Abstract
Hyperkalemic emergency can result from diseases such as chronic kidney disease or related to an adverse event due to commonly prescribed medications. Angiotensin converting enzyme inhibitors and heparin are such examples - both block the Renin-Angiotensin-Aldosterone System (RAAS). Some of the most serious manifestations of hyperkalemia are muscle weakness/paralysis, sinus bradycardia, ventricular fibrillation, and asystole. Prompt recognition through patient history, EKG changes, and laboratory data is of utmost importance when hyperkalemia is suspected. We present a unique case of a patient developing rare, resistant hyperkalemia secondary to heparin use prophylactically. Hyperkalemia was subsequently treated per standard protocol which included Sodium Polystyrene Sulfonate (SPS, or commonly known as Kayexalate), leading to catastrophic ischemic colitis. Ultimately, the hyperkalemia was not resolved until medications were reviewed and changed appropriately.
Keywords: Sodium Polystyrene Sulfonate; Kayexalate; Sorbitol; Ischemic colitis; Hyperkalemia; Heparin
Introduction
Resistant hyperkalemia secondary to heparin is a rare medication complication due to aldosterone being blocked from the mineralocorticoid receptor in the distal tubule and collecting duct of the nephron [1]. Aldosterone blockage prevents the exchange of sodium (Na+) for potassium (K+) leading to hyperkalemia. In a study by Oster et al., hyperkalemia resulting from heparin therapy was reported in about 7% of patients with serum potassium ranging from 5.8 to 8.3 mEq/L. Hyperkalemic emergency is defined as serum potassium >6.5mEq/L or symptoms such as muscle paralysis or cardiac arrhythmias. As for hyperkalemia management, while there are subtle institutional differences, most protocols include the use of Kayexalate (Sodium Polystyrene Sulfate, SPS) as part of the treatment regimen. Review of recent literature shows that treatment with SPS can lead to ischemic colitis. A comprehensive literature review demonstrates at least thirty cases of Kayexalate related ischemic colitis.
Case Presentation
A 75-year-old female was admitted for constipation and treated with stool softener and MiraLAX. Heparin was also administered for DVT prophylaxis per facility standard protocol. Subsequently, she was noted to have persistent hyperkalemia at 6.9mEq/L (ref. 3.5-5mEq//L), and Albuterol, Insulin/Glucose, and multiple doses of SPS were administered. On day two of her hospital admission, she developed sepsis and an acute abdomen. On abdominal Computed Tomography (CT) there was prominent recto-sigmoid distention (Figures 1, 2). The patient underwent diagnostic explorative laparoscopy -this revealed ischemic colitis with necrosis of sigmoid colon and gangrenous changes visually and confirmed on pathology (Figures 2, 3). SPS was felt to be a likely contributor. She underwent subsequent laparotomy and sigmoid resection, with Hartmann’s procedure and colostomy. Meanwhile, despite appropriate medical treatment, persistent hyperkalemia was noted. Medication review revealed heparin as the only potential medication culprit, and it was discontinued. Fondaparinux was subsequently started on day 4 and 24 hours later hyperkalemia was improved to 4.7mEq/L (ref. 3.5-5mEq/L) (Table 1). The patient made a full recovery and was discharged within one week.
