Editorial
Austin J Nutr Food Sci 2014;1(1): 1003.
Extraction and Utilization of Pectin from Purple Star-Apple (Chrysophyllum cainito) and African Star-Apple (Chrysophyllum delevoyi) in Jam Production
Nwosu JN, Udeozor LO, Ogueke CC, Onuegbu N, Omeire GC and Egbueri IS
Department of Food Science and Technology, Federal University of Technology, Owerri, Nigeria
*Corresponding author: : Nwosu JN, Department of Food Science and Technology, Federal University of Technology, Owerri, P.M.B 1526, Owerri, Imo State, Nigeria
Received: December 17, 2013; Accepted: December 27, 2013; Published: December 28, 2013
Abstract
The pectin of African star apple (Chrysophyllum delevoyi) and purple star apple (Chrysophyllum cainito) were extracted using the alkaline hydrolysis method to produce pineapple jam. The jam so produced was used to compare with that of synthetic pectin which was used as a standard. From the analysis of both sensory evaluation and chemical analysis, the result showed that viscosity increased with addition of pectin to jam from 2.25– 3.01 Cp from peels of purple star apple and synthetic pectin respectively. The pH of the jam samples was generally acidic ranging from 3.58 to 3.81. The total dissolved sugar ranged from 55 to 78o Brix. The total titrable acidity varied from 27% to 34% and the ash content ranged from 3.8% to 4.2%; the ascorbic acid content varied from 8.2% to 9.2%mg/1 00g. Finally the total solid varied from 66% to 73%. Experimental result statistically, showed that there was significant difference (p≤0.05) on appearance, spread ability, mouth feel, taste and overall acceptability. However, no differences existed for texture and aroma at p≥0.05.Thus, these fruits could be successfully utilized in the production of fruit preserves.
Keywords: Purple star apple; African star apple; pectin; pineapple jam; extraction; performance.
Introduction
Purple star apple (Chrysophyllum cainito), commonly called “Udara beke” in Anambra State, “Udara Oyigbo in Imo state, “agbulag” in the North, is one of the minor fruits of the family Sapotaceae meaning star apple or gold leaf tree [1]. It has acquired a moderate assortment of regional names. In Spain, it is usually called “caimitto” or “estruella”; in France, generally, “cainute” or “caimtier”. In Colombia, it is called “caimo” or “caimo morado” (purple variety). In Argentina, “aguay” or “olivoa” [1].
African star apple (Chrysophyllum delevoyi) popularly known as Agbalumo among the Yorubas, Agwaluma among the Hausas and Udari among the Efiks and Ibibios in southern Nigeria is a fruit that is commonly eaten raw in the study area. It is a large berry that contains up to five seeds that are flat in shape. The plant belongs to the family sapotaceae. The leaves of the plant are alternate and nearly evergreen elliptic, slightly leathery. The fruit could be ellipsoid, round or pear shaped. It has a milky sweet pulp that houses the seeds. When the fruit is cut transversely it appears like asterisks in the central core or like the pointed stars. This is the origin of the name of the fruit as “STAR APPLE”. The fleshy fruit is eaten raw by most people and relished by many others.
Purple star apple, when matured and ripe was found to contain 25.95% calories, Moisture content of 33.142%, 0.901% protein. It has carbohydrate content of 5.67%, fibre content of 1.276%, 0.278%Ash, 6.6%calcium, 8.5%phosphorus and 0.262% iron. It has a reasonable content of vitamins that is 0.015% carotene, 0.031% thiamine, 0.0154% Riboflavin, 0.52% Niacin, 0.58% Ascorbic Acid. The amino acid content includes 1.547% trypthophan, 0.77% methionine and 0.851% Lysine. It has some amount of saturated fat about 0.90%. The proximate composition of African star apple includes moisturecontent: 66.7%, crude fat: 9.38%, ash: 2.12%, protein: 5.66%, crude fibre: 4.5%, carbohydrate: 78.34%; ascorbic acid: 19.68%; total metabolisable energy: 420.42 kcal as reported by [2].
