Abstract
The moisture isotherm of fruits can be determined at various temperatures range. The equilibrium moisture content of dehydrated fruits increased sharply as the temperature increased. Adsorption isotherm curves of dehydrated fruit showed the characteristics of a type between II-III shapes. More starch contains fruits show type II isotherm curves whereas high sugar contains fruits show type III isotherm. This phenomenon can further be explained by temperature dependent constants of the GAB isotherm model. Some dehydrated fruits have higher monolayer moisture content and specific active surface area, which could be related to their physico-chemical composition, but both decreased with increase in temperature. The net isosteric heat of sorption was higher in more starch containing dehydrated fruits than high sugar content fruits possibly due to different physico-chemical and structural composition with regards to their sorption characteristics.
Keywords: Fruit; Monolayer moisture; GAB; Isosteric heat; Temperature
Abbreviations
aw: Water Activity; C, K: GAB Model Parameters; co, ko: Entropic Accommodation Factors; d.b.: Dry Basis (%); e: Temperature Dependence Constant of Monolayer (K); EMC: Equilibrium Moisture Content (g/100 g dry matter); ERH: Equilibrium Relative Humidity (%); g: Monolayer And Multiplayer Water Enthalpy Difference (K); GAB: Guggenheim-Anderson-Boer Equations; hi: Molar Sorption Enthalpy of Free Water (J mol-1); hm: Monolayer Sorption Enthalpy (J mol-1); hn: Multilayer Molar Sorption Enthalpy (J mol-1); i: Multilayer And Free Water Enthalpy Difference (K); m: Moisture Content (g/100 g dry matter); M: Moisture Content (% d.b.); ma: Temperature Dependence Of Monolayer (K); mo: Monolayer Moisture Content (g/100 g dry matter); qs: Isosteric Heat Of Sorption (J/mol-1); R: Universal Gas Constant (8.314 J mol-1 K-1); r.h.: Relative Humidity (%); S: Active Specific Surface Area (m2 g-1); SD: Standard Deviation; T: Absolute Temperature (K); TSS: Total Soluble Solids Content (oBrix).
Introduction
Dehydration is an important preservation technique with the primary objective of reducing microbial activity, product deterioration and extension of shelf-life during storage. Most fruits contain enough moisture to induce the microbial growth and activity of natural enzymes but by drying can reduce aw and prevent microbial and enzymatic spoilage. Some physical changes may occur in fruit drying operation such as shrinkage, puffing, sugar crystallization, gelatinization of starch and glass transitions etc. In some cases, desirable or undesirable chemical or biochemical reactions may also occur. Structural collapse in fruits due to moisture removal from the fruits product results significant changes in texture [1]. Therefore, reduction of aw in the final product is a very important way to ensure the stability of the dried or dehydrated foods. Dehydrated fruits with sufficient low aw ensure the both low enzymatic activity and microbial spoilage. In general, recommended aw of most dehydrated fruits is relatively <0.6 for safe storage [2]. However, expose to high drying temperature and significant loss of moisture in fruits cause irreversible stresses in the cellular structure of the fruits and may leads to robust textural changes especially during storage.
The properties such as structure, texture and storage time of a dehydrated fruits depend on the moisture adsorption characteristics and are useful in design and modeling of drying, aeration and storage processes. The relationship between aw or ERH and EMC of a product is described by the moisture sorption isotherms. Each commodity has a unique set of isotherms and these isotherms are useful to determine the lowest moisture content attainable under the specific drying or storage conditions [3-8]. The monolayer moisture content (mo) is also recognized as the optimum moisture content for good storage stability and to maintain the overall quality of dehydrated products [3]. The shape of the sorption isotherm curve has resulted due to the physical adsorption of moisture into fine porous structure of the food [9]. In general, type II and III isotherms (Brunauer’s classification) show a lower water sorption with an increase in temperature at constant aw [4,10], indicating loss of hygroscopicity of the fruithigher temperature. During dehydration, structure and physico-chemical nature of the food materials such as starch may undergo in partial gelatinization process [11] and sugar may undergo crystallization at high temperature.
Adsorption Isotherm
The moisture adsorption may reduce significantly due to reduction in moisture affinity sites within the tissue with increasing temperature. The aw of the dehydrated fruits showed a decrease with increasing temperature, which can be explained with the help of a thermodynamic equation. Isotherm behavior characteristics of sugars and sugar containing dehydrated fruits have been reported by several authors [11-18]. The sharp increase in moisture content of dehydrated fruits could be explained by the solubility of sugars and starcheincreasing aw (equilibrium) under constant temperature (Figure 1). Adsorption isotherms of many dehydrated fruits that contain high TSS intersect at aw between 0.5 and 0.8 with increasing storage temperature [10,12,13,16,19,20].
