Research Article
Austin J Nutri Food Sci. 2014;2(1): 1009.
Distribution of Antioxidant Activities and Total Phenolic Contents in Acetone, Ethanol, Water and Hot Water Extracts from 20 Edible Mushrooms via Sequential Extraction
Yuxi Wang and Baojun Xu*
Department of Food Science and Technology Program, Beijing Normal University-Hong Kong Baptist University, China
*Corresponding author: :Baojun Xu, Food Science and Technology Program, Beijing Normal University-Hong Kong Baptist University United International College, China
Received: January 10, 2014; Accepted: February 10, 2014; Published: February 17, 2014
Abstract
Distribution of antioxidative properties and total phenolic content (TPC) of 20 commonly consumed edible mushrooms in four extraction solvents were investigated. The results showed that aqueous extracts (water extracts and hot water extracts) possessed much higher antioxidant properties than organic solvent extracts (acetone extracts and ethanol extracts). Among all extracts, the water extract of Agaricus subrufescens presented the best antioxidant activity. Similar as antioxidants, the TPC values of the samples extracted with water and hot water were much higher than those extracted with acetone and ethanol. In general, water extract of Ophiocordyceps sinensis exhibited the highest TPC value among all extracts.
Keywords: Antioxidant properties; Edible mushrooms; Extraction solvent; Phenolics.
Introduction
Mushrooms have been a part of human diet in many regions of the world for centuries due to their organoleptic characteristics as well as the nutritional values [1,2]. The consumption of mushrooms has even increased remarkably over the past few decades [3]. Generally, mushrooms provide low energy while usually contain large amounts of dietary fibers, proteins, vitamins and minerals [4]. Some mushrooms are even consumed for medicinal purpose as they contain valuable bioactive components, for example, Ganoderma lucidum [5]. Polysaccharides and polysaccharide–protein complexes extracted from some edible mushrooms were found to have stimulation effects on non–specific immune system and have anti–tumor properties by stimulating the defense system of the host in animal experiments [6].
It has been proven that the polysaccharides contained in cell wall of mushroom possess free radical scavenging properties [7]. In general, wild mushrooms contain significant amount of antioxidants and phenols [8]. It has been widely accepted that mushrooms are a good source of nutrients including high level of antioxidants [9]. The intake of dietary antioxidants may help the prevention of free radical damage in human body [10]. Antioxidants can scavenge free radicals through the inhibition of the initiation process or interruption of the propagation process of lipid oxidation and provide preventive function by several actions [10].
The objective of this study was to determinate the antioxidative properties and the antioxidant–related composition of phenolicsin 20 commonly consumed edible mushrooms in China. The study compared the antioxidant activities of different mushroom species and how different solvents affected the effectiveness of the extraction.
Materials and Methods
Mushroom samples
One species (Ophiocordyceps sinensis) (fruiting bodies) was produced in Shanghai Academy of Agricultural Sciences. Other mushroom samples (fruiting bodies) were purchased from several supermarkets in Zhuhai and Harbin, China. Among the samples collected, Boletus pinophilus was produced in Inner Mongolia, China; Agrocybe aegerita and Grifola frondosa were produced in Zhejiang Province, China; Pleurotus citrinopileatus and Pleurotus eryngii were produced in Fujian Province, China; Boletus edulis, Boletus luridus and Boletus aereus were produced in Wutian, Yunnan Province, China; Auricularia auricular, Tremella fuciformis, Hypsizygus tessellates, Agaricus subrufescens, Hericium erinaceus, Coprinus comatus, Phallus indusiatus, Pholiota nameko, Armillaria mellea, Hohenbuehelia reniformis and Lentinus edodes were all produced in Heilongjiang Province, China. All samples were dry mushroom and stored in a cool place before treatment. The information of mushrooms was listed in Table 1.
No.