Based on its widespread nutritional composition, it could be used in many areas of food. The skin which constitutes about 33% of the entire fruit could be a source of pectin which is used as gelling agent, thickener and as a stabilizer in most food and drug industries [3], thus a means of managing waste. The ripe fruit, preferably, chilled, may be cut in halves and the flesh spooned out, leaving the seed cells and core, a combination of the chopped flesh with that of mango, citrus, pineapple, other fruits and coconut water, is frozen and served chilled as Jamaican fruit salad [4]. It could be blended and squeezed to extract juice and served with ice, orange juice or gelled to produce jam or jelly [5].
Despite its food benefits, Purple star apple is highly perishable due to its high moisture content which ranges from 32–33% [1]. It is susceptible to microbial action as it can harbour a wide range of microorganisms due to the high moisture content [6,7]. It is a very seasonal and semi–wild fruit that produces during the dry (Harmattan) season [1]; it is therefore not available all year round. It is very sugary i.e. high in glucose. Due to its high sugar content, non–sugar loving consumers and diabetic patients will not readily demand the fruit for health purposes.
Also the problems associated with African star apple include that of seasonability and perishability. Owing to the above mentioned problems, the fruits could be converted to other forms to meet thedemand of consumers of all types. These forms are preserves, concentrates, wines, extraction of pectin from the fruit, which is a stabilizer. This will go a long way to solve the problems of seasonal glut, rapid spoilage, and unavailability in regions of poor and unfavourablecondition for growth of the fruits, storage and transportation, thus making the fruit not to go into extinction and increasing its utilization in many food formulations.
Pectin refers to the water soluble pectic acids with various degree of methylation (methyl ester content) and degree of neutralization which are capable of forming gels with sugar and acid in a suitab le condition [8]. It is a purified carbohydrate generally obtained from the acid extraction of the inner portion of citrus fruit peels and apple pomance [9]. As a polysaccharide it is an adhesive/firming agent in many fruits and vegetables and therefore could be used as a gelling agent in Jam and jellies [10,11].The objective of this research worktherefore is to extract pectin from purple star apple and African star to compare its performance with commercial pectin on pineapple jam.
Materials and Methods
Matured, semi–ripe purple star apples, (Chrysophyllum cainito) used for this research work were obtained from St. Thomas Acquinas Catholic Church, FUTO. Matured ripe African star apples (Chrysophyllum delevoyi) and fresh pineapples were purchased from Ekeonuwa market in Owerri Town, Imo State, Nigeria. The chemicals used were of analytical grade and were obtained from the Department of Food science and Technology, Federal University of Technology Owerri. The equipment and other materials used were obtained from the Departments of Food science and Technology and Crop science and Technology, Federal University of Technology Owerri, Imo State, Nigeria.
Figure 3: Plate 1: African Star Apple
Figure 4: Plate 2: Purple Star Apple
Sample Preparation
The star apple fruits were sorted to remove over ripe ones, deteriorated ones, and bruised ones. They were graded and washed with potable water and salt. The fruits were pulped with knife and spoon and cut into smaller pieces; the pulp was crushed with blenderfor efficient extraction of pectin from the pomace. The peels too were crushed and kept in another bowl. Also the pineapples were properly sorted and washed with water and salt. It was then peeled using kitchen knife and cut to tiny pieces. With an electric blender, the pulp was crushed properly.
