
Research Article
Austin J Cerebrovasc Dis & Stroke. 2014;1(6): 1030.
Role of Vascular Endothelial Growth Factor in Atherosclerotic Carotid Plaques
Kanno H1,2*, Higashida T1, Miyake S1,2, Kobayashi N1,2 and Nakanowatari S2
1Department of Neurosurgery, Yokohama City University, Japan
2Department of Neurosurgery, Yokosuka City Hospital, Japan
*Corresponding author: Hiroshi Kanno, Department of Neurosurgery, Yokosuka City Hospital, 1-3-2 Nagasaka, Yokosuka 240-0195, Japan.
Received: December 02, 2014; Accepted: December 26, 2014; Published: December 31, 2014
Abstract
Mechanism of atherosclerotic carotid stenosis has not been fully elucidated. Intraplaque hemorrhage is supposed to play an important role in the progression of carotid stenosis and ischemic event. Expression of Vascular Endothelial Growth Factor (VEGF) is related to atherosclerosis. The purpose of this study is to elucidate the role of VEGF in atherosclerotic carotid plaques. We analyzed the expression of VEGF in atherosclerotic carotid plaques obtained at carotid endarterectomy for symptomatic carotid stenosis. Both Immunohistochemical and immunoblotting methods using anti-VEGF antibody were employed. In addition, the correlation between VEGF expression and plaque pathology was examined. Our study showed a high level of VEGF immunoreactivity in carotid plaques with distinct expression of VEGF in foam cells infiltrating carotid plaques. The statistical analyses revealed that the numbers of VEGF expressive cells per unit area in the intimal deep portions of carotid plaques were significantly larger than those in the intimal superficial portions (P<0.01). In addition, it was suggested that the VEGF expression was related to intraplaque hemorrhage. In conclusions, VEGF expression is induced in the deep portion of the atherosclerotic carotid plaques. It may play an important role in induction of intraplaque hemorrhage causing ischemic stroke
Keywords: Vascular endothelial growth factor; Atherosclerotic carotid plaque; Intraplaque hemorrhage; Ischemic stroke
Introduction
Cerebral infarction is often caused by tight stenosis of the carotid artery and ulceration of the carotid endothelium. It has been assumed that carotid intraplaque hemorrhage plays a significant role in the rapid progression of carotid stenosis and the formation of plaque ulceration leading to plaque disruption. Previous studies have demonstrated frequent intraplaque hemorrhage associated with ischemic stroke [1-8]. The mechanism of carotid intraplaque hemorrhage is not clear and it is not known whether it is caused by rupture of newly-formed intraplaque vessels. Vascular Endothelial Growth Factor (VEGF) [9], a potent angiogenesis factor, is produced by a variety of cells including glioma cells [10], smooth muscle cells [11], and macrophages [12]. In addition, VEGF is expressed in atherosclerotic plaques of carotid and coronary arteries [13-20]. However, role of VEGF in atherosclerotic carotid plaque has not been fully elucidated. Since the intraplaque core is hypoxic [21], we hypothesized that VEGF would be easily induced in sclerotic carotid plaques. To this end we investigated VEGF expression in atherosclerotic carotid plaques and examined the relationship between VEGF expression and intraplaque hemorrhage.