Common name
Scientific name
Mode of production
Place of origin
1
Jew's ear
Auricularia auricula
2
Tremella
Tremella fuciformis
Cultivated
Suifenhe, Heilongjiang Province
3
Shimeji
Hypsizygus tessellatus
Cultivated
Suifenhe, Heilongjiang Province
4
Princess matsutake
Cultivated
Suifenhe, Heilongjiang Province
5
Lion's mane mushroom
Cultivated
Suifenhe, Heilongjiang Province
6
Shaggy ink cap
Cultivated
Suifenhe, Heilongjiang Province
7
Bamboo fungus
Phallus indusiatus
Cultivated
Suifenhe, Heilongjiang Province
8
Golden oyster mushroom
Pleurotus citrinopileatus
Cultivated
Gutian, Fujian Province
9
Apricot oyster mushroom
Pleurotus eryngii
Cultivated
Gutian, Fujian Province
10
Porcino
Boletus edulis
Cultivated
Wuding, Yunnan Province
11
Lurid bolete
Boletus luridus
Cultivated
Wuding, Yunnan Province
12
Porcino nero
Cultivated
Wuding, Yunnan Province
13
Caterpillar fungus
Cultivated
Shanghai
14
Nameko
Cultivated
Shangzhi, Heilongjiang Province
15
Stump mushroom
Cultivated
Shangzhi, Heilongjiang Province
16
Winter mushroom
Hohenbuehelia reniformis
Cultivated
Shangzhi, Heilongjiang Province
17
Shiitake
Lentinus edodes
Cultivated
Shangzhi, Heilongjiang Province
18
Pine bolete
Boletus pinophilus
Wild
Chifeng, Inner Mongolia
19
Poplar mushroom
Cultivated
Lishui, Zhejiang Province
20
Maitake
Grifola frondosa
Cultivated
Lishui, Zhejiang Province
Table 1: Scientific name, common name and sources of mushroom samples.
Chemicals and reagents
Absolute acetone and absolute ethanol were offered by Guangzhou Chemical Reagent Factory (Guangzhou, China). 2–Diohenyl–1– picryhydrazyl (DPPH) was supplied by Shanghai Yuanye BiologicalTechnology Co., Ltd (Shanghai, China). Folin–Ciocalteu reagent was provided by Shanghai Sanjie Biotechnology Co., Ltd (Shanghai, China). Trolox was purchased from Aldrich Co. (St. Louis, MO, U.S.A.). Sodium carbonate was supplied by Tianjin Nuoke Technology Development Co., Ltd (Tianjin, China). Gallic acid was purchased from Tianjin Damao Chemical Reagent Co., Ltd (Tianjin, China). All the chemicals were of analytical grade.
Extraction of mushroom samples
Each of the twenty dry mushroom samples was pulverized; 5.0 g of each mushroom powder was accurately weighed into a set of centrifuge tubes (each of the sample prepared in triplicate); 40 mL of absolute acetone was added into each tube for the preparation of acetone extracts. The samples were extracted for 24 hours and then centrifuged by a centrifuge (Weierkang Xiangying Centrifuge Co., Ltd, Changsha, Hunan, China) at 8000 rpm for 10 min. The supernatants were transferred into new centrifuge tubes and stored at −20 degree C. The residues were dried by vaporizing and collected; 40 mL of absolute ethanol was added into the dry residues for the preparation of ethanol extracts. The same extraction procedures were repeated to prepare water extracts as acetone and ethanol extraction and finally hot water extraction was done at 99°C in water bath for 2 hours. All the extracts were stored at −20°C in the dark for further usage.
Determination of antioxidant properties
For determination of organic extracts, 1 mL of each acetone extract, ethanol extract, blank and standard solutions was added into a pre–labeled test tube and mixed with 3 mL of DPPH solution. Fordetermination of aqueous extracts, 0.2 mL of each water extract, hot water extract, blank and standard solutions was added into a prelabeled centrifuge tube and mixed with 3.8 mL of DPPH solution. The mixtures were mixed evenly by vortexing and stood in the dark at room temperature for 20 min. Then the mixtures were centrifuged by a centrifuge (Weierkang Xiangying Centrifuge Co., Ltd, Changsha, Hunan, China) at 3000 rpm for 10 min. The absorbance of each mixture was measured by an UV–Visible spectrophotometer (TI– 1901, Beijing Purkinje General Instrument Co., Ltd, Beijing, China) at 517 nm against solvent blank. The results were expressed as Trolox equivalents (µmole of TE⁄g sample) in accordance to the standard curve of Trolox.