Extraction of pectin
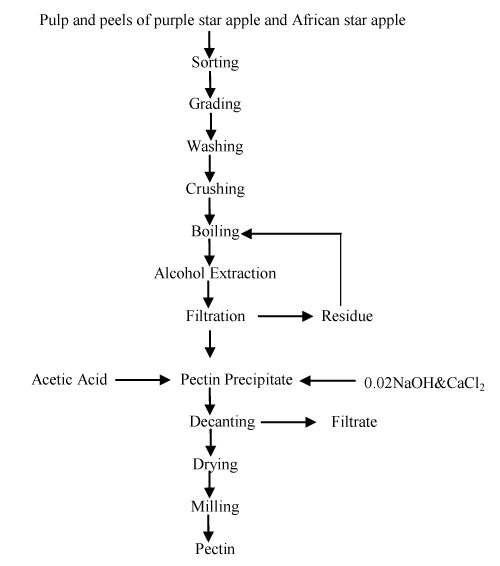
500g of the crushed sample was made up to100ml with hot water while stirring. The mixture was boiled and stirred for 15min in a water bath to disintegrate the tissues of the sample. Afterwards, hot 95% alcohol was added a little at a time till the total volume of slurry was 800ml. The mixture was stirred continuously for 2h while keeping the temperature at about 50°C until no gelatinous particles were visible. After boiling, the different slurries obtained were rapidly filtered using 80μm screen mesh. The residue was washed back into the beaker with hot water; hot 95% ethanol was added a little at a time while stirring in a water bath, for 15min.The mixture was then filtered and the filtrate was combined with the previous filtrate. The extract was cooled rapidly to a temperature below 25°C to minimize heat degradation of pectin, 50mls of 0.02NNaOH was added and the solution was allowed to stand for another 1h. [Figure 1] shows the flow chart for pectin extraction .The precipitated pectin was separated by filtration. Finally the extracted pectin was dried in a conventional Oahus oven for 6h at 35°C. The dried samples were separated to paste and stored for further use as calcium pectate.
Figure 1: Flow chart for pectin extraction
Production of Jam Using Different Pectin Sources
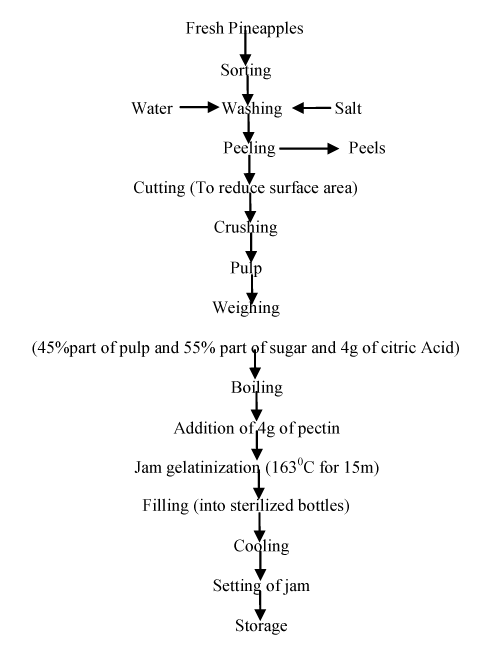
450g of pulp was weighed into a properly sterilized bowl; 550g of sugar was also weighed and dissolved in the pulp. Four grams (4g) of citric acid was added to the mixture and properly dissolved in the mix.
The mixture was then boiled for 10min while stirring constantly. Four grams (4g) of pectin which was blended with ten grams (10g) of granulated sugar (for easy dissolution of pectin in mix) was then added. It was allowed to cook for 5min while stirring constantly. At a temperature of 1630C, the jam gelatinized. (The same procedure was followed for the other pectin sources: peels and pulp of African star apple, peels of purple star apple and commercial pectin). The prepared jams were carefully poured into the sterilized jam bottles and corked immediately. The jam samples were allowed to cool for proper setting of the jam. [Figure 2] shows the flow chart for production of pineapple jam.
Figure 2: Flow chart for production of pineapple jam
Chemical Analysis
Determination of pH
The method of A.O.A.C (1990) was used.