Materials and Methods
Carotid plaques
Sixty-seven sclerotic carotid plaques obtained from carotid endarterectomy were collected at Yokohama City University Hospital and affiliated hospitals. All cases from which sclerotic carotid plaques were obtained showed cerebral ischemic symptoms. They included 18 transient ischemic attacks and 49 minor completed strokes. Intraplaque hemorrhage was found in 46 cases (68.7%) of atherosclerotic carotid plaques. The mean age of patients with carotid stenosis was 63.4 years, and male/female was 58/9. All samples of sclerotic carotid plaques were fixed with 20% formalin solution and provided for the Immunohistochemical examination which was performed by the standard avidin-biotin complex method. The employed primary antibody as a mouse monoclonal antibody, R11 (Immuno-biological Laboratories Co. Ltd, Fujioka, Japan), against recombinant human VEGF165. After sufficient deparaffinization, these sections were treated with 0.3% H2O2 in methanol for 15 minutes to block endogenous peroxidase activity. Nonspecific binding was blocked by treatment with normal horse serum (1:20) for 45 minutes. The sections were next incubated with the primary antibody, R11(1:100) for 60 minutes at room temperature. After sufficient washing in 0.01 M Phosphate- Buffered Solution (PBS), sections were incubated with biotinylated rabbit anti-mouse IgG antibody (1: 200, Vector Laboratories, Inc., CA) for 30 minutes. After washing in 0.01 M PBS, the sections were incubated with avidin-biotinylated horseradish peroxidase complex (1:100, Vector Laboratories, Inc., Burlingame, CA) and developed in 0.03% H2O2 and 0.1% diaminobenzidine tetra hydrochloride (DAB, Wako Pure Chemical Industries, Ltd, Tokyo). Control stain was performed with omission of the primary antibody. The mean number of VEGF immunoreactive cells in randomly sampled 10 areas of 0.5 mm2 in carotid plaques was examined. Then, according to the mean number of immunoreactive cells per unit area (0.5 mm2) in each carotid plaque, we performed the comparison between the level of VEGF expression in the intimal deep portions of carotid plaques (>0.3 mm) and that in the intimal superficial portions (<0.3mm); and the comparison between the level of VEGF expression in the group with intraplaque hemorrhage and that without intraplaque hemorrhage.
Confocal fluorescent immunohistochemistry
In order to identify the origin of VEGF-expressing intraplaque cells, double-immunofluorescence method was performed. Whether VEGF was expressed in macrophage or smooth muscle cells was studied using cell type specific markers. At first, the specimens were exposed to a primary antibody mixture composed of: 1) anti-macrophage monoclonal antibody, KP-1 (1:50, Dakopatts, Glostrup, Denmark) and a rabbit polyclonal antibody against human smooth muscle actin, ACTA2 (1:100) LifeSpan Biosciences, Inc. Seattle, WA,USA); 2) antimacrophage monoclonal antibody, KP-1 (1:50) rabbit anti-human VEGF polyclonal antibody (1:100, Immunobiological Laboratories Company, Gunma, Japan) in PBS. Reaction was then undertaken with a second antibody mixture composed of: 1) a goat anti rabbit immunoglobulin conjugated to tetramethylrhodamine isothianate (TRITC, 1:40, Sigma Chemical Co., St. Louis, MO) in PBS; and 2) a goat anti-mouse immunoglobulin conjugated to fluorescein isothiocyanate (FITC, 1:40, Cappel, West Chester, PA) in PBS. After a reaction for 30 minutes at 37oC, specimens were extensively washed with 0.075% Tween 20 in PBS. A confocal laser scanning microscope (Olympus, FV300, Tokyo, Japan) was employed for observation of double-stained cells. FITC-labeling cells showed green while TRITClabeling ones red.
