Review Article
Austin J Nutri Food Sci. 2014;2(3): 1016.
Alternative Grains as Potential Raw Material for Gluten– Free Food Development in The Diet of Celiac and Gluten– Sensitive Patients
María de Lourdes Moreno, Isabel Comino and Carolina Sousa*
Department of Microbiology and Parasitology, Faculty of Pharmacy, University of Seville, Sevilla, Spain
*Corresponding author: :Carolina Sousa. Department of Microbiology and Parasitology, Faculty of Pharmacy, University of Seville, Sevilla, Spain
Received: February 19, 2014; Accepted: March 05, 2014; Published: March 13, 2014
Abstract
Celiac disease is an autoimmune disorder resulting from gluten intolerance and is based on a genetic predisposition. Gluten is a protein composite found in the cereals wheat, rye, barley and certain oat varieties. A strict gluten–free diet is the only currently available therapeutic treatment for patients with celiac disease. Rising demands for gluten–free products parallels the apparent or real increase in celiac disease, non–celiac gluten sensitivity and gluten allergy. However, gluten removal results in major problems for bakers, and currently, many gluten–free products available on the market are of low quality exhibiting poor mouthfeel and flavor. Thus, an increasing trend in research is focusing on the application of alternative grains potentially healthy to elaborate gluten–free products. A promising area is the use of cereals (rice, corn and sorghum), minor cereals (fonio, teff, millet and job’s tears) or pseudocereals such as amaranth, buckwheat, quinoa. Nevertheless, commercialization of these products is still quite limited. The aim of this work is to review recent advances in research about the nutritional quality and potential health benefits of alternative grains tolerated by patients with gluten related pathologies.
Keywords: Celiac disease; Gluten–free diet; Cereals; Grains; Pseudocereals; Minor cereals.
Abbreviations
CD: Celiac Disease; GFD: Gluten Free Diet; NCGS: Non Celiac Gluten Sensitivity
Introduction and Background
Celiac disease (CD), a common autoimmune enteropathy elicited following gluten ingestion in patients with a genetic predisposition, affects ˜1% of the general population although this percentage is probably an underestimation since the condition often being left undiagnosed [1–4]. Gluten is a complex mixture of proteins called prolamins and glutenins. Depending on the cereal, proteins of the prolamin fraction have specific names: gliadins (in wheat), hordeins (in barley), secalins (in rye) or avenins (in oat). A common characteristic of these proteins is the presence of multiple proline and glutamine residues, making them resistant to gastrointestinal digestion and more exposed to deamination by tissue transglutaminase [3]. Ingestion of these proteins leads to the inflammation, atrophy, and hyperplasia of the small–intestinal crypts of the celiac patient. However, this disease not only affects the gut, but it is a systemic disease that may cause injury to the skin, liver, joints, brain, heart, and other organs [5].
To date, the mainstay of treatment for CD is a strict life–long adherence to a gluten–free diet (GFD). For most patients, CD goes in remission when they adhere to a gluten–exclusion diet, and they relapse when gluten is reintroduced into the diet [3,6]. Complying with a GFD is difficult and affects the patients’ quality of life, but a strict diet is critical to reduce morbidity and mortality [7].
Gluten is a protein that can be separated from flour when the starch and other minor components of the flour are removed by washing out with running water. The resulting gluten contains approximately 65% water. On a dry matter basis, gluten contains 75–86% protein, the remainder being carbohydrate and lipid, which are held strongly within the gluten–protein matrix [8–9]. The unique characteristics of gluten (extensibility, resistance to stretch, mixing tolerance, gas holding ability) favor its use in many food products [9]. There are two main reasons why alternative approaches to the GFD are actively sought. On the one hand, because a large amount of gluten is obtained during the manufacture of starch and the common use of starch as food additive or ingredients, this may turn out to be problematic for celiac patients, since gluten proteins may be found in unexpected sources such as meat, fish or milk products. On the other hand, the removal of gluten presents major problems for bakers, and currently, many gluten–free products available on the market are of low quality exhibiting poor mouthfeel and flavor [9,10]. In this work, we will review the current status of alternative grains with no or low immunogenic content which may be potential tolerated by patients with CD.
Analyses and Interpretation
The viscoelastic network generated by the gluten enables an excellent aerated structure in food products [5]. In contrast, the quality of the gluten–free products available on the market, and food choices, may represent major determinants in the deficiencies in macronutrients and micronutrients of celiac patients [11]. An unbalance in the percentage of energy intake coming from carbohydrates in celiac patients on a GFD may have nutritional implications in relation with dietary intakes of B–vitamins, iron and fiber, as grain foods contribute a large percentage to the daily intake of these nutrients [12]. Gluten–free cereal foods are frequently rich in carbohydrates and fats and they are made using refined glutenfree flour or starch not enriched or fortified [13]. As a result, many gluten–free cereal foods do not contain the same levels of B–vitamins, iron and fiber as their gluten–containing counterparts [13,14]. ifferent proteins have been proposed as alternative for both playing the polymer role and increasing the nutritional value of gluten–free products. The incorporation of other ingredients/nutrients like omega–3 lipids, specific proteins, etc. is a choice to improve the nutritional composition of gluten–free products.
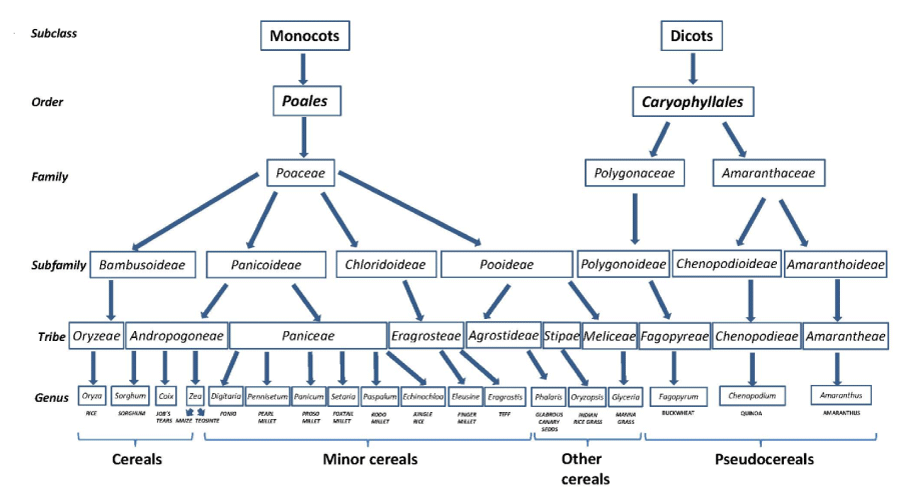
Many family members grains of grasses, closely related to wheat, rye and barley are considered celiac–toxic based on taxonomy. Further studies have supported this hypothesis by molecular evidences focused on protein homology in grains [5,15,16]. Members belonging to other tribes, are considered safe and can serve as substitutes and provide flours for cooking and baking for celiac and non celiac gluten–sensitive individuals (Figure 1). There are protein studies in support of this conclusion, although the studies are not sufficiently complete to provide more than guidance [5].