Determination of Total Titrable Acidity
The total acidity was determined using the method of [12]. 200g of each of the samples was weighed and dissolved in 100ml distilled water. 5ml of each of the dissolved samples was titrated with 0.1N NaOH solution and 2 drops of 1% phenolphthalein was used as an indicator. The results obtained were recorded and multiplied by the factor.
Determination of Viscosity
[13] Adindu et al (2003). A cylinder of 20ml was filled with dissolved sample up to the marked level of 10ml on the cylinder. The T–shaped spindle metal was used to cover the cylinder containing the jam samples and the head end was ensured to touch the sample at the marked level. The narrow part of the spindle was screwy–fixed to the viscometer system while the bottle end of the metal cylinder was screw covered with another metal disc to another container in the viscometer to hold the metal cylinder containing the test samples. A cord connected the viscometer to a meter which converts the viscous force to centipoises unit (Cp)This was done at a particular speed and a chosen spindle number. The Pineapple jam was treated as a non Newtonian fluid.
The meter was connected to a power supply source and switched on; the viscometer began to vibrate and after 5min, the pointer kept vibrating at a steady point which indicated a reading from the viscometer scale.
The system works at a constant speed unit built in with the equipment. Viscosities of the samples were determined with equipment and readings recorded. The viscosities was calculated using the dial reading x factor = viscosity (centipioses).
Determination of Total Soluble Sugar
The refractive index of the dissolved samples was determined according to [14]. Some portions of dissolved samples were placed on an absolutely very dry and clean refractometer prism and the readingwas taken directly at 200C.
Determination of vitamin C (Ascorbic Acid)
Vitamin C (ascorbic acid) was determined by titrometric method as described by [15,16] Mazumdar and Majumder (2003) and James (1995). An amount of 10 ml/g (liquid/solid or semi solid) was taken and made volume up to 100 ml with 3% HPO3 and filtered.10 ml of filtrate was pipetted into a conical flask and titrated with the standard redox dye (2,6–dichlorophenol indophenols) in the burette. When all the ascorbic acid in the solution was used up, there was no electron available to reduce the DCPIPH and solution remained pink due to the DCPIPH. This was an indication of end point as it persisted for 10seconds. The titration reading was calculated by the following formula; Ascorbic acid (mg/100g) =TitrexDye factorxvolume made up volume of filtrate takenxwt of the sample.
Dye standardization
Diluted 5 ml of standard ascorbic acid solution with 5 ml of 3% of metaphosphoric acid. It was titrated with dye solution till pink color persists for 10 seconds. Dye factor was calculated (mg of ascorbic acid per ml of dye) as follow:
Dye Factor (D.F) = 0.5/Titration
Determination of Moisture and Ash content
The determinations of ash and moisture contents were carried out according to the methods of [17].
Determination of Total Solids
Total solids were estimated by deducting percentage moisture from hundred as described by [16].
% total solids = 100 – % moisture.
Sensory Evaluation
The sensory evaluation of the five jam samples was evaluated by a panel of twenty, randomly selected from the university community. They rated the products for overall acceptability and sensory attributes of appearance, spreadability, taste, aroma and mouthfeel on a 9–point hedonic scale where „1 represents „dislike extremely and „9
“like extremely” according to the procedure described by [18].
Statistical Analysis
Results obtained from both the chemical and the sensory evaluations were computed into means and the analysis of variance (ANOVA) was carried out based on the sensory attributes. The null hypothesis and alternative hypothesis were used to access the validity of the results.
Results and Discussion
The result of the chemical analysis on the jam samples is shown in (Table 1) below.