Western blotting
Frozen tissue samples (2 mm3) from randomly sampled 6 carotid plaques were obtained at carotid endarterectomy. Specimens from 3 nonsclerotic carotid walls were processed as control samples. Samples were homogenized in a lysis buffer (0.1 mol/L NaCl, 0.01 mol/L Tris-HCL, 0.01 mol/L EDTA, 1ug/mL aprotinin). Assays to determine the protein concentration of the lysate were performed by comparison with known concentrations of bovine serum albumin. SDS-gel eletrophoresis was performed in 10% polyacrylamide gels under nonreducing conditions. Lysates equivalent to 15μg of protein from samples of carotid plaques and non-sclerotic carotid walls were electrophoresed on each gel, together with prestained molecular weight markers (Amersham, Buckingumshire, UK). The electrophoresis running buffer contained 25 mmol/L Tris base, 250 mmol/L glycine, and 0.1% SDS (pH 8.3). The protein on the gel was subsequently transferred to a Hybond ECL nitrocellulose transfer membrane (Amersham, Buckingumshire, UK) in buffer containing 20% SDS (pH 8.3). The membrane was placed in 5% skim milk in25 mmol/L Tris-buffered saline for 1 hour to block nonspecific binding. The membrane was then incubated for 3 hours with a mouse monoclonal antibody to VEGF, R11(1:200), diluted in TBS-T (50 mmol/L Tris-HCL [pH 7.6], 150 mL NaCl, and 0.05% Tween20). After thorough washing with TBS-T, anti-mouse IgG, biotinylated secondary antibody (1: 400, Vector Laboratories, Inc., Burlingame, CA) was app1ied for 60 minutes. An additional series of washes was followed by incubation with preformed horseradish peroxidasestreptavidin complex (1:200, Vector Laboratories, Inc., Burlingame, CA) and then by detection with DAB. Membranes were finally washed in distilled water and air dried.
Statistical analysis
All statistical results were expressed as the mean ± standard deviation. For comparisons between values for groups, Scheff’s test after the analysis of variance-test was used, with probabilities of less than 0.05 being considered significant.
Results
VEGF expression in carotid plaques
In sclerotic carotid plaques obtained at carotid endarterectomy, VEGF immunoreactivity was detected in the deep layer of the thickened intima, where it was found in the cytoplasm and nucleus of foam and fusiform cells and the extracellular matrix. However, this factor was detected in only a small number of fusiform or round cells in the superficial layer of the intima. Most foam cells and fusiform cells in the deep layer showed distinct expression of VEGF with immunohistochemistry. Expression of VEGF in foam cells was detected at thin cytoplasm, nucleus, and the extracellular matrix around the cells but not at the foamy component including lipid. VEGF expression in fusiform cells was mainly at the cytoplasm. In addition, foam cells around intraplaque hemorrhage and newlyformed vessels showed distinct VEGF immunoreactivity. Nucleus and membranes of foam cells in medium and deep layers showed the distinct immunoreactivity of VEGF. However, elastic and collage nous fibers did not show the VEGF immunoreactivity. Foam cells and some extracellular matrix around newly-formed vessels and intraplaque hemorrhage showed particularly distinct immunoreactivity of VEGF (Figure 1). The statistical analyses revealed that the mean number of immunoreactive cells per unit area (0.5 mm2) in the intimal superficial portion (<0.3 mm) of carotid plaques, 33.5±9.7 was significantly smaller than that in the intimal deep portion (>0.3 mm) of carotid plaques, 94.7±52.8 (P<0.01). The mean number of immunoreactive cells for VEGF per unit area (0. 5 mm2) in carotid plaques with intraplaque hemorrhage (mean age, 64.2 years; male/female, 40/6), 105.5±39.0 was significantly larger than 21 carotid plaques without intraplaque hemorrhage (mean age, 66.1 years; male/female, 18/3), 43.1±27.9 (P<0.01) (Figure 2).

Figure 1: Immunohistochemical studies on atherosclerotic carotid plaques.
A, Atherosclerotic carotid plaque including intimal layer (right), medium layer
(center), and deep layer (left). VEGF immunorectivity is detected in medium
and deep layers of a sclerotic carotid plaque but not in the superficial layer.
B, Deep layer of the atherosclerotic carotid plaque. VEGF immunorectivity is
detected in foam cells, fusiform cells, and surroundings .of the cells.