At low moisture, fruits contain high TSS are hygroscopic and tends to absorb large amount water at high aw and temperature as results of solubilization of sugars [20]. Another reason may associate to high cellulosic and capillary micro-structure of the dehydrated fruits can retain more water within the fruit matrix due to high surface tension [10,14,21]. Therefore, creation of a transient capillary structure along with an increase the number of active binding Site (S) inside the micropores structure of the fruits which helps to adsorb more water [22]. However, at very low moisture content or under very low aw levels, the transient capillary structure may not play an important role in moisture adsorption.
Modeling of the Sorption Isotherms
Although there are several mathematical models to describe the moisture sorption characteristics, six parameter Guggenheim- Anderson-de Boer equation [23] known as GAB model was frequently used best model to analyze the sorption isotherms of fruits (equation 1) for many raw or process biological materials [11-18, 23-28].
It has been shown that parameters K of the GAB model must be in
the range of 0
According to the above equations, g and i values are highly depend on the sorption enthalpy values. Therefore, Arrhenius constants of g and i show the liner relationship with heat of sorption enthalpy values where g = hm-hn/R and i = hi-hn/R.
Therefore, comprehensive temperature dependent six-parameter GAB model fitted more accurately over the wider range of awand the temperature between 0.11-0.9 and 25-60oC, respectively [11]. The GAB model has been used successfully to explain the moisture sorption behavior of many foods [15,16,19,25,28,30,31]. The difference in the degree of suitability of moisture sorption models for the different fruits could be due to inherent differences in the fruits particularly in relation to their biochemical composition, processing conditions [19] and the method of dehydration (Table 1).
Drying method
mo (g/100g)†
Temperature (oC)
qs (kJ/mol-1)‡
S (m2g-1)*
Reference
Osmotically dehydrated fruits
Apples
11
30-50
-
388.3
33
Raisins
14
15-60
10.6
494.2
19
Currents
17.3
15-60
5.7
610.7
19
Prunes
12.6
15-60
18.5
444.8
19
Apricot
11.7
15-60
14.8
413.01
19
Pineapple
7.3-6.8
20-40
-
257.7-219.0
16
Freeze dried fruits
Raspberry
7.4
23
-
261.2
34
Strawberry
5.1
30
-
180
34
Kiwi
4.7
30
165.9
34
Blueberries
17.4
Apr-45
13.8
614.2
35
Passion
6.4-6.3
20-50
40
36
Cabinet dried fruits
Raisins
2.75
15-60
20
97.07
19
Figs
9.7
15-60
22.5
342.4
19
Figs
4.27
15-60
13.2
150.7
15
Prunes
4.54
15-60
20
160.3
15
Apricot
4.13
20-40
20
145.8
15
Apple
6.21
15-45
12.3
219.2
15
Banana
27.6
40-70
16
974.3
15
Papaya
13.8-6.2
May-45
57.35
487.1-219.0
37
Solar dried fruits
Mango
10.0-11.4
50-30
19.5
353-402.4
11
Jackfruit
3.6-5.0
50-30
33
127.1-176.5
11
Vacuum dried fruits
Mango
3.15
26-50
20
111.2
21
Passion
6.4-6.3
20-50
37
36
Table 1: Effect of drying method on monolayer moisture content and isosteric heat of sorption different dehydrated fruits derived from GAB model.
Shape of the Sorption Isotherm
The sorption isotherms of fruits obtained characteristic type either
II or III isotherms with agreement of GAB classification (Figure 1). If
parameter C = 2, the GAB equation gives a sigmoidal shape curve
with a point of type II inflection [25]. Lewicki [29] reported that GAB
model describes well sigmoidal type II isotherms when parameters K
and C are kept in the rages of 0.24
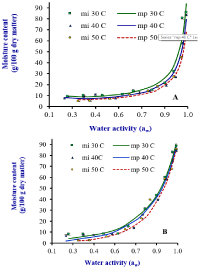
Figure 1: Type II and III moisture adsorption isotherms of dehydrated jackfruit
(A) and mango (B), respectively, at 30, 40 and 50oC. mi is experimental
moisture content and mp is predicted moisture content. Source: Prasantha
and Amunogoda [11].
Monolayer Moisture Adsorption
The mo is the minimum moisture content covering the active hydrophilic binding site on the material and it is necessary information for achieving maximum storage period with minimum quality loss of dehydrated fruits. The mo was calculated using the GAB model and it is a constant of the model. Generally, the mo value decreased with the increase in temperature. According to the reported data GAB constant values of mo content in the rage of 2.7-17.0 g/100 g (d.b.) for many dehydrated fruits in the isothermal range of 15-70oC (Table 1). These differences of mo of fruits could be caused by differences in drying techniques and physico-chemical composition of different varieties of fruits.