Determination of total phenolic content (TPC)
For determination of water extracts, 50 µL of each water extract, hot water extract, blank and standard solution was mixed with 3 mL of distilled water, 250 µL of Folin–Ciocalteu reagent and 750 µL of 7% Na2CO3 solution and the mixtures were mixed evenly by vortexing. After standing at room temperature for 8 min, 950 µL of distilled water was added into each mixture and the mixtures were allowed to stand at room temperature for 1 hour. For organic solvent extraction, 500 µL of each acetone extract, ethanol extract, blank and standard solution was mixed with 2.55 mL of distilled water, 250 µL of Folin–Ciocalteu reagent and 750 µL of 7% Na2CO3 solution. The absorbance of each mixture was measured by the UV–Visible spectrophotometer (TI– 1901, Beijing Purkinje General Instrument Co., Ltd, Beijing, China) at 765 nm by using distilled water as the blank. The total phenolic content (TPC) was expressed as gallic acid equivalents (mg of GAE⁄g sample) in accordance to the standard curve of gallic acid.
Statistical analysis
The data obtained were analyzed by ANOVA with the use of SPSS Statistics (Version 17.0, SPSS Inc., Chicago, IL, U.S.A.). Duncan's multiple range test was used to test whether there was any significant difference in antioxidant activity or total phenolic content among different mushroom species.
Result and discussion
Antioxidant properties of various extracts from mushroom
Free radical scavenging is one of the mechanisms involved in inhibiting lipid oxidation; therefore it is normally used fordetermination of antioxidant activity [11]. The DPPH free radical scavenging activity of extracts from 20 different mushrooms was tested. DPPH provides a violet color when dissolves in ethanol and the discoloration occurs when antioxidants donate protons to DPPH [11,12].
Antioxidant capacities of the 80 extracts from 20 different mushrooms with four different extraction solvents were presented in Table 2. Three mushroom species (Boletus edulis, B. luridus and B. aereus) provided a relative higher antioxidant activity for all four extraction solvents as compared to the other 17 species. Figure 1 presented antioxidant activities of various extracts from mushrooms B. edulis, B. luridus and B. aereus together with their total phenolic contents. In this study, the results showed that the extracts of genus Boletus provided higher antioxidant activities comparing tomushrooms of other genuses.
Scientific name
Acetone extracts
Ethanol extracts
Water extracts
Hot water extracts
1
Auricularia auricula
0.57 ± 0.02g
0.35±0.02i
2.42±0.09h
2.18±0.08j
2
Tremella fuciformis
0.28 ± 0.02m
0.17±0.02k
3.62±0.07d
3.66±0.15f
3
Hypsizygus tessellatus
0.37 ± 0.03kl
0.24±0.01j
1.92±0.06j
3.72±0.12f
4
Agaricus subrufescens
1.04 ± 0.01e
1.18±0.02c
4.94±0.09a
3.76±0.08ef
5
Hericium erinaceus
0.39 ± 0.01jk
0.39±0.03h
2.85±0.06f
2.28±0.14ij
6
Coprinus comatus
0.53 ± 0.04gh
0.71±0.04e
4.88±0.11a
4.07±0.08d
7
Phallus indusiatus
1.21 ± 0.06d
1.53±0.04b
2.35±0.12h
2.17±0.02j
8
Pleurotus citrinopileatus
0.37 ± 0.01k
0.47±0.02fg
4.11±0.10c
3.76±0.20ef
9
Pleurotus eryngii
0.39 ± 0.04jk
0.48±0.05f
3.72±0.07d
4.45±0.13ab
10
Boletus edulis
1.75 ± 0.06b
2.18±0.04a
4.58±0.04b
4.19±0.12cd
11
Boletus luridus
1.79 ± 0.03b
2.16±0.02a
4.82±0.23a
2.84±0.21h
12
Boletus aereus
1.93 ± 0.09a
2.20±0.02a
4.87±0.12a
4.33±0.10bc
13
Ophiocordyceps sinensis
1.29 ± 0.01c
0.95±0.03d
2.68±0.09g
3.40±0.15g
14
Pholiota nameko
0.50 ± 0.03hi
0.43±0.03gh
2.13±0.08i
3.99±0.18de
15
Armillaria mellea
0.31 ± 0.02lm
0.43±0.03fgh
2.50±0.05h
3.78±0.19ef
16
Hohenbuehelia reniformis
0.30 ± 0.03m
0.05±0.01l
1.32±0.06l
1.74±0.04k
17
Lentinus edodes
0.40 ± 0.04jk
0.28±0.02j
1.40±0.09l
2.93±0.05h
18
Boletus pinophilus
0.27 ± 0.01m
1.52±0.01b
3.75±0.08d
3.29±0.08g
19
Agrocybe aegerita
0.66 ± 0.03f
1.51±0.03b
3.32±0.06e
4.64±0.20a
20
Grifola frondosa
0.44 ± 0.03ij
0.41±0.02h
1.75±0.04k
2.47±0.10i
Table 1: Antioxidant activities of mushroom samples extracted by different solvents.
