Research Article
Austin J Nutri Food Sci. 2014;1(3): 1013.
Expressions of Endothelial Cells Adhesion Molecules are Significantly Reduced in the Presence of Minute Amount of Tocotrienols.
Mohd SokhiniAbd Mutalib1*, Huzwah Khaza’ai2, Lim Siang Hui3, Nafeeza Ismail4, Hosseini Khorami1
1Department of Nutrition and Dietetic, University Putra Malaysia, 43400 Serdang, Selangor, Malaysia
2Department of Biomedical Science, University Putra Malaysia, 43400 Serdang, Selangor, Malaysia
3Cancer Research Initiative Foundation, Sime Darby Medical Centre, 47500 Subang Jaya, Selangor, Malaysia
4Faculty of Medicine, University Technology Mara, Shah Alam, Selangor, Malaysia
*Corresponding author: :Mohd SokhiniAbd Mutalib, Department of Nutrition and Dietetic, University Putra Malaysia, 43400 Serdang, Selangor, Malaysia
Received: January 10, 2014; Accepted: February 10, 2014; Published: February 17, 2014
Abstract
Comparative effects of palm tocotrienol rich fractions (TRF) and α–tocopherol on the expression of adhesion molecules by human umbilical vein endothelial cells (HUVECs) were investigated in the present study. Cell based ELISA technique using a monospecific, monoclonal antibodies was employed to measure expression of intracellular cell adhesion molecules–1 (ICAM–1) and vascular cell adhesion molecules–1 (VCAM–1). Primary HUVECs, cultured on a96 wells microtiter plate was incubated for 4 hours with different concentration (ng⁄ml) of TRF or α–tocopherol before subjected to inflammatory stimulation by incubating it with 2 ng⁄ml tumour necrosis factor-α (??F-α) and further incubated for 4 hours. MTS assay was carried out to ascertain the effects of the different dosages on the cells viability. VCAM–1 expression was significantly decreased when HUVECs were incubated with palm TRF between 10–50ng⁄ ml concentrations. Similar effects of the palm TRF were also observed on the expression of ICAM–1. The effect of α–tocopherol however was found to be less consistent. At 10ng⁄ml and 20ng⁄ml, α–tocopherol increased VCAM–1 expression. Higher concentration (30–50ng⁄ml) returned the expression to normal. On the other hand, ICAM–1 was significantly decreased when incubated with 10ng⁄ml of α–tocopherol but gradually increased with increased dosage of α–tocopherol. Our findings suggest that TRF are more potent adhesion molecules expression inhibitor compared to α–tocopherol in–vitro.
Keywords: Palm TRF; a–tocopherol; ICAM–1; VCAM–1; ELISA
Introduction
An important event in the development of atherosclerosis includes the increased interaction of monocytes with endothelial cells lining the vessel wall [1]. This event is mediated by the presence of adhesion molecules expressed on the endothelial cells and the monocytes [2]. These molecules which includes ICAM–1 and VCAM–1 (the product of endothelial and other cells), may be important in controlling the extravasations of leucocytes out of the circulation in acute andchronic inflammation [3]. Cytokines activation of the leucocytes and endothelial cells results in the expression of these adhesion molecules. It has been shown that adhesion molecules expression by the endothelium is highly inducible in the presence of TNFα and Interleukin–β which involved the translocation of the transcription factor, NF–?B [4,5]. Several findings suggest the involvement of intracellularly generated oxygen derived free radicals (ODRF) in the pathway of the NF–?B activation [6,7]. Modulation of the expression of these adhesion molecules by ODRF may therefore, be an important mechanism in initiating atherosclerosis.