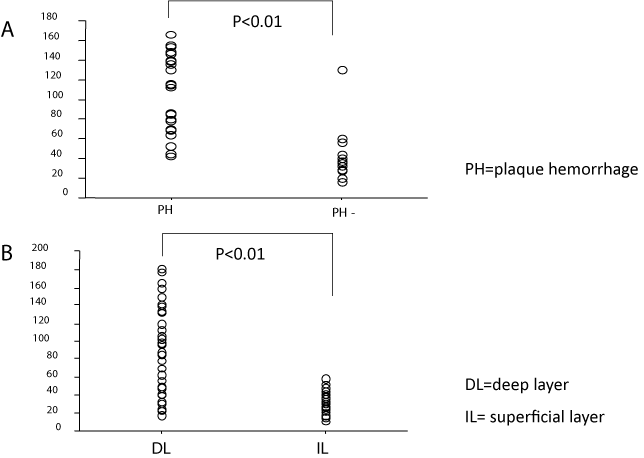
Figure 2: Relationship between VEGF expression and pathology in
atherosclerotic carotid plaques. A, The number of VEGF-expressing cells
per unit area (0.5 mm2) in atherosclerotic carotid plaques with and without
intraplaque hemorrhage. The mean number of VEGF-expressing cells per
unit area in the group with intraplaque hemorrhage was significantly lager
than that without intraplaque hemorrhage (P<0.01). B, The number of VEGFexpressing
cells in the intimal superficial portion (< 0.3 mm) and that in the
intimal deep portion (>0.3 mm) of atherosclerotic carotid plaques. The mean
number of VEGF-expressing cells in the intimal deep portion was significantly
larger than that in the intimal superficial portion (P<0.01). PH, With intraplaque
hemorrhage. PH-, Without intraplaque hemorrhage. d, Deep portion of carotid
plaques. s, Superficial portion of carotid plaques.
Identification of VEGF-immunoreactive cells
In the sclerotic carotid plaques, double-immunofluorence for VEGF (showing red) and macrophage (showing green) or SMA (showing green) revealed that most of foam cells in the medium plaque layer showed both positive in VEGF and in macrophage, and also that some foam cells and fusiform or round cells in the medium or deep plaque layer showed both positive in VEGF and in SMA. In results, VEGF-expressing cells in sclerotic carotid plaques were identified mostly as a macrophage origin or partly as a smooth muscle cell origin (Figure 3).
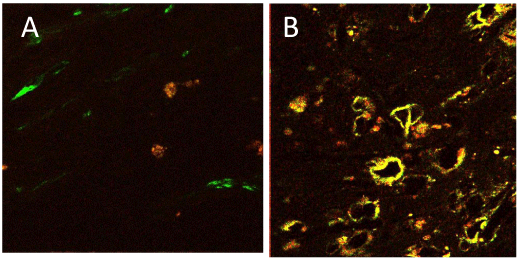
Figure 3: Double-immunofluorescence histochemistry for VEGF and
macrophage in deep layer of a carotid plaque. A,VEGF-positive cells showing
red with TRITC. Macrophage-positive cells showing green with FITC. The
most of foam cells showing both positive. B, Double-immunofluorescence
histochemistry for VEGF and smooth muscle cells in medium layer of a
carotid plaque. VEGF-positive cells showing red with TRITC. SMA-positive
cells showing green with FITC. Some of fusiform cells are identified both
positive.
Western blotting
Western blot analysis of electrophoresed carotid plaque extract and carotid wall lysates reacted with VEGF antibody revealed distinct VEGF expression in all carotid plaques. In every carotid plaque extract, the Western blot showed a significant band, at approximately 45kD, which may correspond to VEGF165 homodimers. The all nonsclerotic carotid wall lysates did not show any VEGF expression (Figure 4).
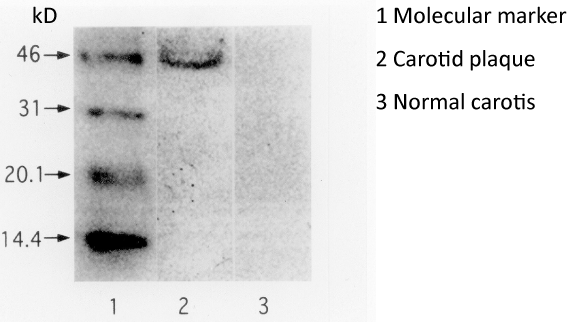
Figure 4: Western blot analysis of electrophoresed carotid plaque extract and
carotid wall lysate reacted with VEGF antibody. Distinct VEGF expression is
identified in a carotid plaques extract. The Western blots showed a significant
band at 45kD, which may correspond to VEGF165 homodimers. The nonsclerotic
carotid wall lysates did not show any VEGF expression. Markers 46,
31, 20.1, and 14.4 indicate molecular weight in kilodaltons.