For all mushrooms, the water extracts and hot water extracts exhibited much higher antioxidant properties than that of acetone extracts and ethanol extracts. However, the differences between water extracts and hot water extracts and the differences between acetone extracts and ethanol extracts were not significant (p > 0.05). Among all the extracts, the water extracts of Agaricus subrufescens provided the highest antioxidant activity with 4.94 µmole of TE⁄g sample.
Total phenolic content of mushrooms
Phenols are known as important botanical constituents due to their scavenging ability provided by the hydroxyl groups [13]. Phenolic compounds may have direct contribution to antioxidative action [14]. Phenolic compounds were reported to be associated with antioxidant activity and play important roles in the stabilizing of lipid peroxidation [15]. Natural phenolics are able to provide antioxidative function through various ways, such as intercepting singlet oxygen, decomposing primary products of oxidation, preventing continued hydrogen abstraction from substances, etc [16]. In addition, total polyphenols were considered as the major naturally occurring antioxidant compounds in the wild edible mushrooms [17].
The results of TPC values of mushrooms were shown in Table 3. The TPC of the samples extracted with water and hot water were much higher than those extracted with acetone and ethanol. Among the four different solvents, water extraction provided the highest TPC. There were no significant differences between acetone extracts and ethanol extracts; they presented in low amounts. Meanwhile, there were significant (p < 0.05) differences between different mushrooms. In organic extracts, Boletus pinophilus provided the highest amount of TPC with 0.91 mg GAE⁄g in acetone extracts and 0.62 mg GAE⁄g in ethanol extracts. However, comparing to other mushroom samples, it only provided medium TPC in aqueous extracts with 4.26 mg GAE⁄g in water extracts and 2.86 mg GAE⁄g in hot water extracts. In inorganic extracts, water extract and hot water extracts of Ophiocordyceps sinensis had the highest amount of TPC with 10.82 mg GAE⁄g and 9.44 mg GAE⁄g. The distribution of TPC in different extraction solvents depended on species of the mushrooms.