Inhibiting the expression of adhesion molecules during an inflammatory and oxidative stress condition has been considered to be an alternative way in preventing the development of atherosclerosis. Antioxidants have been suggested to confer inhibitory effect on controlling the expression of the adhesion molecules by their respective cells. α –tocopherol is shown to be one of the most potent natural antioxidant with the highest biological activity of the vitamin E family. α–tocopherol has been shown in many studies to reduce the adhesion of monocytes to endothelial cells through reduction in the expression of the adhesion molecules [4,8-13]. However, some studies have shown that the unsaturated phytyl chain of tocotrienol might confer the tocotrienol molecules with better antioxidative potential than tocopherol. α–tocotrienol has been reported to possess higher antioxidant activity in liver microsomes and to elicit better protection of intrinsic membrane proteins (cytochrome P–450) against oxidative damage than α–tocopherol [14]. Further evidences of the greater antioxidant efficacy of tocotrienols compared with tocopherols include higher efficiency in protecting erythrocytes against oxidative hemolysis in vitro [15], stronger anti–tumour action [16,17], greater protection against cardiotoxicity and anti–tumour redox cycling for drug adriamycin [18,19] and higher inhibitory effects on lipid peroxidation and protein oxidation in rat liver microsomes and brain mitochondria [20,21]. Recently, α–tocotrienol has been reported to provide the most potent neuroprotection among the vitamin E analogs against oxidative stress–induced cell death [22-25]. We have reported that palm TRF inhibits LDL oxidation and endothelial cell lipid peroxidation more effective than α–tocopherol in vitro [26]. In the present study the potential effect of palm TRF and α–tocopherol in inhibiting the expression of adhesion molecules in endothelial cells was investigated.
Materials and Methods
Culture of endothelial cell
Human umbilical cord were collected from Hospital Universiti Kebangsaan Malaysia after written consents were obtained from the patients prior to the commencement of the study. Endothelial cells was isolated from the umbilical cord veins according to the method of Jaffe [27]. Only cells cultured up to the passage 6 were used for the study. Cells were primarily cultured in T75 cultured flask before consecutively subcultured into 96 well microtiter plate in the presence of M199 medium with 20% fetal calf serum and grown into confluence at 37°C, and 5% CO2 .
Treatment of cells and cell based ELISA
Stock solution of α–tocopherol and palm TRF was diluted to two sets of concentrations; µg⁄ml and ng⁄ml concentrations. Cells were incubated with either 10, 20, 30, 40, or 50 ng⁄ml (or µg⁄ml) for 4 hours before TNFα (2ng⁄ml) were added. The cells were further incubated for 4 hours.
Treated cells were measured for the expression of the adhesion molecules by the ELISA technique according to Krakauer [28]. Briefly, cells were washed with PBS before fixing with either Acetone citrate buffer (for VCAM–1) or 1% glutaraldehyde in PBS (for ICAM– 1) at room temperature. Cells were washed and dried at 37°C for 20 minutes and incubated with monoclonal antibodies against VCAM–1 or ICAM–1. Secondary antibody conjugated to biotin was added and the cells were further incubated for 1 hour at room temperature. Another conjugate; HRP–streptavidin was added before finally the luminescence substrate was pipetted and the cells were further incubated at 37°C for 30 minutes. Colour development was terminated with the addition of sulfuric acid (3M). The plate was read in the ELISA plate reader at 490nm wavelength. Condition for control was similar with the exception that the cells were not treated with either of the vitamin E compounds.
Cell viability was measured by MTS assay according to protocol provided by the manufacturer (Promega Corporation USA).
Result
Cytotoxicity effect of α–tocopherol and palm TRF
Cell viability was not affected when the cells were incubated with either TRF or α–tocopherol at ng⁄ml concentration. Increasing concentration of TRF to µg⁄ml level killed the cells at 20µg⁄ml or higher. No cytotoxicity effect was observed when α–tocopherol was used at the µg⁄ml concentration (figures 1a & 1b).
