 2.5 cm. These were felt to most likely represent the adnexa. No ascites was seen. The appendix was normal in caliber with its tip somewhat in the region of the cluster of lucencies in the right hemipelvis. No focally prominent infiltration of fat was seen in this area compared to the remainder of the abdomen and pelvis. A small fat-containing periumbilical hernia was seen; the small bowel protruded towards this area although no frank bowel herniation or obstruction was evident. In view of the increasing pain and uncertain diagnosis, the patient underwent laparoscopy for diagnosis. On entry, omental adhesions to the anterior abdominal wall were seen; the upper abdomen was unremarkable and free of adhesive disease. A hyperemic bulky uterus was present. There were bilateral periovarian and peritubal adhesions with normal ovaries bilaterally. The fallopian tubes previously had been transected distally. There were bilateral proximal small hydrosalpinges adherent to the anterior abdominal wall, underlying the patient’s reported sites of maximal pain. The appendix was adherent to the right fallopian tube. (Figures 1 and 2) Laparoscopic lysis of adhesions, bilateral salpingectomy and an appendectomy were performed; the site of peri-umbilical herniation was also repaired, although no incarceration was present. The patient has an unremarkable postoperative course with rapid resolution of her pain. She was discharged home on postoperative Day 2on oflaxacin, due to a multiple drug allergy history. She was seen 2 weeks later for follow-up and was without complaints. Histopathology revealed bilateral pyosalpinges with acute and chronic salpingitis and evolving microabscesses. The appendix showed obliteration of the distal lumen, but was otherwise without significant change. Her outpatient cervical cultures and nucleic acid testing were negative; cultures of the fallopian tubes also were negative.
Figure 1: Appendix with adhesions to right adnexae.
Figure 2: Right Adnexae adhesed to anterior abdominal wall.
Discussion
PID describes an acute infection of the upper reproductive tract that causes an inflammatory reaction from the cervix to the peritoneal cavity; this results in endocervicitis, endometritis, salpingitis, and peritonitis. If left untreated, PID can lead to long-term sequelae such as chronic pelvic pain, tubal factor infertility, ectopic pregnancy, and tubo ovarian abscess formation. PID is generally regarded as a community-acquired infection, most often incited by sexual transmission; the most frequent such pathogens are Neisseria gonorrhoeae and Chlamydia trachomatis. Each of these 2 organismshas been reported toaccount individually for one third of PID cases, collectively accounting for two-thirds of cases overall [1,2]. However, in most situations the microbiology of PID is polymicrobial.In the majority of women with PID, facultative aerobic bacteria and anaerobic bacteria have been isolated from the upper genital tract, with and without concomitant documentation of N. gonorrhoeaeor C. trachomatis. In addition, the putative sexually transmitted pathogen Mycoplasma genitaliumhas been detected in the endocervix or endometrium in 14% of women with PID unrelated to gonococcal or chlamydial etiologies [5]. Bacterial vaginosis (due to Gardnerella vaginalis and Mycoplasma) is present in up to 2/3 of women with PID [6]; it is thought to facilitate the spread of vaginal microorganisms by interfering with host defenses [7]. Risk factors for the acquisition of PID are well known. Women with multiple partners are at greatest risk for developing PID, whereas celibate women typically are not at risk for PID [8]. Methods of protection against PID include abstinence, condom use/barrier protection, and reducing the number of sexual partners. Since PID is regarded as an ascending infection from the lower to upper genital tract, it logically follows that disruption of this ascending infection wouldprevent against the development of PID. Based on this theory, surgical sterilization in the form of a tubal transection generally has been considered protective against the development of PID [9-12]. In 1946, Falk et al., followed 1,000 cases of tubal ligation for more than 8 years & reported no instances of pelvic infection [10]. In addition, Vessey et al., and Poma et al., similarlyreported no cases of PID after surgical sterilization [11,12]. Despite these studies, in 2000 Levgur et al., cited 109 case reports of PID identified since 1975 [13]. In many case reports, authors have described PID related to “proximal stump salpingitis” not involving the distal fallopian tubes [14-16]. In a retrospective study Green et al., reviewed the charts of 364 inpatients that were discharged with a diagnosis of PID [17]. Acute PID was identified in 21patientswho had previously undergone surgical sterilization. Nine of the surgically diagnosed cases had systemic toxicity that warranted surgical evaluation; these patients had not only “proximal stump” involvement but also significant inflammation of distal portions of the tubes. Similarly, Abbuhl et al., performed a retrospective chart review of 209 patients diagnosed with PID over a 1 year period and found 24 patients (11.7%) had undergone a previous bilateral tubal ligation [18]. These authors, along with those from case reports identified in the past, propose that PID in patients with prior surgical sterilization may occur more frequently than previously thought. Due to the fact that PID represents a spectrum of infection, there is no gold standard for the diagnosis of PID; hence, emphasis is placed on clinical diagnosis. The