Figure 1: Taxonomic relation of known non-toxic cereals, minor cereals, pseudocereals and other cereals in the context of celiac disease.
Non–gluten–containing sources available in product formulation include cereals (rice, corn and sorghum), minor cereals (fonio, teff, millet and job’s tears), pseudocereals (buckwheat, quinoa and amaranth) and other cereals. Some of these grains are also nutrientdense and thus, their incorporation in the GFD could not only add variety but also improve its nutritional quality. As the environmental conditions for growing these grains are variable, availability of regular supplies is not always assured.
The proximate chemical composition of alternative gluten–free grains is presented in Table 1.
Protein
(g/100 g)
Fat
(g/100 g)
Carbohydrate
(g/100 g)
Crude fiber
(g/100 g)
Dietary fiber
(g/100 g)
Minerals
(mg/100 g)
Cereals
Rice
7.7017
2.2017
73.7 017
1.6318
2.2017
1.2017
Corn
8.8017
3.8017
65.0017
ND
9.8017
1.3017
Sorghum
10.4019
1.9019
72.6019
1.6019
ND
1.6019
Minor cereals
Fonio
8.0520
3.2520
79.0520
5.8520
ND
3.5020
Teff
9.6021
2.0021
73.0021
3.0021
ND
2.9021
Pearl millet
14.8022
4.8622
59.8022
12.1922
ND
1.6422
Proso millet
11.5823
4.9023
80.1023
0.7023
ND
ND
Finger millet
8.2024
1.80024
83.3024
3.5024
ND
2.7024
Foxtail millet
11.5025
2.3825
75.2025
ND
ND
3.3026
Kodo millet
9.8026
3.6026
66.6026
5.2026
37.0026
3.3026
Teosinte
4.527
ND
ND
32.227
ND
10.827
Jungle rice
5.227
ND
ND
34.827
ND
12.427
Job's tears
6.7028
2.5028
85.9028
ND
15.1028
ND
Pseudocereals
Buckwheat
12.5029
2.1029
75.7430
0.7030
29.5029
1.4230
Quinoa
16.5031
5.2029
69.0031
2.3032
14.2029
2.7029
Amaranth
16.5029
5.7029
70.3029
3.9032
20.6029
3.2532
Other cereals
Glabrous canary seed
23.7033
7.9033
60.9333
ND
7.3033
2.3033
Table 1: Average value based on dry matter basis (protein: N x 6.25) [17-33].
Cereals
Rice: Rice is one of the most important foods in the human diet due to its cultivation is widespread. Rice is the seed of the monocot plant of the genus Oryza, belonging to the grass family Poaceae (formally Graminae), which includes twenty wild species and two cultivated ones, Oryza sativa (Asian rice) and Oryza glaberrima (African rice). In spite of rice is mainly consumed as white grain in the last decade, dozens of products containing rice as an ingredient have appeared on the food market [34]. The hypoallergenic qualities of rice have encouraged a significant increase in the use of rice flour in the formulation of gluten–free products. However, the use of hydrocolloid, emulsifier, enzyme or protein is necessary to confer viscoelastic properties in the products [34,35].
Corn: Maize or corn (Zea mays subsp. mays L) has been considered a safe cereal for celiac patients and used as alternative to elaborate gluten–free foodstuffs [36]. A study confirmed the improvement of some patients with refractory celiac disease on GFD when a corn–free diet was prescribed [37]. However, there is controversy over the maize safety. Studies have demonstrated that zeins, the maize prolamins, were able to induce an inflammatory response through contact with mucosa in some CD patients [38]. Indeed, a high degree of identity in maize prolamins to the celiac toxic peptides was found and their integrity after gastrointestinal proteolysis is unknown [36,39].
The reaction to maize prolamins in CD patients appears to be a rare event. The confirmation that zeins play a role in the pathogenesis of CD will be useful information for the follow–up of some nonresponsive celiac patients. Despite the low content of zeins in maizecontaining foods compared with that of gliadins in wheat–containing foods, maize could be responsible for persistent mucosal damage in a very limited subgroup of CD patients [40].
Sorghum: Sorghum is considered a safe cereal for celiac patients due to it is more closely related to maize than to wheat, rye and barley. Sorghum (genus of numerous species of grasses) is a drought–and heat–tolerant cereal grain that grows in semiarid conditions. Whereas sorghum traditionally has been used primarily as animal feed in Western countries, nearly 40% of the world sorghum production is used for human food in Africa and India.
Different studies based on immunological assays and in vitro and in vivo challenges of sorghum food products have supported that sorghum might provide a good basis for gluten–free breads and other baked products such as pasta, cookies or snacks [41,42]. Recently, Pontieri et al. [43] demonstrated that sorghum may be definitively considered safe for consumption by people with CD for the absence of toxic gliadin–like peptides using in silico approaches and biochemical/immunochemical experiments.
Minor cereals
Minor cereals, so called because they are less common and are only grown in a few small regions of the world, include teff, millet (pearl, proso, finger, foxtail and Kodo), fonio (white and black), teosinte, jungle rice and Job’s tears [5,44,45].
Teff: Teff (Eragrostis tef) is a cereal traditionally grown in Ethiopia and used to make injera or flat bread. Teff is the smallest of all grains in the world and it is classified on the basis of seed color, ranging from milky white to almost black. Comparing to the available gluten–free products, teff has a higher vitamin, mineral (calcium, iron, magnesium and zinc) and fiber content. Therefore, teff could be used for the same purposes than wheat flour, as valuable supplement to a GFD [46,47].
Millet: Millet refers to a number of different species belong to the order Poales. Discrepancies exist concerning classification of family millet, with some references giving the family name Gramineae, and others classifying it in the family Poaceae. There are many varieties of millets and the four major types are pearl millet, proso millet, finger millet and foxtail millet. Other minor millets are barnyard millet (Echinochloa spp.), kodo millet (Paspalum scrobiculatum), little millet (Panicum sumatrense), guinea millet (Brachiaria deflexa = Urochloa deflexa) or browntop millet (Urochloa ramosa = Brachiaria ramosa = Panicum ramosum) [48].
In many African and Asian areas, millet serves as the major food component. Various traditional foods and beverages, such as bread (fermented or unfermented), porridges, and snack foods are made of millet [49–51].
