Editorial
Austin J Nutr Food Sci 2014;1(1): 1005.
Phenolipids: New Generation of Antioxidants with Higher Bioavailability
Mohamed Fawzy Ramadan*
Department of Biochemistry, Faculty of Agriculture, Zagazig University, Egypt
*Corresponding author: :Mohamed Fawzy Ramadan. Faculty of Agriculture, Biochemistry Department, Zagazig University, Zagazig 44519, Egypt
Received: December 07, 2013; Accepted: December 10, 2013; Published: December 16, 2013
The effectiveness in food systems of any antioxidant depends on its chemical reactivity, interaction with food components, environmental conditions, and physical location of the antioxidant. The polarity of the environment, in particular, strongly affects antioxidant activity. This observation led to the prevailing hypothesis known as the antioxidant polar paradox: hydrophilic antioxidants are more effective in bulk oils, whereas lipophilic antioxidants are more effective in systems of high surface⁄volume ratio, such as emulsions.
Because phenolic antioxidants are highly polar, their lipophilization can potentially extend their application in oil–based foods and cosmetics and make them more efficient in emulsions [1]. Consequently, lipophilized surface–active phenolic antioxidants have been developed to improve their ability to counteract lipid oxidation in emulsions. These functionalized molecules resulting from the grafting of lipid onto a phenolic moiety are prepared via different synthesis strategies such as esterification, amidation, and etherification. Phenolic compounds have received much attention due to their biological activities. The flavonoid quercetin is found as an aglycone in many foods–including apple, tea, onion, and berries. Quercetin is anti–inflammatory and antiallergenic, and it has positive effects on cardiovascular health. However, it is generally recognized that flavonoid glycosides are poorly absorbed in the small intestine because of sugar moieties that elevate their hydrophilicity.
Several factors affect flavonoid absorption, such as the presence or absence of glycosylation on hydroxyl groups, the type of the attached sugar moiety, plant⁄food matrix and interactions with proteins, micelles, and emulsifiers. Thus, the efficiency of intestinal absorption of quercetin is strongly affected by the compound's solubility. In vitro studies revealed the health–promoting properties of quercetin. However, there is limited knowledge about the bioavailability of quercetin in humans. A recent study suggested that absorption of quercetin glycosides in grape juice is less than from pure aglycones. Little quercetin was observed in the plasma after ingestion of pure aglycone or grape juice. A combination of lipids and emulsifiers is necessary to increase quercetin absorption. Similarly, green tea catechins were absorbed to a greater extent when administered as a phospholipid complex compared to the absorption of free catechins.Therefore, quercetin dispersion in lipid micelles may be an important factor for increasing its absorption from the alimentary tract.
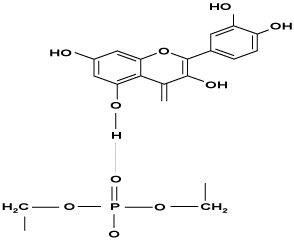
Phospholipids are constituents of cell membranes and present in many foods. Soy lecithin is a mixture of the phospholipids phosphatidylcholine, phosphatidylethanolamine, and phosphatidylinositol. Lecithin serves as an emulsifying agent in margarine, chocolate, caramels, and chewing gum. Production of cosmetics and pharmaceuticals is an example of the growing field of lecithin application as emulsifier. Antioxidant characteristics of phospholipids have been reported. Two crucial polyunsaturated fatty components of phospholipids are linoleic acid (C18:2) and linolenic acid (C18:3), and their carbon chains are the most sensitive unsaturation sites for oxidation. Thus, phospholipids are also easily oxidized, and this oxidation is likely to modify some characteristics of phospholipids. The ability of quercetin and phosphatidylcholine to form chain–like structures linked by hydrogen bonds (Figure 1) has been demonstrated with nuclear magnetic resonance spectroscopy.
Figure 1: Chain like strucutrue of quercetin linked to phosphatidylcholine
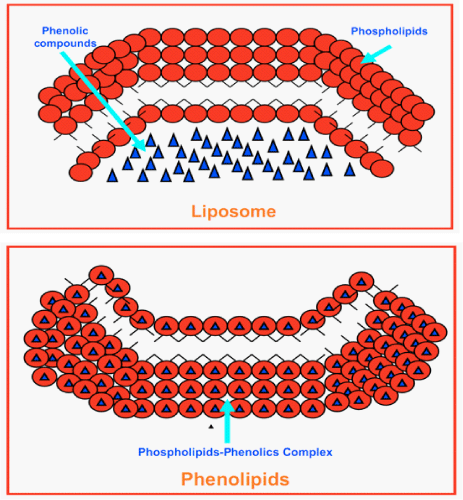
Liposomes are formed by mixing water–soluble substances with phospholipids; no chemical bonds are formed. Phenolipids, unlike liposomes, result from the reaction of phospholipids with selected phenolic compounds. They are lipophilic and freely soluble in some solvents and in fats (Figure 2).
Figure 2: Structural differences between liposomes and phenolipids
Novel phenolipid formulations (quercetin–enriched lecithin) were prepared in ethyl acetate. To study the interaction between lecithin and quercetin, the antioxidant potential of the lecithin as well as phenolipid formulations (quercetin–enriched lecithin) when added to sunflower oil (SFO) during an accelerated oxidation test were determuned. The antimicrobial and antiviral effects of the phenolipidsand lecithin were also investigated [2, 3]. The results might be applied to increase the biological activity and health impact of lecithin and phenolics in food and pharmaceutical products.
Phenolipids open potential new applications for phenolipids in the food, pharmaceutical, and cosmetic or personal care industries. We are looking forward to exciting and fruitful discussions and would like to invite you to participate and to share your opinion and results on bioavailability and functionality of Phenolipids on this occasion.
References
- Ramadan MF. Quercetin increases antioxidant activity of soy lecithin in a triolein model system, LWT-Food Sci. Technol.2008; 41:581-587.
- Ramadan MF and Asker MMS. Antimicrobial and antiviral impact of novel quercetin-enriched lecithin, J. Food Biochem.2009; 33:557-571.
- Ramadan MF. Antioxidant characteristics of phenolipids (quercetin-enriched lecithin) in lipid matrices, Ind. Crops Prod.2012; 36:363-369.