clinical criteria for diagnosis of acute PID usually include a triad of moderate-to-severe lower abdominal pain, associated with cervical motion tenderness, and fever. Hagerproposedcriteria for diagnosis using different modalities – clinical and laparoscopic [19]. In the latter scheme, visual confirmation of tubal salpingitis involves erythema of the fallopian tube, edema and swelling of the tube or purulent exudate from the fimbriated end or on the serosal surface of the fallopian tube [19]. Jacobson performed laparoscopy in the presence of a high clinical suspicion of PID and confirmed visual evidence of salpingitis approximately 65% of the time. 3Furthermore, 12% of patients with suspected PID had other pelvic pathology, and in 24% of patients, no disease process was found. Due to the relative inaccuracy of clinicalcriteria in diagnosing PID, Jacobson proposed laparoscopic staging criteria for PID; however this may be impractical due to cost, invasiveness of laparoscopy, and hospitalization. Therefore, clinicians rely mostly on clinical criteria to diagnose PID and often maintain a low threshold for the diagnosis due to the consequences of untreated PID, such as chronic pelvic pain, tubal factor infertility, ectopic pregnancy, and tuboovarian abscess formation. Laparoscopy has been found to have sensitivity as low as 50%, and a specificity approaching 100%; since screening diagnostic tests generally require high sensitivity, whereas confirmatory tests require high specificity, laparoscopy may be more suitable for confirmation rather than diagnosis of PID [4,6]. As a result of the limitations of laparoscopy its use is generally confinedto patientswho have failed initial treatment or in whom the diagnosis remains cryptic. Our patient was unusual in that a laparoscopically-confirmed visual diagnosis of PID was determined in a woman with a low suspicion of PID based on clinical exam and prior surgical sterilization. Furthermore, due to the absence of other risk factors, we suspect the ascending infection was triggered by the retained tampon. The adhesions of her tubes to the anterior abdominal wall hindered establishment of the diagnosis and also obscured imaging findings. Interestingly, these tubal agglutinations that altered the normal anatomic positionsimultaneously may have allowed for isolating the infection; this limited signs of peritonitis. Due to our patient’s atypical presentation and findings, laparoscopy was necessary to confirm the diagnosis of acute salpingitis. Our caseemphasizes that, PID should be consideredin symptomatic patients regardless of surgical history, even though the incidence of PID after tubal ligation is rare. It further servesas a reminder that any vaginal or cervical nidus of infection may result in an ascending infection. Laparoscopy should be utilized if the diagnosis is in question.
References
- Eschenbach DA, Buchanan TM, Pollock HM, Forsyth PS, Alexander ER, Lin JS, Wang SP. Polymicrobial etiology of acute pelvic inflammatory disease. N Engl J Med. 1975; 293: 166-171.
- Bowie WR, Jones H. Acute pelvic inflammatory disease in outpatients: association with Chlamydia trachomatis and Neisseria gonorrhoeae. Ann Intern Med. 1981; 95: 685-688.
- Jacobson L, Weström L. Objectivized diagnosis of acute pelvic inflammatory disease. Diagnostic and prognostic value of routine laparoscopy. Am J Obstet Gynecol. 1969; 105: 1088-1098.
- Peipert JF, Boardman LA, Sung CJ. Performance of clinical and laparoscopic criteria for the diagnosis of upper genital tract infection. Infect Dis Obstet Gynecol. 1997; 5: 291-296.
- Cohen CR, Mugo NR, Astete SG, Odondo R, Manhart LE, Kiehlbach JA, et al. Detection of Mycoplasma genitalum infection versus Neisseria gonorrhoeae infection among women with pelvic inflammatory disease. Clin Infec Dis. 2009; 48: 41-47.
- Soper DE, Brockwell NJ, Dalton HP, Johnson D. Observations concerning the microbial etiology of acute salpingitis. Am J Obstet Gynecol. 1994; 170: 1008-1014.
- Paavonen J, Critchlow CW, DeRouen T, Stevens CE, Kiviat N, Brunham RC, et al. Etiology of cervical inflammation. Am J Obstet Gynecol. 1986; 154: 556-564.
- Suss AL, Homel P, Hammerschlag M, Bromberg K. Risk factors for pelvic inflammatory disease in inner-city adolescents. Sex Transm Dis. 2000; 27: 289-291.
- Hajj SN. Does sterilization prevent pelvic infection? J Reprod Med. 1978; 20: 289-290.
- Falk HC. Interpretation of the pathogenesis of pelvic infection as determined by cornual resection. Am J Obstet Gynecol. 1946; 52: 66-73.
- Vessey M, Huggins G, Lawless M, McPherson K, Yeates D. Tubal sterilization: findings in a large prospective study. Br J Obstet Gynaecol. 1983; 90: 203-209.
- Poma PA. Tubal sterilization and later hospitalizations. J Reprod Med. 1980; 25: 272-278.
- Levgur M, Duvivier R. Pelvic inflammatory disease after tubal sterilization: a review. Obstet Gynecol Surv. 2000; 55: 41-50.
- Semchyshyn S. Letter: Fallopian tube stump perforation. Can Med Assoc J. 1975; 113: 278-281.
- Fletcher V Jr. Proximal stump salpingitis. Am J Obstet Gynecol. 1986; 155: 496-500.
- Vermesh M, Confino E, Boler LR, Friberg J, Gleicher N. Acute salpingitis in sterilized women. Obstet Gynecol. 1987; 69: 265-267.
- Green MM, Vicario SJ, Sanfilippo JS, Lochhead SA. Acute pelvic inflammatory disease after surgical sterilization. Ann Emerg Med. 1991; 20: 344-347.
- Abbuhl SB, Muskin EB, Shofer FS. Pelvic inflammatory disease in patients with bilateral tubal ligation. Am J Emerg Med. 1997; 15: 271-274.
- Hager WD, Eschenbach DA, Spence MR, Sweet RL. Criteria for diagnosis and grading of salpingitis. Obstet Gynecol. 1983; 61: 113-114.