Several potential health benefits (preventing cancer and cardiovascular diseases, reducing tumor incidence, lowering blood pressure, etc.) have been reported for millet [51–53]. However, millet is most recognized nutritionally for being a good source of minerals magnesium, manganese and phosphorus. The presence of all the required nutrients in millet makes it suitable for large–scale utilization in the manufacture of food products (baby foods, snacks, and dietary food).
Millet grains are usually processed to improve their edible, nutritional, and sensory properties by commonly used traditional processing techniques. Nevertheless, negative changes in these properties occur due to the industrial methods for the formulation of millet products are not well developed, being necessary formidable challenge to both the cereal technologist and the baker.
Pearl millet: The most important millet type for food consumption is pearl millet or bajra (Pennisetum glaucum), comprising 40% of the world production, which is similar in texture to rice flour [44,48]. Pearl millet is an important food across the Sahel although India is its largest producer [54]. Pearl millet is rich in resistant starch, soluble and insoluble dietary fibers, minerals, and antioxidants [48,55]. The bioavailability of zinc and iron from pearl millet was compared with that of other important grain food staples in India in a carefully designed trial reported in 1999 [56]. Absorption and liver amounts of both micronutrients from pearl millet and wheat were superior tothose from sorghum and rice. New varieties of pearl millet are being conventionally bred to provide more dietary iron to rural forming communities in arid drought–prone regions where few other crops thrive [57]. Iron supplementation in this alternative cereal would provide an advantage in celiac patients where anemia is a repeated manifestation. However, more complete characterization of pearl millet proteins and its functionality would provide useful information for marketing gluten–free foods [58].
Proso millet: Proso millet (Panicum miliaceum) is also referred to common millet, hog millet, broom corn, yellow hog, hershey, and white millet [59]. Today, proso millet plays an important role in northwest China and Kazakhstan, as well as the central and southern states of India, Eastern Europe, USA and Australia. The protein content of proso millet (11.6% of dry matter) was found to be comparable with that of wheat. Nevertheless, proso millet was significantly richer in essential amino acids (leucine, isoleucine, and methionine) than wheat protein [51,60]. A recent study has proposed proso millet malt wort as raw material for the manufacture of foods and beverages, especially on the way to producing gluten–free beers [61].
Finger millet: Finger millet (Eleusine coracana), commonly known as Ragi in India, is widely gluten–free cereal grown in Asian and African countries. Of all the cereals and millets, finger millet has the highest amount of calcium (344 mg⁄100g) and potassium (408 mg⁄100g) [62]. What particularly stands out is its minerals, sulfur containing amino acids and dietary fiber content compared to white rice, the current major staple in India [63]. Tatham et al. [64] demonstrated that the prolamins of finger millet were closely related to the a–prolamins of the Andropogoneae (maize, sorghum and Job's tears) despite being classified in different subfamilies of the Poaceae by SDS–PAGE, amino acid analysis and N–terminal amino acid sequencing.
Foxtail millet: Foxtail millet is an economically important crop grown and consumed all over the world, especially in India, China, and other parts of Asia, North Africa, and the Americas [65]. There are over 12 varieties of foxtail millet, Setaria italica, which commonly occur as weeds. They bear different names, such as German, Italian, Hungarian and Siberian millet. The plants are small compared to other cultivated grasses. Foxtail millet has been cultivated especially as a forage crop and widely used in crop rotation and as a supplementary or catch crop after some other crop has failed. The reported starch content of foxtail millet is about 50–55%, which is relatively low for cereals [66]. Foxtail contains polyphenols, phytic acid, and oxalate as anti–nutritional factors [67]. Foxtail millet was determined by immunological test to have prolamin content below 10 mg⁄100 g grains and to be suitable for the diet in celiac disease. This was also confirmed by electrophoretic analysis [68].
Kodo millet: Kodo millet (Paspalum scrobiculatum), indigenus to India, is rich in glutamic acid, alanine, leucine, and serine, but deficient in the essential amino acid lysine. Especially noteworthy is the high dietary fiber content (37% to 38%) and polyunsaturated fatty acids in kodo millet among the cereals [51,64,69]. Karuppasamy et al. [70] found that incorporation of kodo millet as raw material for bread resulted in highly acceptable on the physical, sensory and nutritional characteristics. Fibre, calcium and iron content of this small millet incorporated bread were higher than for the refined wheat bread.
Fonio: Fonio is one of the oldest cultivated cereals in Africa and is believed to be a healthy and cheap addition to European diets and at the time generating incomes for local producers in Africa [71,72]. This cereal can survive in poor soil conditions such as sandy and acidic soils and its composition is similar to millets (limited in lysine, but rich in methionine) [67]. This cereal is highly demanded in Englishspeaking countries, has received increasing attention in research and development in recent years.
Among fonio, we can distinguish two species, acha (Digitaria exilis) and iburu (Digitaria iburua). Acha and iburu proteins have composition similar to that of white rice. Fonio cereal grains are mostly consumed whole, perhaps because of their small size, each seed is only slightly larger than a grain of sand [72]. As rich source of fibre and other phytonutrients, they can be used as ingredients helping to improve nutritional profiles without compromising taste and quality in products. There is scarce information available regarding the technological quality of these cereals [73]. The use of acha and iburu is mainly limited to traditional foods. Wholemeal acha and iburu flours can be used in the preparation of a number of biscuits and snacks that could be useful for individuals with gluten intolerance [74]. Indeed, Jideani et al. [75] produced wheatless bread from acha flour and determined consumer acceptability of the product. This study demonstrated a loaf comparable to wheat bread in terms of crumb color, crumb texture and overall acceptability.
Teosinte: Teosinte (Zea spp.) is an annual and perennial grass endemic to Mexico and Central America (Guatemala and Nicaragua). It is a critical component of maize evolution. There are five recognized species of teosinte: Zea diploperennis, Zea perennis, Zea luxurians, Zea nicaraguensis, and Zea mays [76]. Teosinte grains have high nutritional value with higher total nitrogen and methionine content than maize. There is no difference in lysine, tryptophan or niacin [77].
Jungle rice: Jungle rice (Echinochloa colona) is considered a noxious gluten–free weed in several crops and particularly in rice fields as it mimics rice in its vegetative growth stage. Jungle rice originated from India but it is now widespread in the tropics and subtropics. It has relatively low crude protein content (dry matter ˜5%) and high crude fibre content (˜35%) [78].