Discussion
A previous Immunohistochemical study with anti-VEGF polyclonal antibody revealed that the VEGF expression in pathological vessel walls was present in activated T cells in macrophage-rich areas and that its expression in normal vessels was found in smooth muscle cells and in the vasa vasorum [13]. Our immunoblot result revealed that atherosclerotic carotid plaques contained a high level of VEGF, while VEGF expression was not detected on immunoblot probably because of little VEGF in normal carotid artery or limited sensitivity of the immunoblot. The Immunohistochemical studies showed that most foam cells and some SMCs distinctly expressed VEGF in sclerotic carotid plaques but that nonsclerotic carotid walls scarcely contained the factor. In addition, foam cells around intraplaque hemorrhage and newly-formed vessels showed distinct VEGF expression and were mostly identified as a macrophage origin. Therefore, our results for the carotid artery are similar to those for other large pathological vessels. Since expression of VEGF followed by hypoxia-inducible factor-1 a is enhanced under the hypoxic condition [17,18], we propose that the expression and secretion of VEGF in foam cells which originate from macrophages and SMCs are promoted in the hypoxic core of the sclerotic carotid plaque. With progression of atherosclerosis, SMCs transform and wander in the vessel wall. SMCs in slightly sclerotic areas where the wandering of SMCs was not found also showed distinct VEGF expression. The different patterns of VEGF expression between highly and slightly sclerotic areas may be possibly due to various VEGF functions. It is likely that VEGF functions to maintain or restore the vessels in normal to slightly sclerotic vessel walls, whereas it promotes atherosclerosis in moderately to highly sclerotic vessel wall.
It is possible that the newly-developed vessels may easily rupture, resulting in intraplaque hemorrhage, which would lead to rapid progression of carotid stenosis and plaque disruption. VEGF secreted from foam cells in sclerotic plaques may induce intraplaque angiogenesis, leading to newly-formed vessels. This intraplaque vascularization may stimulate further carotid atherosclerosis. These carotid plaque changes can cause cerebral ischemic lesions. Therefore, we suppose that VEGF may play an important role in carotid intraplaque neovascularization as one of the various angiogenesis factors.
Other angiogenesis factors such as basic Fibroblast Growth Factor (bFGF), [22,23] platelet-derived growth factor, insulin-like growth factor-1[24], transforming growth factor-ß 1[25], soluble vascular cell adhesion molecule-1[26], interleukin-8, and tumor necrosis factor-a[27] are less specific for endothelial cells than VEGF and also less able to permeate membranes than it. VEGF also promotes extravasation of plasma proteins, resulting in the formation of extra vascular fibrin deposition. Since the above factors are not so pronounced as VEGF under hypoxic conditions, our findings in carotid plaques support our contention that VEGF is one of the most important angiogenesis factors in atherosclerotic carotid plaques. However, since bFGF expression was identified in smooth muscle cells in normal media and in the endothelium of new vessels, [25,28] we suppose that bFGF may also play an important role in atherosclerotic angiogenesis of the factor along with VEGF. Basic FGF does not contain a signal peptide for secretion into the extracellular environs, whereas VEGF does have one [29]. These facts probably suggest that VEGF may play a more important role than bFGF in intraplaque angiogenesis. An experimental trial of VEGF gene transfer into the rabbit carotid wall using the Sendai virus vector demonstrated a thickened wall containing newly-formed vessels in the intima [30]. This model also supports our view that VEGF may promote angiogenesis in carotid plaque. If VEGF plays a critical role in the carotid atherosclerosis, anti-VEGF drug may be effective against the progression of carotid arteriosclerosis.