Effect of a–tocopherol and palm TRF on the expression of VCAM–1 and ICAM–1
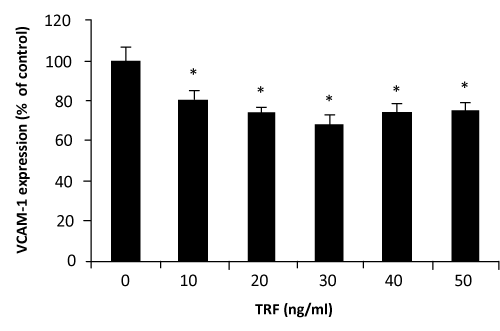
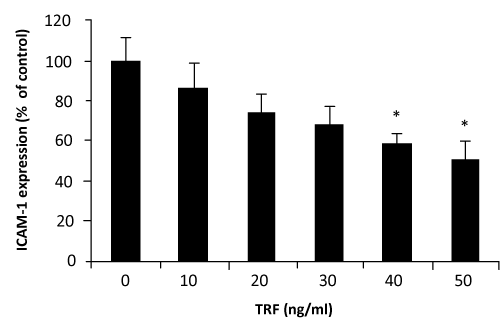
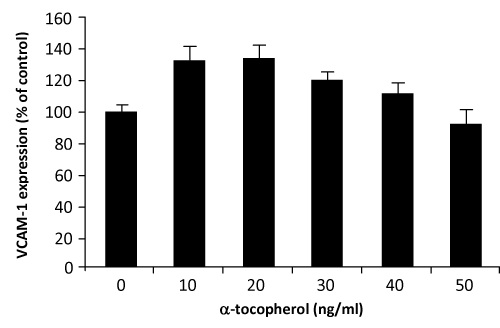
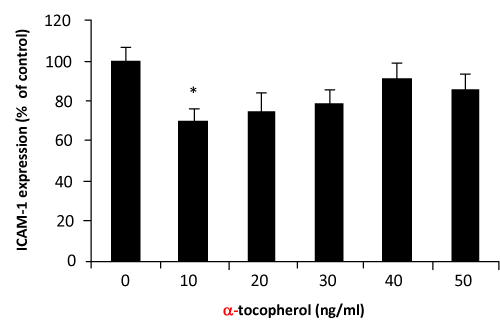
VCAM–1 expression was significantly decreased when HUVECs were incubated with palm TRF between 10–50ng⁄ml concentrations (figure 2). Similar effects of the TRF were also observed on the expression of ICAM–1 (figure 3). Effect of α–tocopherol however, was found to be less consistent. At 10ng⁄ml and 20ng⁄ml, α–tocopherol increased VCAM–1 expression (figure 4). Higher concentation (30– 50ng⁄ml) returned the expression to normal. On the other hand, ICAM–1 was significantly decreased when incubated with 10ng⁄ml of α–tocopherol but gradually increased when α–tocopherol increased (figure 5).manner. Even at 10ng⁄ml concentration of palm TRF, VCAM–1 expression is 20% lower than the control and reached ˜35% when the dosage was increased to 30ng⁄ml. No cytotoxicity was observed at these levels; therefore the reduction cannot be accorded to cell death. Similar results was observed on ICAM–1 expression when the cells were treated with palm TRF, indicating that the reduction in adhesion molecules are very much due to the inhibiting effect of the TRF on adhesion molecules expression. Earlier studies on almost identical experiment demonstrated a higher reducing effect of α–tocotrienol on VCAM–1 expression (77% reduction on VCAM–1 expression by 25µmol⁄l α–tocotrienol) [23,29], confirming the inhibitory effect of tocotrienols on VCAM–1 expression. Although this work produced higher reduction, we demonstrated that the inhibiting effect can even be achieved at the levels 500 times lower than the dose used by the previous group. Our attempt to work at the µg⁄ml concentration was hampered with the decrease number of viable cells when they were treated with the palm TRF.
Figure 1: Effects of α–tocopherol and palm TRF on cells viability. HUVEC were incubated for 18 hours with different concentration of either α–tocopherol or palm TRF. Cell viability was determined by the MTS assay. Data is presented as percent of viable cells as compared to control. Cells incubated with a–tocopherol or palm TRF either a) at ?g⁄ml concentration or b) at ng⁄ml concentration.