Job’s tears: Job’s tears (Coix lacryma–jobi), also known as Chinese Pearl Barley or adlay, is a type of grain wild tropical Asian grass related to maize. Job’s tears is widely appreciated as a health food supplement. It is naturally gluten–free, but similar to other grains it may be contaminated during processing by commingling with gluten grains such as wheat. The adlay consumption decreases level of serum cholesterol, triglycerides, and low–density lipoprotein cholesterol, increases high–density lipoprotein cholesterol, lowers liver lipids, prevents fatty liver, and increases lipid excretion [79].
Pseudocereals
Pseudocereals are defined as starchy food grains excluding those currently classified as cereals, legumes, oilseeds and nuts [80,81]. Pseudocereals are dicotyledonous plants that resemble in function and composition those of the true cereals. These grains have been increasingly researched as nutritious ingredients in gluten–free formulations and as source of bioactive compounds with healthpromoting effects. Some of the most attractive features of these seeds include their high quality protein and the presence of abundant quantities of fiber and minerals such as calcium and iron [11]. Therefore, pseudocereals not only add to gluten–free diet but also improve its nutritional quality.
Recently, the use of pseudocereals producing small, grain–like seeds like amaranthus and quinoa (Amaranthaceae family), and buckwheat (Polygonaceae family), belonging to Caryophyllales order, have been considered for the preparation of gluten–free food products because they lack of toxic seed proteins and is of high nutritional value [82]. The known lack of toxicity for most of these pseudocereals was based on their taxonomical classification rather than a direct evaluation of their immunostimulatory activity [5].
Several studies affirmed that amaranth and quinoa have high quality protein in terms of digestibility, efficiency ratio and nutrition balance, almost equivalent to that of milk protein casein [83,84]. Additionally, these pseudocereals are also rich in polyunsaturated fatty acids (high linolenic:linoleic acid ratio) and bioactive compounds such as ?– and β–tocopherol, polyphenols and flavonoids [5].
Amaranth: The genus Amaranthus L. contains more than 60 species. The main amaranth species being cultivated for their seeds and most used for human nutrition are A. caudatus in Peru and other Andean countries, A. cruentus in Guatemala and A. hypochondriacus in Mexico [85]. Amaranth proteins consist mainly of albumins and globulins, whereas prolamins are very scarce. The essential amino acids content is high in amaranth seeds and the amino acid composition is better balanced than in most cereals. It is a good source of riboflavin, vitamin E, calcium, magnesium and iron, among minerals [82]. The high calcium content in amaranth seeds has special relevance for celiac subjects due to the well known prevalence of osteopenia and osteoporosis among newly diagnosed celiac patients [86–88]. Different studies, focused on investigations concerning the protein patterns from different amaranth cultivars, suggested that amaranth may be safely included in the GFD of subjects suffering from CD. However, controlled clinical studies are necessary to confirm the results and support the inclusion in the celiac’s diet [82]. Gambus et al. [89] replaced corn starch with amaranth flour to enhance the protein and fibre contents of gluten–free breads. At a 10% replacement level, protein and fiber levels increased by 32% and 152% respectively, whilst sensory quality was unaffected.
Quinoa: sQuinoa (Chenopodium quinoa) was a staple food of the ancient civilizations of the Andes of South America, and is nowadays mainly grown in the Andean Countries of Peru and Bolivia. Sometimes quinoa is called pseudocereal because of its grain like appearance and sometimes a pseudo–oilseed because of its high content of fat [90]. Quinoa has been authorized to be sown in Europe, North America, Asia and Africa [91]. Recently, France has reported an area of 200 ha with yields of 1080 kg⁄ha and Kenya has shown high seed yields (4 t⁄ha) [92]. There are hundreds of varieties of quinoa, ranging in color from white to red and purple to black [44].
Quinoa is an excellent example of “functional food” that aims at lowering the risk of various diseases [90]. The nutritional value of quinoa protein is comparable to that of milk protein [83]. Quinoa has a high biological value (83%) because of its high concentration of proteins, providing all of the essential amino acids [93–95]. Quinoa is rich in minerals such as calcium, iron, zinc, magnesium and manganese, which give the grains high value for different target populations: for instance, adults and children benefit from calcium for bones and from iron for blood functions [84,90]. Due to its excellent nutritional value and a potential for production in various climates, quinoa has been classified as one of the humanity’s most promising crops [96].
Several studies to examine the suitability of quinoa for patients with CD have been carried out in last years and concluded that quinoa could be a safe addition to a GFD [95]. However, two cultivars had celiac–toxic epitopes that could activate the adaptive and innate immune responses in some patients with CD [95]. Since quinoa is an ancient crop, available technical information regarding the properties of chemical composition and functional properties is limited [90]. A complete in vivo characterization of quinoa protein reactivity is needed to recommend their consumption to patients with CD [96,98,99].
Buckwheat: Buckwheat (Fagopyrum spp.) is one of the traditional crops cultivated in Asia, Central and Eastern Europe [100,101]. It is botanically classified as a fruit. Buckwheat can be consumed as grains or as flour. The toasted grains are known as Kasha. Two species of buckwheat are cultivated for food consumption, Fagopyrum esculentum or common buckwheat and Fagopyrum tartaricum or tartary buckwheat. Common buckwheat is the most common cultivated buckwheat species [102–104] and it is primarily consumed in Asian countries.
Buckwheat is a highly nutritious pseudocereal known as a dietary source of protein with favorable amino acid composition and vitamins [105], starch, dietary fiber [104], essential minerals [106], trace elements [105] and rutin [101]. The consumption of buckwheat in western countries including the United States is increasing due to being the substitute for wheat flour for gluten–sensitive patients and as a health food by its nutrient content [107–109]. Recently, cases of buckwheat allergy have been reported in Japan, Korea and Europe [110].
Other Cereals
Glabrous canary seed: An alternative grain that may potentially be considered for celiac patients is glabrous canary seed (Phalaris canarienses L.) that belongs to the Poaceae (Gramineae) family. Canary seed is originally from the Mediterranean region but is now grown in many parts of the world for birdseed. In a recent study carried out by Boye et al. [111], glabrous canary seeds were reported as a good alternative gluten–free cereal. This study confirmed no presence of celiac–related gluten fragments from wheat, rye, barley, or their derivatives in glabrous canary seed. Three different techniques (ELISA, mass spectrometry and immunoblotting) were reported to assess the presence of gluten–specific protein fragments in glabrous canary seeds and to support gluten–free labeling of products that contain it. The chemical composition of canary seed shows that it has potential as a food crop. Indeed, canary seed is a richer source of most required minerals. Glabrous canary seed contains an average of 24% protein, 8% crude fat, 56% starch, and 7% total dietary fiber. There are also smaller amounts of soluble sugars and ash in this grain [111].