Finally, there are two limitations in this study. First, although the histological assessment was performed on the lesion with maximum stenosis, the location of intraplaque hemorrhage may not be always at this part because there were variations in carotid plaques. Second, some artifacts after the removal of plaque specimens were not completely excluded.
Conclusion
In conclusion, VEGF expression is induced in the deep portion of the atherosclerotic carotid plaques. It may play an important role in the growth of atherosclerotic carotid plaques and induction of intraplaque hemorrhage causing ischemic stroke.
Acknowledgment
This study was supported by a Grant-in-Aid (No.11671375 ) from the Ministry of Education, Science, Sport and Culture of Japan.
References
- Imparato AM, Riles TS, Gorstein F. The carotid bifurcation plaque: pathologic findings associated with cerebral ischemia. Stroke. 1979; 10: 238-245.
- Lusby RJ, Ferrell LD, Ehrenfeld WK, Stoney RJ, Wylie EJ. Carotid plaque hemorrhage. Its role in production of cerebral ischemia. Arch Surg. 1982; 117: 1479-1488.
- Fisher M, Blumenfeld AM, Smith TW. The importance of carotid artery plaque disruption and hemorrhage. Arch Neurol. 1987; 44: 1086-1089.
- von Maravic C, Kessler C, von Maravic M, Hohlbach G, Kömpf D. Clinical relevance of intraplaque hemorrhage in the internal carotid artery. Eur J Surg. 1991; 157: 185-188.
- Park AE, McCarthy WJ, Pearce WH, Matsumura JS, Yao JS. Carotid plaque morphology correlates with presenting symptomatology. J Vasc Surg. 1998; 27: 872-878.
- Lindsay AC, Biasiolli L, Lee JM, Kylintireas I, MacIntosh BJ, Watt H, et al. Plaque features associated with increased cerebral infarction after minor stroke and TIA: a prospective, case-control, 3-T carotid artery MR imaging study. JACC Cardiovasc Imaging. 2012; 5: 388-396.
- Sun J, Underhill HR, Hippe DS, Xue Y, Yuan C, Hatsukami TS. Sustained acceleration in carotid atherosclerotic plaque progression with intraplaque hemorrhage: a long-term time course study. JACC Cardiovasc Imaging. 2012; 5: 798-804.
- Turc G, Oppenheim C, Naggara O, Eker OF, Calvet D, Lacour JC, et al. HIRISC study investigators. Relationships between recent intraplaque hemorrhage and stroke risk factors in patients with carotid stenosis: the HIRISC study.Arterioscler Thromb Vasc Biol. 2012; 32: 492-499.
- Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989; 246: 1306-1309.
- Plate KH, Breier G, Weich HA, Risau W. Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature. 1992; 359: 845-848.
- Ferrara N, Winer J, Burton T. Aortic smooth muscle cells express and secrete vascular endothelial growth factor. Growth Factors. 1991; 5: 141-148.
- Berse B, Brown LF, Van De Water L, Dworak HF, Senger DR. Vascular permeability factor (vascular endothelial growth factor) gene is expressed differentially in normal tissues, macrophages, and tumors. Mol Biol Cell. 1992; 3: 211-220.
- Couffinhal T, Kearney M, Witzenbichler B, Chen D, Murohara T, Losordo DW, et al. Vascular endothelial growth factor/vascular permeability factor (VEGF/VPF) in normal and atherosclerotic human arteries. Am J Pathol. 1997; 150: 1673-1685.
- Inoue M, Itoh H, Ueda M, Naruko T, Kojima A, Komatsu R, et al. Vascular endothelial growth factor (VEGF) expression in human coronary atherosclerotic lesions: possible pathophysiological significance of VEGF in progression of atherosclerosis. Circulation. 1998; 98: 2108-2116.