Scientific name
Acetone extracts
Ethanol extracts
Water extracts
1
Auricularia auricula
0.12 ± 0.01h
0.09 ± 0.01i
0.82 ± 0.04o
0.35 ± 0.07m
2
Tremella fuciformis
0.11 ± 0.01hi
0.06 ± 0.01j
0.81 ± 0.06o
0.66 ± 0.03l
3
Hypsizygus tessellatus
0.09 ± 0.01i
0.07 ± 0.01j
2.16 ± 0.03n
2.37 ± 0.06g
4
Agaricus subrufescens
0.25 ± 0.01d
0.30 ± 0.01d
2.82 ± 0.01i
1.01 ± 0.03k
5
Hericium erinaceus
0.16 ± 0.01g
0.13 ± 0.01h
3.08 ± 0.09k
1.75 ± 0.08i
6
Coprinus comatus
0.19 ± 0.01f
0.18 ± 0.01g
2.41 ± 0.06m
4.12 ± 0.05b
7
Phallus indusiatus
0.28 ± 0.01c
0.27 ± 0.01e
3.21 ± 0.16k
2.58 ± 0.02f
8
Pleurotus citrinopileatus
0.10 ± 0.01i
0.10 ± 0.01i
9.42 ± 0.16d
2.96 ± 0.10d
9
Pleurotus eryngii
0.09 ± 0.01j
0.07 ± 0.01j
3.65 ± 0.11j
1.52 ± 0.06j
Boletus edulis
0.28 ± 0.01c
0.30 ± 0.01d
5.17 ± 0.09g
3.98 ± 0.15bc
11
Boletus luridus
0.36 ± 0.01b
0.38 ± 0.01b
10.43 ± 0.01b
2.72 ± 0.11ef
12
Boletus aereus
0.29 ± 0.01c
0.32 ± 0.01c
10.18 ± 0.13c
3.95 ± 0.07c
13
Ophiocordyceps sinensis
0.22 ± 0.01e
0.24 ± 0.01f
10.82 ± 0.19a
9.44 ± 0.24a
14
Pholiota nameko
0.06 ± 0.01j
0.06 ± 0.01j
7.31 ± 0.09e
2.15 ± 0.10h
15
Armillaria mellea
0.10 ± 0.01hi
0.11 ± 0.01i
4.81 ± 0.09n
2.13 ± 0.10h
16
Hohenbuehelia reniformis
0.06 ± 0.01j
0.03 ± 0.01k
2.35 ± 0.04m
1.37 ± 0.07j
17
Lentinus edodes
0.06 ± 0.01j
0.06 ± 0.01j
4.26 ± 0.01i
1.82 ± 0.01i
18
Boletus pinophilus
0.91 ± 0.06a
0.62 ± 0.01a
4.26 ± 0.07i
2.86 ± 0.10de
19
Agrocybe aegerita
0.13 ± 0.01h
0.27 ± 0.01e
5.71 ± 0.07f
2.99 ± 0.09d
20
Grifola frondosa
0.11 ± 0.01hi
0.11 ± 0.01i
3.78 ± 0.12j
2.34 ± 0.09g
Table 3: Total phenolic content of mushroom samples extracted by different solvents
Figure 1: #
Figure 2: Antioxidant activities (A) and total phenolic contents (B) of various extracts from mushrooms B. edulis, B. luridus and B. aereus.
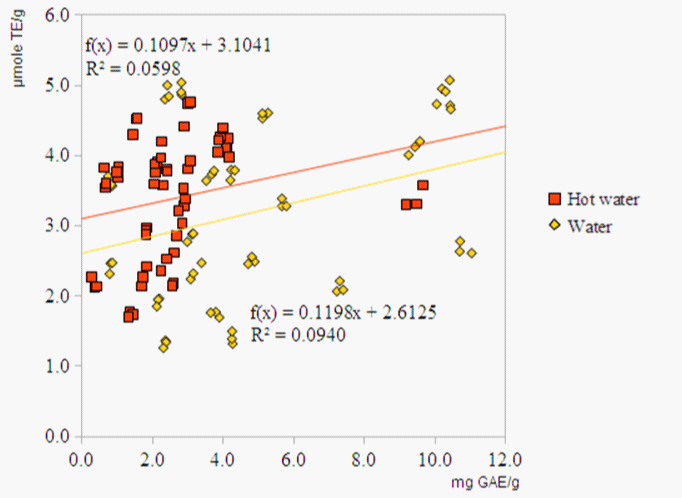
The correlations of phenolic levels and antioxidant capacities in triplicate aqueous extracts were shown in Figure 2. According to the results, the overall trend was that the antioxidant capacities were positively related to the phenolic levels. However, as the values of the correlation analysis were 0.31 and 0.24 for water extracts and hot water extracts, respectively, the correlation was not highly significant.
Figure 3: Correlation between antioxidant activities and TPC values of water extracts and hot water extracts.