Figure 2: Effect of palm TRF on VCAM–1 expression in HUVEC. Human umbilical vein endothelial cells, more than 95% confluence were incubated with different concentrations of the palm TRF for 4 hours at 37°C before stimulated with TNFα. Adhesion molecules expression was measured by the ELISA technique as discussed in the text. Effect of the treatment was expressed as % of changes from control (no treatment). (∗ P< 0.05)
Figure 3: Effect of palm TRF on ICAM–1 expression in HUVEC. Human umbilical vein endothelial cells, more than 95% confluence were incubated with different concentrations of the palm TRF for 4 hours at 37°C before stimulated with TNF<. Adhesion molecules expression was measured by the ELISA technique as discussed in the text. Effect of the treatment was expressed as % of changes from control (no treatment). (∗ P< 0.05)
Figure 4: Effect of α–tocopherol on VCAM–1 expression in HUVEC. Human umbilical vein endothelial cells, more than 95% confluence were incubated with different concentrations of the α–tocopherol for 4 hours at 37°C before stimulated with TNα. Adhesion molecules expression was measured by the ELISA technique as discussed in the text. Effect of the treatment was expressed as % of changes from control (no treatment).
Figure 5: Effect of α–tocopherol on VCAM–1 expression in HUVEC. Human umbilical vein endothelial cells, more than 95% confluence were incubated with different concentrations of the palm TRF for 4 hours at 37°C before stimulated with TNFα. Adhesion molecules expression was measured by the ELISA technique as discussed in the text. Effect of the treatment was expressed as % of changes from control (no treatment). (∗ P< 0.05).
Many previous work have reported the inhibiting effect of α–tocopherol on adhesion molecules expression [30–32]. In the present study, effects of α–tocopherol at the level of µg⁄ml (µmol⁄L) was observed to significantly decrease adhesion molecules expression (data not shown), but when the concentration were lowered to ng⁄ ml the effect were less consistent. This particularly evident on the VCAM–1 expression. On treating the cells at concentration between 10–40 ng⁄ml, α–tocopherol tends to increase VCAM–1 expression, although what was observed on the ICAM–1 is more positive.
Our observation that only minute amount (ng⁄ml) of tocotrienolrich fraction needed to produce the necessary effect is physiologically significant considering that the amount of tocotrienols detected in human plasma is extremely low which raised the benefits of taking the tocotrienols. It has been shown that α–tocotrienol at nM concentration readily block glutamate–induced death of the HT neuronal cells in vitro [33,34] indicating the minute amount of tocotrienol needed to exert it effect. Our findings and the mentioned groups are the few preliminary studies to suggest that tocotrienol exhibits its therapeutic effects at concentrations very much lower than effect that is expected from α–tocopherol. Our attempt to work at the µg⁄ml concentration were hindered with the reduced number of viable cells treated with the TRF.
The usage of α–tocopherol as therapeutic agent has long been recognised. On the other hand, despite the very close chemical similarity between tocotrienol and tocopherol, the potential of tocotrienol in therapeutic usage has not been comprehensively explored.
Few years back, we have reported the higher potential of palm -TRF as an inhibitor of LDL oxidation and endothelial cell lipid peroxidation compared to α–tocopherol [35,36]. Many earlier studies have also reported higher antioxidant potential of either palm TRF or pure α–tocotrienol than α–tocopherol [20,21]. The explanation on these growing evidences is yet to be resolved. It was suggested that the effects of α–tocotrienol might not due to its antioxidant property [33,34]. Rather, it has been hypothesised that the better intercalation and anchorage of the tocotrienol unsaturated phytyl side chain to the cell membrane compared to tocopherol make it more readily available to the cells [14]. This hypothesis is supported by recent study which showed that tocotrienols were more readily transferred between membranes and incorporated into cell membrane when compared to tocopherols [37]. This observation might as well describe the low levels of tocotrienol detected in the plasma even after a very high intake of tocotrienols. We observed that rats given high amount of tocotrienol tend to take up only minute amount of the compound initially compared to α–tocopherol which is readily absorbed from day one (data to be published). Despite the observed low absorption and the very low concentration detected in the plasma, the levels found in adipose tissue and certain vital organs of the animals suggesting a different metabolic pathway of tocotrienol from tocopherol.
In conclusion, the present study clearly demonstrated that palm TRF when administered at a very low dosage is highly effective in inhibiting adhesion molecules expression by HUVEC in vitro suggesting the higher potency of TRF compared to α–tocopherol alone. How the finding might be of relevance to in vivo application is yet to be determined.