Wild graminoids: Indian ricegrass (Achnatherum hymenoides=Oryzopsis hymenoides), also known as Indian millet or montina [112,113], was a widely used food plant of Indian tribes in the USA and nowadays gluten–free all–purpose baking flour ismarketed from this cultivated cereal.
The Middle European traditions of culinary use of wild graminoids (manna grass–Glyceria fluitans, plicate sweet–grass– Glyceria nocata, cheat–Bromus secalinus, tribe Bromeae, and green bristle grass–Setaria glauca, tribe Paniceae) form an area for future research which may provide valuable gluten–free cereals. Glyceria is one of the main genera in the small, isolated tribe Meliceae [114]. Manna grass was used to make gruel (boiled with milk), desserts with butter and bread, which were highly valued. The importance of manna grass products as a Polish cuisine specialty was reported by foreigners visiting the country in the past [115]. Based on taxonomy, manna grass can be considered gluten–free; however, further studies are needed to measure its safety and usefulness for celiac patients. It needs to be stressed that it can take years to select cultivars of Glyceria fluitans suitable for intensive agriculture [45].
Conclusion
The health of a significant number of people depends on the absence of gluten in the diet. An increasing number of CD patients diagnosed, a new gluten–associated clinical condition named NGCS and an important group of the population with wheat allergies have enabled an estimated prevalence in Europe that may rise up to 1%, 6% and 0.2–1.3%, in the general population respectively, but large variations among countries have been shown. Therefore, the complete elimination of gluten proteins contained in the known cereals wheat, rye, barley and certain oat varieties from the diet is the key to glutenfree related pathologies management.
Although official data are lacking, the number of patients embracing a GFD is rapidly growing as well as a global market of gluten–free products. Here, we review the status of potential alternative grains tolerated by patients with celiac disease, non–celiac gluten sensitivity and gluten allergy. Non–gluten–containing sources frequently used in product formulation include cereals (rice, corn and sorghum), minor cereals (fonio, teff, millet, and job's tears) and pseudocereals (buckwheat, quinoa and amaranth). However, new studies seem to show that certain grains, traditionally considered safe for celiac patients, could activate the immune response in some celiac patients. Although the application of pseudocereal flours as gluten–free ingredients is increasing, the commercial production of pseudocereal–containing gluten–free products is limited, and only a small number of products containing these flours are available.
Important challenge to both alternative grains technologist and the baker is necessary for industrial scale–up. Availability of palatable gluten–free grain foods is expected to grow in coming years, providing many opportunities for agriculture companies to market new tasty and affordable grains.
Acknowledgments
This work was supported by Junta de Andalucía (Project P09AGR–4783).
References
- Rubio-Tapia A, Kyle R A, Kaplan E L, Johnson D R, Page W, et al. Increased prevalence and mortality in undiagnosed celiac disease. Gastroenterology. 2009; 137: 88-93.
- Lionetti E, Catassi C. New clues in celiac disease epidemiology, pathogenesis, clinical manifestations, and treatment. Int Rev Immunol. 2011; 30: 219-231.
- Bernardo D, Pe&nTilde;a A S. Developing strategies to improve quality of life of patients with gluten intolerance in patients with and without celiac disease. Eur J Intern Med. 2012; 23: 6-8.
- Sapone A, Bai JC, Ciacci C, Dolinsek J, Green PH. Spectrum of gluten-related disorders: consensus on new nomenclature and classification. BMC Med. 2012; 10: 13.
- Comino I, Moreno Mde L, Real A, Rodríguez-Herrera A, Barro F. The gluten-free diet: testing alternative cereals tolerated by celiac patients. Nutrients. 2013; 5: 4250-4268.
- Sollid LM. Coeliac disease: dissecting a complex inflammatory disorder. Nat Rev Immunol. 2002; 2: 647-655.
- Corrao G, Corazza GR, Bagnardi V, Brusco G, Ciacci C. Mortality in patients with coeliac disease and their relatives: a cohort study. Lancet. 2001; 358: 356-361.
- Bloksma AH, Bushuk W (1998). Rheology and chemistry of dough. In: Wheat: chemistry and technology (Pomeranz, ed), pp131-200. Minnesota: AACC.
- Gallagher E, Gormley TR, Arendt EK. Recent advances in the formulation of gluten-free cereal-based products. Trends Food Sci Tech. 2004; 15: 143-152.
- Rashtak S, Murray JA. Review article: coeliac disease, new approaches to therapy. Aliment Pharmacol Ther. 2012; 35: 768-781.
- Alvarez-Jubete L, Auty M, Arendt E K, Gallagher E. Baking properties and microstructure of pseudocereal flours in gluten-free bread formulations. Eur Food Res Technol. 2010; 230: 437-445.
- Thompson T, Dennis M, Higgins LA, Lee AR, Sharrett MK. Gluten-free diet survey: are Americans with coeliac disease consuming recommended amounts of fibre, iron, calcium and grain foods? J Hum Nutr Diet. 2005; 18: 163-169.
- Thompson T. Thiamin, riboflavin, and niacin contents of the gluten-free diet: is there cause for concern? J Am Diet Assoc. 1999; 99: 858-862.
- Thompson T. Folate, iron, and dietary fiber contents of the gluten-free diet. J Am Diet Assoc. 2000; 100: 1389-1396.
- Real A, Comino I, de Lorenzo L, Merchán F, Gil-Humanes J. Molecular and immunological characterization of gluten proteins isolated from oat cultivars that differ in toxicity for celiac disease. PLoS One. 2012; 7: 48365.
- Tye-Din JA, Stewart JA, Dromey JA, Beissbarth T, van Heel DA. Comprehensive, quantitative mapping of T cell epitopes in gluten in celiac disease. Sci Transl Med. 2010; 2: 41.
- Koehler P, Wieser H. Chemistry of Cereal Grains (2013). In: Handbook on Sourdough Biotechnology (Gobbetti M, Gänzle M, eds), pp 1-45. USA: Springer US.
- Oko AO, Ubi BE, Efisue AA, Dambaba N. Comparative Analysis of the Chemical Nutrient Composition of Selected Local and Newly Introduced Rice Varieties Grown in Ebonyi State of Nigeria. Int J Agric Forest. 2012; 2: 16-23.
- Nambiar, VS, Dhaduk JJ, Sareen N, Shahu T, Desai R. Potential Functional Implications of Pearl millet (Pennisetum glaucum) in Health and disease. J Appl Pharma Sci. 2011; 1: 62-67.
- Vénérande BY, Mohamed SM, Fatiou T, Hounhouigan JD. Structure and nutritional composition of Fonio (Digitaria exilis) grains: a review. Int Res J Biol Sci. 2013; 2: 73-79.