Discussion
Our unique case highlights two rare events that must be included in the differential diagnoses when simple, more common answers do not explain the clinical picture. While rare, both events – hyperkalemia caused by heparin and intestinal ischemia caused by SPS – occur in the context of routinely utilized medications which are otherwise thought to be relatively benign. The patient in our facility was treated with SPS which removes potassium by exchanging sodium ions for potassium ions in the intestine. This cation exchange resin is usually mixed with sorbitol, a cathartic, to avoid constipation and fecal impaction, but hypertonic sorbitol may directly damage intestinal mucosa. With this recognition, in September 2009, the US Food and Drug Administration (FDA) added a new warning of this drug. Specifically, it stated that, “Cases of colonic necrosis and other serious gastrointestinal adverse events (bleeding, ischemic colitis, perforation) have been reported in association with Kayexalate use”. Most of the cases reviewed in the FDA warning reported the “concomitant use of sorbitol” and because of this “concomitant administration of sorbitol is not recommended.” In fact, it is considered a Risk X and combination should be avoided [2]. Despite this warning, in many hospitals the premixed sorbitol–SPS preparation is the only SPS product available, with an estimated 5 million SPS doses given annually in the United States (based on an unpublished single-hospital survey result) [3,4]. Interestingly, per Sterns et al., there is no convincing evidence that SPS increases fecal potassium losses in experimental animals or humans, nor is there evidence that adding sorbitol to the resin increases its effectiveness as a treatment for hyperkalemia [3]. SPS thus may be a relatively ineffective tool to manage hyperkalemia, yet literature suggests that clinicians rely heavily on it. In one study of 1189 emergency department patients who received SPS in sorbitol for hyperkalemia, only 188 patients received other potassium-lowering therapies. The patient in our facility was given SPS mixed with water and not sorbitol, which nonetheless led to ischemic colitis.
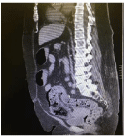
Figure 1: CT abdomen, sagittal view. Distention of the recto-sigmoid colon.
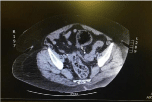
Figure 2: CT abdomen, cross sectional view.
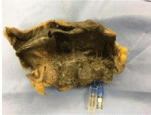
Figure 3: Intestinal necrosis with gangrenous changes.
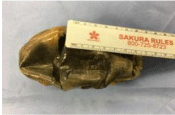
Figure 4: Intestinal necrosis with gangrenous changes.
Day 1
Day 2
Day 3
Day 4
Day 5
Day 6
Day 7
Heparin
Administered
Administered
Administered
Not administered
Not administered
Not administered
Not administered
Potassium mEq/L
6.9
5.5
6
5.4
5.3
4.7
4.4
Fondaparinux
Not administered
Not administered
Not administered
Administered
Administered
Administered
Administered
Table 1: Potassium levels with concurrent heparin and fond aparinux administration during the stay of patient.
Historically, SPS was approved by the FDA in 1962 after an uncontrolled study by Scherr et al. showed 23 of 30 cases where the plasma potassium fell by at least 0.4mEq/L in the first 24 hours. Surprisingly, there was no statistical analysis done, and the results are only significant because the authors declared them as useful anecdotally [5]. Why is this medication still a cornerstone treatment for hyperkalemia and what can be done to better educate regarding its relative efficacy and potential hazards? There is no easy answer, but more rigorous research is needed to propose safer and more effective alternative therapies and to promote a cultural change in standard practices. Incremental steps have already been taken to reduce the risk of adverse reactions -the most recent is changing the formulation from 70% sorbitol to 33% sorbitol [5]. However, serious adverse effects may still happen, and continue to be reported. Currently, Sodium Zirconium Cyclosilicate (ZS-9, ZS Pharma) is undergoing clinical trial evaluation and is not yet available for use in the United States [6]. ZS-9 was recently given a recommendation from the European Medicines. It is an orally administered, non-absorbable, and inorganic microporous compound composed of zirconium and silicate atoms with approximately 8% sodium by total weight. It acts as a sodium–potassium cation exchanger much like SPS, selectively trapping potassium ions as it moves through the GI tract. It has shown promising results with very few adverse effects reported in clinical trials with the most common being diarrhea and constipation. It was reported by Packham et al. that these events were similar between the ZS-9 and placebo group [7]. Most importantly, rigorous methods of study were used for ZS-9 compared to SPS. For example, Ash and colleagues conducted a phase 2, randomized, double-blind, placebo-controlled trial to assess the safety and efficacy of three doses of ZS-9 [8]. Kosiborod et al. led the HARMONIZE (Hyperkalemia Randomized Intervention Multi-Dose ZS-9 Maintenance) trial, a phase 3, randomized, double-blind, placebo-controlled study that evaluated the safety and efficacy of four weeks of treatment with ZS-9 in 258 ambulatory patients with hyperkalemia [9]. Packham et al. conducted a two-stage, phase 3, double-blind, randomized, placebo-controlled study to evaluate the effects of ZS-9 in 753 ambulatory patients with hyperkalemia [9]. Patiromer should also be considered as an alternative. It is a non-absorbable, cation exchange polymer that contains a calcium-sorbitol counter-ion, which increases fecal potassium excretion through binding of potassium in the lumen of the gastrointestinal tract, resulting in a reduction of serum potassium levels. There have been no reports of adverse effects secondary to sorbitol. In fact, the AMETHYST-DN trial indicates that the most common adverse events include hypomagnesemia, mild or moderate constipation, diarrhea, nausea, abdominal discomfort, or flatulence [10]. Patiromer is currently available for use in the United States.