Acknowledgements
We would like to thank the Malaysian Palm Oil Board who generously provides the grant for the project.
References
- Kelishadi R . Inflammation-induced atherosclerosis as a target for prevention of cardiovascular diseases from early life. Open Cardiovasc Med J. 2010; 4: 24-29.
- Cook-Mills JM, Marchese ME, Abdala-Valencia H . Vascular cell adhesion molecule-1 expression and signaling during disease: regulation by reactive oxygen species and antioxidants. Antioxid Redox Signal. 2011; 15: 1607-1638.
- Hortelano S, López-Fontal R, Través PG, Villa N, Grashoff C, et al. "ILK mediates LPS-induced vascular adhesion receptor expression and subsequent leucocyte trans-endothelial migration.," Cardiovasc. Res.201; 86: 283-292.
- Berdnikovs S, Abdala-Valencia H, McCary C, Somand M, Cole R, et al. "Isoforms of vitamin E have opposing immunoregulatory functions during inflammation by regulating leukocyte recruitment.," J. Immunol. 2009; 182: 4395-4405.
- Brasier AR . The nuclear factor-kappaB-interleukin-6 signalling pathway mediating vascular inflammation. Cardiovasc Res. 2010; 86: 211-218.
- Morgan MJ, Liu ZG . Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011; 21: 103-115.
- Park HJ, Jeong SK, Kim SR, Bae SK, Kim WS, et al. "Resveratrol inhibits Porphyromonas gingivalis lipopolysaccharide-induced endothelial adhesion molecule expression by suppressing NF-kappaB activation.," Arch. Pharm. Res. 2009; 32: 583-591.
- Jialal I, Devaraj S, Kaul N . The effect of alpha-tocopherol on monocyte proatherogenic activity. J Nutr. 2001; 131: 389S-94S.
- Rahman A, Fazal F . Blocking NF-κB: an inflammatory issue. Proc Am Thorac Soc. 2011; 8: 497-503.
- Jung WJ, Sung MK . Effects of major dietary antioxidants on inflammatory markers of RAW 264.7 macrophages. Biofactors. 2004; 21: 113-117.
- Munteanu A, Zingg JM, Azzi A . Anti-atherosclerotic effects of vitamin E--myth or reality? J Cell Mol Med. 2004; 8: 59-76.
- Atkinson J, Epand RF, Epand RM . Tocopherols and tocotrienols in membranes: a critical review. Free Radic Biol Med. 2008; 44: 739-764.
- Theriault A, Chao JT, Gapor A . Tocotrienol is the most effective vitamin E for reducing endothelial expression of adhesion molecules and adhesion to monocytes. Atherosclerosis. 2002; 160: 21-30.
- Serbinova E, Kagan V, Han D, Packer L . Free radical recycling and intramembrane mobility in the antioxidant properties of alpha-tocopherol and alpha-tocotrienol. Free Radic Biol Med. 1991; 10: 263-275.
- Yoshida Y, Hayakawa M, Habuchi Y, Itoh N, Niki E . Evaluation of lipophilic antioxidant efficacy in vivo by the biomarkers hydroxyoctadecadienoic acid and isoprostane. Lipids. 2007; 42: 463-472.
- Ashfaq MK, Zuberi HS, Anwar Waqar M . Vitamin E and beta-carotene affect natural killer cell function. Int J Food Sci Nutr. 2000; 51 Suppl: S13-20.
- Nesaretnam K, Dorasamy S, Darbre PD . Tocotrienols inhibit growth of ZR-75-1 breast cancer cells. Int J Food Sci Nutr. 2000; 51 Suppl: S95-103.
- Qureshi AA, Mo H, Packer L, Peterson DM . Isolation and identification of novel tocotrienols from rice bran with hypocholesterolemic, antioxidant, and antitumor properties. J Agric Food Chem. 2000; 48: 3130-3140.
- Komiyama K, Iizuka K, Yamaoka M, Watanabe H, Tsuchiya N, et al. Studies on the biological activity of tocotrienols. Chem Pharm Bull (Tokyo). 1989; 37: 1369-1371.