- Shewry P R (2002). The Major Seed Storage Proteins of Spelt Wheat, Sorghum, Millets and Pseudocereals . In: Pseudocereals and Less Common Cereals: Grain Properties and Utilization Potential (Belton P S, Taylor J R N, eds), pp 1-24. Berlin: Springer.
- Taylor JRN, Barrion SC, Rooney LW. Pearl millet-New developments in ancient food grain. Cereal Foods World. 2010; 55:16-19.
- Bagdia A, Balázsa G, Schmidt J, Szatmária M, Schoenlechner R, et al. Protein characterization and nutrient composition of Hungarian proso millet varieties and the effect of decortication. Acta Alimentaria. 2011; 40:128-141.
- Devi PB, Vijayabharathi R, Sathyabama S, Malleshi NG, Priyadarisini VB. Health benefits of finger millet (Eleusine coracana L.) polyphenols and dietary fiber: a review. J Food Sci Technol. 2011; 2: 73-79.
- Kamara MT, Zhou HM, Zhu KX, Amadou I, Tarawalie F. Comparative Study of Chemical Composition and Physicochemical Properties of Two Varieties of Defatted Foxtail Millet Flour Grown in China. Am J Food Technol. 2009; 4: 255-267.
- Saleh ASM, Zhang Q, Chen J, Shen Q. Millet Grains: Nutritional Quality, Processing, and Potential Health Benefits. Comprehensive Reviews in Food Science and Food Safety. 2013; 12: 281-295.
- Patel A. Nutritive value of commonly available feeds and fodders in India. Animal nutrition group. Anand: NDDB; 2012.
- Lin MH, Wu MC, Lu S, Lin J. Glycemic index, glycemic load and insulinemic index of Chinese starchy foods. World J Gastroenterol. 2010; 16: 4973-4979.
- Alvarez-Jubete L, Arendt EK, Gallagher E. Nutritive value and chemical composition of pseudocereals as gluten-free ingredients. Int J Food Sci Nutr. 2009; 60 Suppl 4: 240-257.
- Baljeet SY, Ritika BY, Roshan LY. Studies on functional properties and incorporation of buckwheat flour for biscuit making. International Food Research Journal. 2010; 17: 1067-1076.
- Koziol MJ. Chemical composition and nutritional evaluation of quinoa (Chenopodium quinoa wild). J Food Composition Analysis. 1992; 5: 35-68.
- Schoenlechner R, Siebenhandl S, Berghofer E (2008). Pseudocereals. In: Gluten-free cereal products and beverages (Arendt E K, Bello F D, ed), pp. 149-190. Massachusetts: Academic press.
- Cogliatti M. Canaryseed Crop. Scientia Agropecuaria. 2012; 1: 75-88.
- Rosell CM, Marco C (2008). Rice. In: Gluten free cereal products and beverages (Arendt EK and dal Bello F, eds), pp81-100. UK: Elsevier Science.
- Boye JI, Achouri A, Raymond N, Cleroux C, Weber D, et al. Analysis of Glabrous Canary Seeds by ELISA, Mass Spectrometry, and Western Blotting for the Absence of Cross-Reactivity with Major Plant Food Allergens. J Agric Food Chem. 2013; 61: 6102-6112.
- Cabrera-Chávez F, Iamett S, Miriani M, Calderón de la Barca AM, Mamone G, et al. Maize Prolamins Resistant to Peptic-tryptic Digestion Maintain Immune-recognition by IgA from Some Celiac Disease Patients. Plant Foods Hum Nutr. 2012; 67: 24-30.
- Accomando S, Albino C, Montaperto D, Amato GM, Corsello G. Multiple food intolerance or refractory celiac sprue? Dig Liver Dis. 2006; 38: 784-785.
- Kristjánsson G, Högman M, Venge P, Hällgren R. Gut mucosal granulocyte activation precedes nitric oxide production: studies in coeliac patients challenged with gluten and corn. Gut. 2005; 54: 769-774.
- Darewicz M, Dziuba J, Minkiewicz, P. Computational characterization and identification of peptides for in silico detection of potentially celiac-toxic proteins. Food Sci Technol Int. 2007; 13: 125-133.
- Ortiz-Sánchez JP, Cabrera-Chávez F, de la Barca AM. Maize prolamins could induce a gluten-like cellular immune response in some celiac disease patients. Nutrients. 2013; 5: 4174-4183.
- Carson L, Setser C, Sun X S. Sensory characteristics of sorghum composite bread. Int J Food Sci Technol. 2000; 35: 465-471.
- Ciacci C, Maiuri L, Caporaso N, Bucci C, Del Giudice L. Celiac disease: in vitro and in vivo safety and palatability of wheat-free sorghum food products. Clin Nutr. 2007; 26: 799-805.
- Pontieri P, Mamone G, De Caro S, Tuinstra MR, Roemer E. Sorghum, a healthy and gluten-free food for celiac patients as demonstrated by genome, biochemical, and immunochemical analyses. J Agric Food Chem. 2013; 61: 2565-2571.
- Saturni L, Ferretti G, Bacchetti T. The gluten-free diet: safety and nutritional quality. Nutrients. 2010; 2: 16-34.
- Hozyasz KK. Letter to the editor re: Comino, I., et al. Nutrients 2013, 5, 4250-4268. Nutrients. 2013; 5: 4964-4965.
- Hopman E, Dekking L, Blokland ML, Wuisman M, Zuijderduin W. Tef in the diet of celiac patients in The Netherlands. Scand J Gastroenterol. 2008; 43: 277-282.
- Pagano AE (2006) Whole Grains and the Gluten-Free Diet. In: The Celiac Diet (Parrish C R, ed.). pp 66-78.
- Amadou I, Gounga ME, Le G-W. Millets: Nutritional composition, some health benefits and processing-A Review. Emir J Food Agric. 2013; 25: 501-508.
- Chandrasekara A, Shahidi F. Determination of antioxidant activity in free and hydrolyzed fractions of millet grains and characterization of their phenolic profiles by HPLC-DAD-ESI-MSn. J Funct Foods. 2011; 3: 144-158.
- Chandrasekara A, Shahidi F. Bioaccessibility and antioxidant potential of millet grain phenolics as affected by simulated in vitro digestion and microbial fermentation. J Funct Foods. 2012; 4: 226-237.
- Saleh ASM, Zhang Q, Chen J, Shen Q. Millet Grains: Nutritional Quality, Processing, and Potential Health Benefits. Comprehensive Reviews in Food Science and Food Safety. 2013; 12: 281-295.
- Gupta N, Srivastava AK, Pandey VN (2012) Biodiversity and nutraceutical quality of some indian millets. Proceedings of the National Academy of Sciences.