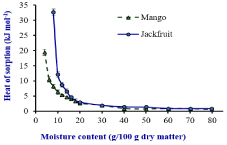
Figure 2: Net Isosteric heat of sorption vs moisture content (g/100g dry
matter) of the dehydarated Mango and Jackfruit. Source: Prasantha and
Amunogoda [11].
In general, fruits that contain high amount of amorphous sugars in the tissue matrix have high affinity to surface adsorption of moisture that comes into direct contact with the fruit matrix. Quirijns et al. [30] and Togrul and Arslan [27] have reported that reduction of total number of S as a result of physical or chemical changes in the existing porous structure of the products. This phenomenon can further be explained by using S of active binding site (Table 1). The active binding sites of S of dry matter, could be determined by S = mo × 3.53 × 103 m2 g-1 (3.53 × 103 = Avogadro number×surface area of a water molecule/molar weight of water). The total S available for hydrophilic binding in adsorption decreased with increasing temperature. The large S of active binding sites was attributed to the existence of many intrinsic micro-nano porous structures of dehydrated fruits and dehydrated fruits that contain high TSS [9,27,32] such as osmotically dehydrated, freeze drayed and solar dehydrated fruits (Table 1).
The Isosteric Heat of Moisture Sorption
Net isosteric heat of sorption qs (J/mol-1) is the amount of energy required to remove water from the products in excess of the energy required for free water evaporation. Clausisus-Clapeyron equation (5) is used to calculate qs. The value of qs is calculated from the slope of the linear regression line plotted between ln (aw) and 1/T at constant moisture [27,33-38].
The value of qs is generally depending on the physico-chemical and structural composition of the materials with regards to their sorption characteristics (Figure 2). The very high qs at low moisture content was an indication of strong water-starch component interactions in the dehydrated fruits (Table 1).
At low moisture content, moisture is adsorbed at the strongest binding sites on the active surface of the solids perhaps to the surface of Nano size capillaries [32]. As moisture increases, the food material tends to swells and opening up new sites for moisture to bind in the food. Therefore, low level of the heat of sorption required at high moisture content in the food materials [22,27,38] than low moisture containing dehydrated fruits.
Acknowledgement
The authors thanks to Faculty of Agriculture, University of Peradeniya.
References
- Yan Z, Sousa-Gallagher MJ, Oliveira FAR. Shrinkage and porosity of banana, pineapple and mango slices during air-drying. Journal of Food Engineering. 2008; 84: 430-440.
- Ong S P, Law C L. Hygrothermal properties of various foods, vegetables and fruits. Jangam SV, Law C L, Mujumdar AS, editors. In: Drying of foods, vegetables and fruits, Singapore. 2010; 31-58.
- Labuza TP, Kannane A, Chen J. Effect of temperature on the moisture sorption isotherm and water activity shift of two dehydrated foods. Journal of Food Science. 1985; 50: 385-392.
- Sun DW. Comparison and selection of EMC/ERH isotherm equations for rice. Journal of Stored Products Research. 1999; 35: 249-264.
- Basunia MA, Abe T. Moisture desorption isotherm of medium- grain rough rice. Journal of Stored Products Research. 2001; 37: 205-219.
- Singh B, Panesar PS, Gupta AK, Kennedy JF. Sorption isotherm behavior of osmo convectively dehydrated carrot cubes. Journal of Food Processing and Preservation. 2006; 30: 684-698.
- Yanniotis S, Blahovec J. Model analysis of sorption isotherm. LWT- Food Science and Technology. 2009; 42: 1688-1695.
- Sato Y, Wada Y, Higo A. Relationship between monolayer and multilayer water contents, and involvement in gelatinization of baked starch products. Journal of Food Engineering. 2010; 96: 172-178.
- Ngoddy PO, Bakker-Arkema FW. A generalized theory of sorption phenomena in biological materials (Part I. The isotherm equation). Transactions of the ASAE. 1970; 13: 612-617.
- Mir AM, Nath N. Sorption isotherms of fortified mango bars. Journal of Food Engineering. 1995; 25: 141-150.
- Prasantha BDR, Amunogoda PNRJ. Moisture adsorption characteristics of solar dehydrated mango and jackfruit. Food and Bioprocess Technology: An International Journal. 2013; 6: 1720-1728.
- Saravacos GD, Tsiourvas DA, Tsami E. Effect of temperature on the water adsorption isotherms of sultana raisins. Journal of Food Science. 1986; 51: 381-383.
- Verma VK, Narain M. Moisture absorption isotherms of jaggery. Journal of Stored Product Research. 1990; 26: 61-66.
- Hubinger M, Menegalli FC, Aguerre RJ, Suarez C. Water vapor adsorption isotherms of guava, mango and pineapple. Journal of Food Science. 1992; 57: 1405-1407.