Conclusion
The current study compared the antioxidant activities among different mushroom samples and the effects of different extraction solvents on distribution of antioxidants and phenolics. The antioxidant activity determination indicated that water extracts and hot water extracts had much higher antioxidant properties than acetone extracts and ethanol extracts. The TPC of the samples extracted with water and hot water were also much higher than those extracted with acetone and ethanol. Due to their high content of antioxidants,aqueous extracts of some mushrooms, especially B. edulis, B. luridus and B. aereus, may be used as materials of dietary supplements.
Acknowledgements
This research was jointly supported by Natural Science Foundation of Guangdong Province, China (Project code: S2012010008961), and a research grant (UICRG 201402) from Beijing Normal University– Hong Kong Baptist University United International College, China.
References
- De Román M, Boa E, Woodward S . Wild-gathered fungi for health and rural livelihoods. Proc Nutr Soc. 2006; 65: 190-197.
- Cheung PK. The nutritional and health benefits of mushrooms. Nutrition Bulletin. 2010; 35: 292-299.
- Gan, CH, Nurul AB and Asmah R. Antioxidant analysis of different types of edible mushrooms (Agaricus bisporous and Agaricus brasiliensis). International Food Research Journal 20, 2013; 1095-1102.
- Crisan EV and Sands A (1978) Nutritional values. In: The Biology and Cultivation of Edible Mushrooms, (ST Chang, WA Hayes, eds), pp.137-168. New York: Academic Press.
- Wasser SP. Medicinal mushroom science: History, current status, future trends and unsolved problems. International Journal of Medicinal Mushrooms. 2010; 12: 1-16.
- Reshetnikov SV, Wasser SP and Tan KK. Higher basidiomycetes as a source of antitumor and immunostimulating polysaccharides (review). International Journal of Medicinal Mushrooms. 2001; 3: 361-394.
- Liu F, Ooi VE, Chang ST . Free radical scavenging activities of mushroom polysaccharide extracts. Life Sci. 1997; 60: 763-771.
- Kumari DD, Reddy MS and Upadhyay RC. Nutritional composition and antioxidant activities of 18 different wild Cantharellus mushrooms of Northwestern Himalayas. Food Science & Technology International. 2011; 17: 557-567.
- Zeng X, Suwandi J, Fuller J, Doronila A, Ng K . Antioxidant capacity and mineral contents of edible wild Australian mushrooms. Food Sci Technol Int. 2012; 18: 367-379.
- Olajire AA and Azeez L. Total antioxidant activity, phenolic, flavonoid and ascorbic acid contents of Nigerian vegetables. African Journal of Food Science and Technology. 2011; 2: 022-029.
- Elmastas M, Isildak O, Turkekul I and Temur N. Determination of antioxidant activity and compounds in wild edible mushrooms. Journal of Food Compostition and Analysis. 2007; 20: 337-345.
- Xu BJ, Chang SK . A comparative study on phenolic profiles and antioxidant activities of legumes as affected by extraction solvents. J Food Sci. 2007; 72: S159-166.
- Hatano T, Edamatsu R, Mori A, Fujita Y and Yasuhara E. Effect of interaction of tannins with co-existing substances. VI. Effects of tannins and related polyphenols on superoxide anion radical and on DPPH radical. Chemical and Pharmaceutical Bulletin. 1989; 37: 2016-2021.
- Duh PD, Tu YY and Yen GC. Antioxidant activity of water extract of harn jyur (Chyrsanthemum morifolium Ramat). Lebensmittel-Wissenschaft und Technologie. 1999; 32: 269-277.
- Yen GC, Duh PD and Tsai CL. Relationship between antioxidant activity and maturity of peanut hulls. Journal of Agricultural and Food Chemistry. 1993; 41: 67-70.
- Shahidi F and Naczk M (2004) Antioxidant properties of food phenolics. In: Phenolics in food and nutraceuticals, (F Shahidi, M Naczk, eds). p1, 403. Florida: , Boca Raton, CRC Press.
- Choi SW and Sapers GM. Effects of washing on polyphenols and polyphenol oxidase in commercial mushrooms (Agricus bisporus). Journal of Agricultural and Food Chemistry 1994; 42: 2286-2290.
.gif)
.gif)