- Truswell AS. Cereal grains and coronary heart disease. Eur J Clin Nutr. 2002; 56: 1-14.
- Bhattacharjee R, Khairwal IS, Bramel PJ, Reddy KN. Establishment of a pearl millet (Pennisetum glaucum (L.) R. Br.) core collection based on geographical distribution and quantitative traits. Euphytica. 2007; 155: 35-45.
- Ragaee S, Abdel-Aal E M, Noaman M. Antioxidant activity and nutrient composition of selected cereals for food use. Food Chem. 2006; 98: 32-38.
- Agte VV, Khot S, Girigosavi ST, Paknikar KM, Chiplonkar SA. Comparative performance of pearl millet- and sorghum- based diets vs. wheat- and rice-based diets for trace metal bioavailability. J Trace Elem Med Biol. 1999; 13: 215-219.
- Kodkany BS, Bellad RM, Mahantshetti NS, Westcott JE, Krebs NF, et al. Biofortification of pearl millet with iron and zinc in a randomized controlled trial increases absorption of these minerals above physiologic requirements in young children. J Nutr. 2013; 143: 1489-1493.
- Nambiar VS, Dhaduk JJ, Sareen N, Shahu T, Desai R. Potential Functional Implications of Pearl millet (Pennisetum glaucum) in Health and disease. J Appl Pharma Sci. 2011; 1: 62-67.
- Baltensperger DD (1996) Foxtail and proso millet. In: Progress in New Crops (Janick J, ed). Alexandria VA: ASHS Press.
- Kalinova J, Moudry J. Content and quality of protein in proso millet (Panicum miliaceum L.) varieties. Plant Foods Hum Nutr. 2006; 61: 45-49.
- Zarnkow M, Keβler M, Back W, Arendt E K, Gastl M. Optimisation of the Mashing Procedure for 100% Malted Proso Millet (Panicum miliaceum L.) as a Raw Material for Gluten-free Beverages and Beers. Jnl Institute Brewing. 2010; 116: 141-150.
- Devi PB, Vijayabharathi R, Sathyabama S, Malleshi NG, Priyadarisini VB. Health benefits of finger millet (Eleusine coracana L.) polyphenols and dietary fiber: a review. J Food Sci Technol. 2011; 1-20.
- Shobana S, Krishnaswamy K, Sudha V, Malleshi NG, Anjana R M (2013). Finger Millet (Ragi, Eleusine coracana L.): A Review of Its Nutritional Properties, Processing, and Plausible Health Benefits, In: Advances in Food and Nutrition Research (Jeyakumar H, ed), pp1-39. Massachusetts : Academic Press.
- Tatham AS, Fido RJ, Moore CM, Kasarda DD, Kuzmicky DD et al. Characterisation of the Major Prolamins of Tef (Eragrostis tef) and Finger Millet (Eleusine coracana). J Cereal Sci. 1996; 24: 65-71.
- B VS, Muthamilarasan M, Misra G, Prasad M. FmMDb: a versatile database of foxtail millet markers for millets and bioenergy grasses research. PLoS One. 2013; 8: e71418.
- Kumar KK, Parameswaran K P. Characterisation of storage protein from selected varieties of foxtail millet (Setaria italica (L) Beauv). J Sci Food Agric. 1998; 77: 535-542.
- Arendt EK, Dal Bello F. Gluten-free cereal, products and beverages. Food Science and Technology. Massachusetts : Academic Press; 2011.
- Petr J, Michalík I, Tlaskalová H, Capouchova I, Famera O, et al. Extention of the spectra of plant products for the diet in coeliac disease. Czech J Food Sci. 2003; 21: 59-70.
- Hegde PS, Chandra TS. ESR spectroscopic study reveals higher free radical quenching potential in kodo millet (Paspalum scrobiculatum) compared to other millets. Food Chem. 2005; 92: 177-82.
- Karuppasamy P, Malathi D, Banumathi P, Varadharaju N, Seetharaman K. Evaluation of quality characteristics of bread from kodo, little and foxtail millets. Int J Food Sci. 2012; 2: 2320-7876.
- Dury S, Meuriot V, Fliedel G, Blancher BGF, Drame D, et al (2007) The retail market prices of fonio reveal the demand for quality characteristics in Bamako, Mali. In: Communication at 106th seminar of the Europian Association of Agricultural Economists. Pro-poor development in low income countries: food, agriculture, trade, and environment.
- Jideani IA, Jideani VA. Developments on the cereal grains Digitaria exilis (acha) and Digitaria iburua (iburu). J Food Sci Technol. 2011; 48: 251-259.
- Jideani IA. Traditional and possible technological uses of Digitaria exilis (acha) and Digitaria iburua (iburu): a review. Plant Foods Hum Nutr. 1999; 54: 363-374.
- Ayo J A, Nkama I. Effect of acha (Digitaria exilis Staph) grain flours on the physical and sensory quality of biscuit. Nutr Food Sci. 2003; 33: 125-130.
- Jideani VA, Alamu R, Jideani IA. Preliminary study into the production of non-wheat bread from acha (Digitaria exilis). Nutr Food Sci. 2007; 37: 434-441.
- Loáisiga C H, Brantestam A K, Diaz O, Salomon O, Merker A. Genetic diversity in seven populations of Nicaraguan teosinte (Zea nicaraguensis Iltis et Benz) as estimated by microsatellite variation. Genet Resour Crop Ev. 2011; 58: 1021-1028.
- Melhus IE, Aguirre F, Scrimshaw NS. Observations on the nutritive value of teosinte. Science. 1953; 117: 34-35.
- Ahmed MMM, El-Hag, F M. Degradation characteristics of some Sudanese forages and tree pods using in sacco and gas production techniques. Small Rumin Res. 2004; 54: 147-156.
- Hung PV, Morita N. Distribution of phenolic compounds in the graded flours milled from whole buckwheat grains and their antioxidant capacities. Food Chem. 2008; 109: 325-331.
- Fletcher RJ (2004) Pseudocereals, overview, In: Encyclopedia of Grain Science (Wrigley G, Corke H, Walker C E, ed), pp488-493. Oxford: Elsevier.
- Aghamirzaei M, Heydari-Dalfard A, Karami F, Fathi M. Pseudo-cereals as a functional ingredient: effects on bread nutritional and physiological properties- . Intl J Agri Crop Sci. 2013; 5; 1574-1580.
- Ballabio C, Uberti F, Di Lorenzo C, Brandolini A, Penas E, et al. Biochemical and immunochemical characterization of different varieties of amaranth (Amaranthus L. ssp.) as a safe ingredient for gluten-free products. J Agric Food Chem. 2011; 59: 12969-12974.