- Singh PC, Singh RK. Application of GAB model for water sorption isotherms of food products. Journal of Food Processing and Preservation. 1996; 20: 203-220.
- Falade KO, Olukini I, Adegoke GO. Adsorption isotherm and heat of sorption of somatically pretreated and air-dried pineapples slices. European Food Research Technology. 2004; 218: 540-543.
- Pavan MA, Schmidt SJ, Feng H. Water sorption behavior and thermal analysis of freeze-dried, Refractance Window-dried and hot-air dried açaí (Euterpeoleracea martius) juice. LWT-Food Science and Technology. 2012; 7: 75-81.
- Caballero-Cerón C, Serment-Moreno V, Velazquez G, Torres JA, Welti-Chanes J. Hygroscopic properties and glass transition of dehydrated mango, apple and banana. Journal of Food Science and Technology. 2018; 55: 540-549.
- Tsami E, Marinos-Kouris D, Maroulis Z B. Water sorption isotherms of raisins, currants, figs, prunes and apricots. Journal Food Science. 1990; 55: 1594-1597.
- Hayoglu I, Gamli OF. Water sorption isotherms of pistachio nut paste. International Journal of Food Science and Technology. 2007; 43: 224-227.
- Isse MG, Schuchmann H, Schubert H. Divided sorption isotherm concept: An alternative way to describe sorption isotherm data. Journal of Food Process Engineering. 1993; 16: 147-157.
- Aguerre RJ, Suarez C, Viollaz PE. Swelling and pore structure in starchy materials. Journal of Food Engineering. 1989; 9: 71-80.
- Van den Berg C. Description of water activity of food for engineering purposes by means of the GAB model of sorption. Mckenna BM, editor. In: Engineering and Food. Elsevier Applied Science, London. 1984; 211-311.
- Blahovec J. Sorption isotherms in materials of biological origin mathematical and physical approach. Journal of Food Engineering. 2004; 65: 489-495.
- Blahovec J, Yanniotis S. GAB generalized equation for sorption phenomena. Food and Bioprocess Technology. 2008; 1: 82-90.
- Kiranoudis CT, Maroulis ZB, Tsami M, Marinos-Kouris D. Equilibrium moisture content and heat of desorption of some vegetables. Journal of Food Engineering. 1993; 20: 55-74.
- Togrul H, Arslan N. Moisture sorption behaviour and thermodynamic characteristics of rice stored in a chamber under controlled humidity. Biosystems Engineering. 2006; 95: 181-195.
- Bellagha S, Sahli A, Farhat A. Desorption isotherms and isoseteric heat of three tunisian date cultivars. Food and Bioprocess Technology. 2008; 1: 270-275.
- Lewicki PP. The applicability of the GAB model to food water sorption isotherms. International Journal of Food Science and Technology. 1997; 32: 553-557.
- Quirijns EJ, JB van Boxtel A, van Loon WKP, van Straten G. Sorption isotherms, GAB parameters and isosteric heat of sorption. Journal of the Science of Food and Agriculture. 2005; 85: 1805-1814.
- Palipane KB, Droscoll RH. Moisture sorption characteristics of in-shell macadamia nuts. Journal of Food Engineering. 1992; 18: 63-76.
- Ngoddy PO, Bakker-Arkema FW. A generalized theory of sorption phenomena in biological materials. Part III-A pore size distribution function of the power law type for biological materials. Transactions of the ASAE. 1972; 15: 1160-1164.
- Bellagha S, Sahli A, Zid MB, Farhat A. Desorption isotherms of fresh and osmotically dehydrated apples (Golden delicious). Revue des Energies Renouvelables. 2008; 8: 45-52.
- Syamaladevi RM, Sablani SS, Tanga J, Powers J, Swanson BG. Water sorption and glass transition temperatures in red raspberry (Rubusidaeus). Thermochimica Acta. 2010; 503-504: 90-96.
- Lim LT, Tang J, He J. Moisture sorption characteristics of freeze dried blueberries. Food Science. 1995; 60: 810-814.
- Pedro MAM, Telis-Romero J, Telis VRN. Effect of drying method on the adsorption isotherms and isosteric heat of passion fruit pulp powder. Ciência e Tecnologia de Alimentos. 2010; 30: 993-1000.
- Vega-Gálvez A, Palacios M, Lemus-Mondaca RA. Moisture sorption isotherms and isosteric heat determination in Chilean papaya (Vasconcellea pubescens). Quimica Nova. 2008; 31: 1417-1421.
- Tsami E, Maroulis ZB, Marinos-Kouris D, Saravacos GD. Heat of sorption of water in dried fruits. International Journal of Food Science & Technology. 1990; 25: 350-359.