Sample
Viscosi
ty(Cp)
PH
TSS
(mg)
TTA
(ml)
MC
(%)
VitC
(mg)
Ash
(%)
TS
(mg)
A
2.5a
3.58a
70ab
8.4a
27c
9.2a
3.8b
73a
B
2.25b
3.71a
72a
9.2a
29b
8.7a
3.6c
71a
C
2.30ab
3.60a
67b
8.6a
30ab
8.2a
4.2a
70b
D
2.35a
3.97a
65b
9.8a
34a
8.4a
4.0a
66b
E
3.01a
3.81a
75a
9.0a
28bc
9.0a
3.9ab
72a
LSD
0.815
0.019
3.293
0.06
4.02
0.76
0.69
6.21
Table 1: Effect of different sources of pectin on the chemical properties of the jam samples
pH (Hydrogen Ion Concentration)
The pH of the jam samples ranged from 3.58 to 3.97 which showed that the jam samples were acidic. From (Table 1), it could be seen that jam sample with pectin from peels of udara (D) had the highest pH value of 3.97 while sample (A) with pectin from pulp of star apple had the lowest pH value of 3.58. The difference in pH of the samples could be from the variation in quantity of pectinic acid in the pectin from the different samples. However, since the pH values of the jam samples could be used in determining the type of pectin as well as gel firmness [19] (Fishman and Jen, 1986), it could be deduced here that pectin produced from both purple star apple and African star apple and the commercial pectin are rapid set pectin. This finding is in agreement with the work of [19] Fishman and Jen (1986) who reported that the upper limit for a successful gel set for rapid set pectin is greater or equal to 3.6. Thus, the jam set rapidly since all the values reached this level.
Total Titrable Acidity (TTA)
The total titrable acidity ranged from 8.4 to 9.8. The jam sample prepared with pectin from African star apple peel (D) gave the highestTTA value of 9.8 while the jam sample produced with pectin from pulp of African star apple (sample A) gave the least TTA value of 8.4. This implies that as the source of pectin had an effect on pH so also there is a variation in the TTA values of the jam samples due to difference in concentration of pectinic acid present in the different pectin sources.
Viscosity
The viscosity result of the jam samples ranged from 2.25Cp for sample B and D to 3.01Cp for sample E. From table I below, the viscosity of the jam samples increased with addition of pectin. This was observed during boiling of the jam while stirring the mixture. It became harder to stir after pectin was added. This was expected since pectin is commercially added to improve the consistency of jams and jellies and to ensure product of uniform quality, appearance and stability [20] (Guichard et al., 1991).
Total Soluble Sugar (TSS)
From the table I, it could be inferred that total soluble solids of the jam samples increased with decrease in moisture content. This is because pectin absorbed the moisture in the jam samples and the sugar concentration per gram increased. Sample E had the highest TSS value of 75% while sample D had the least TSS value of 65%. But sample B had the hardest gel structure with TSS value of 72%. This result agreed with standard procedure of manufacturing of jams and jellies which states that for optimum gel formation, the total soluble sugar (TSS) shou ld range between 65% and 68% [10] (Macrae et al., 1993a).
Moisture Content
The moisture content of the jam samples varied from 27% to 34%. Sample A had the least moisture content of 27% compared to D which had the highest moisture content of 34%. This implies that sample D with the highest moisture is more susceptible to spoilage than the other samples by microbial invasion especially fungi and mould [6] (Ihekoronye and Ngoddy, 1985). The variability is due to the effect of different sources of pectin on the jam samples. The water holding capacity of the pectin in the jam samples varies.
Ash Content
The ash content of the jam samples ranged from 3.6% to 4.2%.Sample C had the highest ash content value of 4.2% while sample B had the least value of 3.6% from (Table 1). Ash indicates the measure of minerals in a food commodity. The variation in ash content is due to variation in inorganic compounds especially calcium ion present in pectin extracted from different fruits.
Vitamin C (ASCORBIC ACID CONTENT)
From (Table 1), it could be observed that the vitamin C content of the jam samples varied, it ranged from 3.6 to 4.2. Sample A had the highest vitamin C content while sample E had the least vitamin C content. Vitamin C is very unstable since it oxidizes easily thus it is used as an index of quality [21] (Onyeka, 2008). Pectin, a gelling agent [22] (Eisenbrand and Schrerier, 2006), enabled the jam samples to gel fast at lesser temperatures thus preventing heat liable nutrientsfrom being lost. This explains why vitamin C was present though in small amount.