- Ranhotra GS, Gelroth JA, Glaser BK, Lorenz KJ, Johnson DL. Composition and protein nutritional quality of quinoa. Cereal Chem. 1993; 70: 303-305.
- Repo-Carrasco R, Esponiza C, Jacobsen S-E. Nutritional value and use of the Andean crops: quinoa (Chenopodium quinoa) and ka&nTilde;iwa (Chenopodium pallidicaule). Food Rev Int. 2003; 19: 179-189.
- Bressani R (2003). Amaranth. In: Encyclopedia of food sciences and nutrition (Caballero B, ed), pp166-173. Oxford: Academic Press.
- Chand N, Mihas AA. Celiac disease: current concepts in diagnosis and treatment. J Clin Gastroenterol. 2006; 40: 3-14.
- Fasano A, Catassi C. Current approaches to diagnosis and treatment of celiac disease: an evolving spectrum. Gastroenterology. 2001; 120: 636-651.
- Rodrigo L . Celiac disease. World J Gastroenterol. 2006; 12: 6585-6593.
- Gambus H, Gambus F, Sabat R. The research on quality improvement of gluten-free bread by amaranthus flour addition. Zywnosc. 2002; 9: 99-112.
- Vega-Gálvez A, Miranda M, Vergara J, Uribe E, Puente L . Nutrition facts and functional potential of quinoa (Chenopodium quinoa willd.), an ancient Andean grain: a review. J Sci Food Agric. 2010; 90: 2541-2547.
- Bhargava A, Shukla S, Ohri D. Chenopodium quinoa-An Indian perspective. Ind Crop Prod. 2006; 23: 73-87.
- Nascimento AC, Coelho CI, Gueif&aTilde;o S, Santos M, Matos AS, et al. Characterisation of nutrient profile of quinoa (Chenopodium quinoa), amaranth (Amaranthus caudatus), and purple corn (Zea mays L.) consumed in the North of Argentina: Proximates, minerals and trace elements. Food Chemistry. 2014; 148: 420-426.
- Abugoch James LE. Quinoa (Chenopodium quinoa Willd.): composition, chemistry, nutritional, and functional properties. Adv Food Nutr Res. 2009; 58: 1-31.
- Gonzalez JA, Konishi Y, Bruno M, Valoy M, Prado FE. Interrelationships among seed yield, total protein and amino acid composition of ten quinoa (Chenopodium quinoa) cultivars from two different agroecological regions. J Sci Food Agric. 2012; 92: 1222-1229.
- Zevallos VF, Ellis HJ, Suligoj T, Herencia LI, Ciclitira PJ . Variable activation of immune response by quinoa (Chenopodium quinoa Willd.) prolamins in celiac disease. Am J Clin Nutr. 2012; 96: 337-344.
- Mäkinen OE, Zannini E, Arendt EK . Germination of oat and quinoa and evaluation of the malts as gluten free baking ingredients. Plant Foods Hum Nutr. 2013; 68: 90-95.
- Bergamo P, Maurano F, Mazzarella G, Iaquinto G, Vocca I . Immunological evaluation of the alcohol-soluble protein fraction from gluten-free grains in relation to celiac disease. Mol Nutr Food Res. 2011; 55: 1266-1270.
- De Vincenzi M, Silano M, Luchetti R, Carratù B, Boniglia C . Agglutinating activity of alcohol-soluble proteins from quinoa seed flour in celiac disease. Plant Foods Hum Nutr. 1999; 54: 93-100.
- Berti C, Ballabio C, Restani P, Porrini M, Bonomi F, et al. Immunochemical and molecular properties of proteins in Chenopodium quinoa. Cereal Chem. 2004, 81: 275-277.
- Wijngaard H H, Arendt E K. Buckwheat. Cereal Chem J. 2006; 83: 391-401.
- Vojtíšková P, Kmentová K, Kubán V, Krácmar S. Chemical composition of buckwheat plant (Fagopyrum esculentum) and selected buckwheat products. J Microbiol Biotechnol Food Sci. 2012; 1: 1011-1019.
- Ikeda K. Buckwheat: composition, chemistry, and processing. Adv Food Nutr Res. 2002; 44: 395-434.
- Mazza G, Oomah B D. (2003). Buckwheat. In: Encyclopedia of food sciences and nutrition B (Caballero, ed), pp692-69. Oxford: Academic Press.
- Skrabanja V, Kreft I, Golob T, Modic M, Ikeda S, et al. Nutrient content in buckwheat milling fractions. Cereal Chem. 2004; 81: 172-176.
- Bonafaccia G, Marocchini M, Kreft I. Composition and technological properties of the flour and bran from common and tartary buckwheat. Food Chem. 2003; 80: 9-15.
- Steadman KJ, Burgoon MS, Lewis BA, Edwardson SE, Obendorf RL. Buckwheat seed milling fractions: description, macronutrient composition, and dietary fiber. J Cereal Sci. 2001; 33: 271-278.
- Stember RH . Buckwheat allergy. Allergy Asthma Proc. 2006; 27: 393-395.
- Cogliatti M. Canaryseed Crop. Scientia Agropecuaria. 2012; 1: 75-88.
- Wieslander G . Review on buckwheat allergy. Allergy. 1996; 51: 661-665.
- Panda R, Taylor SL, Goodman RE. Development of a Sandwich Enzyme-Linked Immunosorbent Assay (ELISA) for Detection of Buckwheat Residues in Food J Food Sci. 2010; 75: 110-117.
- Boye JI, Achouri A, Raymond N, Cleroux C, Weber D. Analysis of glabrous canary seeds by ELISA, mass spectrometry, and Western blotting for the absence of cross-reactivity with major plant food allergens. J Agric Food Chem. 2013; 61: 6102-6112.
- Dunmire WW, Tierney GD. Wild plants and native peoples of the Four Corners. Santa Fe: Museum of New Mexico Press; 1997.
- Hozyasz KK (2012). Malo Znane Odmiany i Gatunki Zbóz Bezglutenowych (Minor Cultivars and Species of Gluten-Free Cereals). In: Celiakia. Dieta Bezglutenowa-Poradnik; Stowarzyszenie Przyjaciól Chorych na Celiakie Przekreslony Klos. Poland: Bydgoszcz.
- Tsvelev NN. Synopsis of the mannagrass genus, Glyceria (Poaceae). Bot. Zhurn. (Moscow and Leningrad). 2006; 91: 255-276.
- Luczaj LJ, Dumanowski J, Kohler P, Mueller-Bieniek A. The use and economic value of manna grass (Glyceria) in Poland from the Middle Ages to the twentieth century. Hum Ecol. 2012; 40: 721-733.