- Papalambros E, Sigala F, Georgopoulos S, Panou N, Kavatzas N, Agapitos M, et al. Vascular endothelial growth factor and matrix metalloproteinase 9 expression in human carotid atherosclerotic plaques: relationship with plaque destabilization via neovascularization. Cerebrovasc Dis. 2004; 18: 160-165.
- Migdalski A, Kotschy M, Jawien A. Tissue factor, tissue factor pathway inhibitor and vascular endothelial growth factor-A in carotid atherosclerotic plaques. Eur J Vasc Endovasc Surg. 2005; 30: 41-47.
- Sluimer JC, Gasc JM, van Wanroij JL, Kisters N, Groeneweg M, Sollewijn Gelpke MD, et al. Hypoxia, hypoxia-inducible transcription factor, and macrophages in human atherosclerotic plaques are correlated with intraplaque angiogenesis. J Am Coll Cardiol. 2008; 51: 1258-1265.
- Higashida T, Kanno H, Nakano M, Funakoshi K, Yamamoto I. Expression of hypoxia-inducible angiogenic proteins (hypoxia-inducible factor-1alpha, vascular endothelial growth factor, and E26 transformation-specific-1) and plaque hemorrhage in human carotid atherosclerosis. J Neurosurg. 2008; 109: 83-91.
- Hiyama T, Tanaka T, Endo S, Komine K, Kudo T, Kobayashi H, et al. Angiogenesis in atherosclerotic plaque obtained from carotid endarterectomy: association between symptomatology and plaque morphology. Neurol Med Chir. (Tokyo). 2010; 50: 1056-1061.
- Pelisek J, Well G, Reeps C, Rudelius M, Kuehnl A, Culmes M, et al. Neovascularization and angiogenic factors in advanced human carotid artery stenosis. Circ J. 2012; 76: 1274-1282.
- Zemplenyi T, Crawford DW, Cole MA. Adaptation to arterial wall hypoxia demonstrated in vivo with oxygen microcathodes. Atherosclerosis. 1989; 76: 173-179.
- Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992; 359: 843-845.
- Hughes SE, Crossman D, Hall PA. Expression of basic and acidic fibroblast growth factors and their receptor in normal and atherosclerotic human arteries. Cardiovasc Res. 1993; 27: 1214-1219.
- Cuevas P, Gonzalez AM, Carceller F, Baird A. Vascular response to basic fibroblast growth factor when infused onto the normal adventitia or into the injured media of the rat carotid artery. Circ Res. 1991; 69: 360-369.
- Pepper MS, Vassalli JD, Orci L, Montesano R. Biphasic effect of transforming growth factor-beta 1 on in vitro angiogenesis. Exp Cell Res. 1993; 204: 356-363.
- Koch AE, Halloran MM, Haskell CJ, Shah MR, Polverini PJ. Angiogenesis mediated by soluble forms of E-selectin and vascular cell adhesion molecule-1. Nature. 1995; 376: 517-519.
- Szekanecz Z, Shah MR, Harlow LA, Pearce WH, Koch AE. Interleukin-8 and tumor necrosis factor-alpha are involved in human aortic endothelial cell migration. The possible role of these cytokines in human aortic aneurysmal blood vessel growth. Pathobiology. 1994; 62: 134-139.
- Brogi E, Winkles JA, Underwood R, Clinton SK, Alberts GF, Libby P. Distinct patterns of expression of fibroblast growth factors and their receptors in human atheroma and nonatherosclerotic arteries. Association of acidic FGF with plaque microvessels and macrophages. J Clin Invest. 1993; 92: 2408-2418.
- Dvorak HF, Brown LF, Detmar M, Dvorak AM. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol. 1995; 146: 1029-1039.
- Yonemitsu Y, Kaneda Y, Morishita R, Nakagawa K, Nakashima Y, Sueishi K. Characterization of in vivo gene transfer into the arterial wall mediated by the Sendai virus (hemagglutinating virus of Japan) liposomes: an effective tool for the in vivo study of arterial diseases. Lab Invest. 1996; 75: 313-323.