Sample
Appearance
Taste
Aroma
Spreadability
Texture
Mouthfeel
Overall acceptability
A
7.10a
7.50a
6.50a
7.05a
6.90a
7.33a
7.30a
B
6.30b
6.10b
6.10a
6.30b
6.60a
6.55b
6.50b
C
]7.20a
7.15a
6.60a
7.05a
6.95a
7.10a
7.45a
D
6.00b
6.25ab
6.20a
6.00d
6.10a
6.45b
6.60ab
E
6.75ab
6.80a
6.50a
6.15c
6.10a
6.30b
6.90a
LSD
0.70
0.83
0.80
0.79
0.85
0.74
0.71
Table 1: Mean scores for sensory parameters as judged by twenty (20) panelists
Total Solids
From table I, it can be inferred that total solids varied from 66% to 73%. Sample D had the least concentration of total solid per gram while sample A had the highest concentration of total solids per gram of the sample. The variation in the total solids is due to variation in moisture content of the samples. This variation implies that the water absorption capacity of pectin from different sources varies. Total solid refers to amount of carbohydrate, protein, crude fibre, fat and ash content of the sample. An increase in total solids implies decrease in moisture content of the sample thus increase in the nutritional composition of the jam samples.
Sensory Evaluation
The mean sensory scores of the samples produced with pectin from different sources: pulp of purple star apple, peel of purple star apple, pulp of African star apple, peel of African star apple and synthetic pectin are shown in table 2. This was analyzed in terms of appearance, taste, aroma, spreadability, texture, mouthfell and overall acceptability. There was significant difference (p≤0.05) on appearance, taste, mouthfeel, spreadability and overall acceptance. However, no significant difference (p=0.05) existed in the aroma and texture ofbe attributed to the hydrolysis of pectin, source of pectin (appearance of fruit) and caramelization during cooking, resulting in loss of natural colour and taste respectively (Fishman and Jen, 1986), since they are from the peels. There was no significant difference (p≥0.05) betweenthe jam samples in terms of aroma and texture. Absence of difference in the aroma of the sample could be due to the inherent aroma of pineapple which dominated and over shadowed other aroma in the jam samples. Absence of difference in the texture of the samples could be due to gel nature of the pectin in the jam samples. Slight difference in the result of spreadability, mouthfeel, taste, appearance and overallacceptability of the jam samples with mean scores ranging from: 7.05 to 6.00, 7.33 to 6.3, 7.5 to 6.1, 7.2 to 6.0, and 7.45 to 6.5 respectively may have resulted as a difference in degree of variation of the different sources of pectin, variation in dissolution of total solid and degree of caramelization. In general, the overall acceptance of the jam samples as judged by the panelists could be said to be above average.
It could be deduced from the results that there was an increasing trend on the chemical properties tested. For instance, the viscosity was lower on the jam with pectin from purple star apple as well as the other parameters as pH, total soluble solids, moisture contents,vitamin c. It was only the ash content that had lower value on jam with synthetic pectin while the other parameters were high on jams with pectin from purple star apple peel and those of African star apple and its feels.
Conclusion
The result obtained from this study showed that purple star apple and African star apple peels and pulps are potential pectin sources which can successfully be applied in food gel systems like fruit preserves. Thus indigenous fruits and wastes, if optimally utilized for pectin production could significantly reduce the present waste and waste– disposal problems encountered while handling wild fruits like purple star and African star apples. This will go a long way in preventing the fruit from going into extinction. It could therefore be said that pectin from African Star apple is better and can be used in Jam production than pectin from these other examined sources.