- Kamat JP, Sarma HD, Devasagayam TP, Nesaretnam K, Basiron Y . Tocotrienols from palm oil as effective inhibitors of protein oxidation and lipid peroxidation in rat liver microsomes. Mol Cell Biochem. 1997; 170: 131-137.
- Kamat JP, Devasagayam TP. "Tocotrienols from palm oil as potent inhibitors of lipid peroxidation and protein oxidation in rat brain mitochondria.," Neurosci. Lett. 1995; 195: 179-182.
- Khanna S, Patel V, Rink C, Roy S, Sen CK . Delivery of orally supplemented alpha-tocotrienol to vital organs of rats and tocopherol-transport protein deficient mice. Free Radic Biol Med. 2005; 39: 1310-1319.
- Sen CK, Khanna S, Roy S . Tocotrienols: Vitamin E beyond tocopherols. Life Sci. 2006; 78: 2088-2098.
- Osakada F, Hashino A, Kume T, Katsuki H, Kaneko S, et al. Alpha-tocotrienol provides the most potent neuroprotection among vitamin E analogs on cultured striatal neurons. Neuropharmacology. 2004; 47: 904-915.
- Packer L, Weber SU, Rimbach G . Molecular aspects of alpha-tocotrienol antioxidant action and cell signalling. J Nutr. 2001; 131: 369S-73S.
- M S A Mutalib, H Khaza'ai and K W J Wahle, "Palm-tocotrienol rich fraction (TRF) is a more effective inhibitor of LDL oxidation and endothelial cell lipid peroxidation than a-tocopherol in vitro," Food Res. Int. 2003; 36: 405-413.
- Jaffe EA, Nachman RL, Becker CG, Minick CR . Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973; 52: 2745-2756.
- Krakauer T . A sensitive ELISA for measuring the adhesion of leukocytic cells to human endothelial cells. J Immunol Methods. 1994; 177: 207-213.
- Theriault A, Chao JT, Gapor A . Tocotrienol is the most effective vitamin E for reducing endothelial expression of adhesion molecules and adhesion to monocytes. Atherosclerosis. 2002; 160: 21-30.
- Faruqi R, de la Motte C, DiCorleto PE . Alpha-tocopherol inhibits agonist-induced monocytic cell adhesion to cultured human endothelial cells. J Clin Invest. 1994; 94: 592-600.
- Erl W, Weber C, Wardemann C, Weber PC . alpha-Tocopheryl succinate inhibits monocytic cell adhesion to endothelial cells by suppressing NF-kappa B mobilization. Am J Physiol. 1997; 273: H634-640.
- Martin A, Foxall T, Blumberg JB, Meydani M . Vitamin E inhibits low-density lipoprotein-induced adhesion of monocytes to human aortic endothelial cells in vitro. Arterioscler Thromb Vasc Biol. 1997; 17: 429-436.
- Tocotrienols (2008) Vitamin E Beyond Tocopherols (Google eBook). p. 424. CRC Press.
- Sen CK, Khanna S, Roy S, Packer L . Molecular basis of vitamin E action. Tocotrienol potently inhibits glutamate-induced pp60(c-Src) kinase activation and death of HT4 neuronal cells. J Biol Chem. 2000; 275: 13049-13055.
- Packer L, Weber SU, Rimbach G . Molecular aspects of alpha-tocotrienol antioxidant action and cell signalling. J Nutr. 2001; 131: 369S-373S.
- M S A Mutalib, H Khaza'ai and K W J Wahle, "Palm-tocotrienol rich fraction (TRF) is a more effective inhibitor of LDL oxidation and endothelial cell lipid peroxidation than a-tocopherol in vitro," Food Res. Int. 2003; 36: 405-413.
- Yoshida Y, Hayakawa M, Habuchi Y, Itoh N, Niki E . Evaluation of lipophilic antioxidant efficacy in vivo by the biomarkers hydroxyoctadecadienoic acid and isoprostane. Lipids. 2007; 42: 463-472.
.gif)