Conclusion
Providers at the bedside should be familiar with adverse effects of commonly used medications such as heparin and SPS as seen in our case. With regards to SPS, given the lack of mounting clinical evidence and limited research historically, we suggest reconsidering SPS as a standard treatment option for hyperkalemia. While SPS has been used for many years, several new alternatives are becoming available that decrease patient risk of serious adverse effects. Recent data should be taken into consideration among clinicians and other alternative therapies exhausted before turning to therapies that are known to be potentially harmful, possibly deadly and at best, less effective. A periodic, critical review of the way in which we practice medicine is vital, particularly when patient benefit and safety is at jeopardy.
References
- Oster JR, Singer I, Fishman LM. Heparin-induced aldosterone suppression and hyperkalemia. Am J Med. 1995; 98: 575-586.
- US Food and Drug Administration: Kayexalate (sodium polystyrene sulfonate) powder.
- Richard H. Sterns, Maria Rojas, Paul Bernstein, Sreedevi Chennupati. Ion-Exchange Resins for the Treatment of Hyperkalemia: Are They Safe and Effective? JASN. 2010; 21: 5733-5735.
- Josh Batterink, Jane Lin, Sarah Hin Mui Au-Yeung, Tara Cessford. Effectiveness of Sodium Polystyrene Sulfonate for Short-Term Treatment of Hyperkalemia. Can J Hosp Pharm. 2015; 68: 296-303.
- Dennis J Malone. Taking a Second Look at Kayexalate. Hosp Pharm. 2015; 50: 959-960.
- Martin Chaitman, Deepali Dixit, and Mary Barna Bridgeman. Potassium-Binding Agents for the Clinical Management of Hyperkalemia. P T. 2016; 41: 43-50.
- Packham DK, Rasmussen HS, Lavin PT, El-Shahawy MA, Roger SD, Block G, et al. Sodium zirconium cyclosilicate in hyperkalemia. N Engl J Med. 2015; 372: 222-231.
- Ash SR, Singh B, Lavin PT, Stavros F, Rasmussen HS. A phase 2 study on the treatment of hyperkalemia in patients with chronic kidney disease suggests that the selective potassium trap, ZS-9, is safe and efficient. Kidney Int. 2015; 88: 404-411.
- Kosiborod M, Rasmussen HS, Lavin P, Qunibi WY, Spinowitz B, Packham D, et al. Effect of sodium zirconium cyclosilicate on potassium lowering for 28 days among outpatients with hyperkalemia: the HARMONIZE randomized clinical trial. JAMA. 2014; 312: 2223-2233.
- Patiromer in the Treatment of Hyperkalemia in Patients With Hypertension and Diabetic Nephropathy (AMETHYST-DN) (AMETHYST-DN). 2017; Available from: ClinicalTrials.gov, Ipswich, MA. 2017.