References
- Morton, J. (1987). Star apple In: Fruits of warm climates.Macmillan press publisher.Pp 408-410
- Christopher, E.A and Dosunmu, M.I. (2011). Chemical Evaluation of Proximate, Ascorbic Acid and Anti-Nutrients Content of African Star Apple (Chrysophyllum Africana) Fruit. IJRRAS 9(1): 146-149.
- Diana, S.(2010).Pectin In: Hydrocolloids in tropical fruits roots and tubers of the tropics Press publishers, 281 -287.
- Broomfield, R.N.; Ranken, M.D. and Blackie, G. (2000). Preserves in Food Industries Manual.New York press, Pp. 368-369.
- Cruess, W.V. (1966). Pectin, Jellies and marmalades, In: Commercial fruits and vegitable products. 4th ed.Pub. Mc GrawHill Book Co New York, Pp. 434-440.
- Ihekoronye, A.I. and Ngoddy, P.O. (1985). Carbohydrate In: Integrated Food Science and Technology for the Tropics. Macmillan Press publisher, Pp 10-26, 293-300.
- Carpenter, L.S. (1991).Post harvest handling of tropical fruits. 3rd ed.Macmillan press, London, Pp 82-89.
- Pederson, J.K. (1974). Pectin In: Encyclopedia of Food Science and Technology series. Johnson, A.H and Peterson, M.S.(eds). AVI pub. Co Inc Westport Connecticut, Pp.684-688.
- Meloan, C. and Pomeranz, Y. (1980). Pectin In: Food Analysis Laboratory Experiment. 2nd ed. Avi Pub. Co. Inc Westport, Connectiut U.S.A. Pp 136-137.
- Macrae, R.; Robinson, R.K. and Sadler, M.J. (1993a). Encyclopaedia of Food Science, Food Technology and Nutrition.Academic Press Publ. Ltd., London, 3:2120-2123.
- Zerk, A.C. (1985).Food Biochemistry. AVI Publ. Westport connecticut. Pp. 108-120.
- Aspinal, G.O. (1980).Chemistry of Plants. Academic press,New York NY, Pp. 473.
- Adindu, M,.N.; Williams J.O. and Adiele, E.C,. (2003). Preliminary Storage Study on African Star Apple (Chrysophyllum albidium). Plant Food for Human Nutrition Vol. 58(3) 1 - 9 .
- Awolu, O.O, Aderinola, T.A. and Adebayo, I.A. (2013). Physiochemical and Rheological behavior of African Star Apple (Chrysophyllum albidium) Juice as affected by concentration and temperature variation. J. Food Process Tech. 4(5): 1-6.
- Mazumdar, S.M. and Majumder (2003). Quality Control in the Food Chemistry. AVI Publ. Co. Inc. Westport-connecticut, U.S.A., Pp. 151-152.
- James, C.S. (1995). Agricultural Chemistry of Foods. 1st ed. Chapmani and Hall, New York.
- A.O.A.C., (1990). Official method of Analysis 15th (ed). Association of official Analytical Chemist, Washington D.C. U.S.A. Pp.223-225.
- Land, D.G. and Shepherd, R. (1988). Scaling and Ranking Methods. In "Sensory Evaluation of Foods" J. R.Piggott (Ed). Elsevier Applied Science. London.
- Fishman,G. and Jen, F. (1986). Thickening and gelling Agents for food. 1st ed. Publ. Blackies Academic and professional, London, P. 175.
- Guichard, D.W.,Leas, G. and William, S. (1991). Making jams, Marmalades, Preserves and Conserves. University of Minnesota, extension.umn.edu/distribution/nutrition/DJ1088.html.Retrieved 2008-09-20.
- Onyeka, U.E. (2008). Food and Nutrition. 2nd ed. Characteristic Forum Publications. No. 21. Mbaise Road, Owerri, Nigeria, Pp. 157.
- SEisenbrand, G. and Schrerier, P. (2006). Extraction of pectin from citrus peels and conversion of pectin to pectin acid. In:Carbohydrate chemistry. Academic Press New York 5:23-24.