Research Article
Austin J Nutri Food Sci. 2014;2(5): 1026.
Comparative Microbial Analysis and Storage of Tigernut-Soy Milk Extract
Udeozor LO1* and Awonorin SO2
1Department of Food Science and Technology, Federal University of Technology, Nigeria
2Department of Food Science and Technology, University of Agriculture Abeokute, Nigeria
*Corresponding author: :Udeozor LO, Department of Food Science and Technology, Federal University of Technology, P.M.B 1526, Owerri, Imo State, Nigeria
Received: March 18, 2014; Accepted: April 07, 2014; Published: April 08, 2014
Abstract
Fresh tigernuts and soybean seeds were processed and blended at different proportions to formulate six new products of natural Tigernut–Soy Milk Extract (TSME) samples: (TME: SME)– 100:0, 90:10, 80:20, 70:30, 60:40 and 50:50 without addition of spices and chemical preservatives. The samples were evaluated for their microbiological status. Microbiological examination of the products was carried out for a period of 14 days under refrigeration storage (4°C) and ambient storage (28±2°C). A total of 7 different genera of microorganisms were isolated from the blends and a direct relationship existed between the microbial content of the samples and the rate of substitution. Varying proportions, length of storage and storage conditions significantly (p<0.05) affected the microbiological status of the samples. The refrigerated milk products were microbiologically stable during storage and had no coli form growth throughout the storage period. The bacterial and fungal growth at zero days of storage was not higher than 102cfu⁄ml; however, the samples stored under ambient condition had 104cfu⁄ml. The microbial status of the samples revealed that the different storage conditions (refrigeration and ambientstorage) affected the quality of beverages differently, thus the beverage should be consumed immediately after processing.
Keywords: Microbiological Status; Milk Extract; Storage; Soybean; Tigernuts
Introduction
Tiger–nut (Cyperus esculentus L.) belongs to the Division– Magnoliophyta, Class–Liliopsida, Order–cyperales and Family– Cyperaceae and was found to be a cosmopolitan, perennial crop of the same genus as the papyrus plant. The tubers are about the size of peanuts and are abundantly produced in Nigeria. It has many other names like Zulu nut, yellow nut grass, ground almond, and chufa, edible rush and rush nut. Other names of the plant are earth almond as well as yellow nut grass [1]. In Nigeria, the Hausas call it “Aya”, Yorubas “imumu”, the Igbos “aki Hausa”, “ofio” in southern Nigeria [2]. Tiger–nut has been cultivated since early times (chiefly in south Europe and West Africa) for its small tuberous rhizomes which are eaten raw or roasted, used as hog feed or pressed for its juice to make a beverage. Non–drying oil (usually called chufa) is equally obtained from the rhizome. The nuts which are cultivated throughout the world are also found in the Northern part of Nigeria and other West Africa Countries like Guinea, Cote d'ivore, Cameroon, Senegal, America and other parts of the World (Irvine, 1969).
The nut was found to be rich in myristic acid, oleic acid, linoleic acid [3], with oleic acid being the most abundant [4]. Tiger–nut was reported as very healthy as it helps in preventing heart attacks, thrombosis and activates blood circulation. It is believed that they help to prevent cancer especially of the colon due to high content of soluble glucose. Tiger–nut was equally reported to have positive effect on cholesterol level due to high content of vitamin E. They are thought to be beneficial to diabetics and those seeking to reduce cholesterol or lose weight. The very high fibre content combined with a delicious taste; make them ideal for healthy eating [2]. The nut is rich in energy content (starch, fat, sugars & protein), mineral (phosphorus, potassium) and vitamins E and C. The nut was found to be ideal for children, older persons and sportsmen [5]. The inclusion of 33.33% of tiger–nut in the diet of cockerel starters was reported by [6]. Since the tubers contain 36% oil, C. esculentus has been suggested as potential oil crop for the production of biodiesel [4]. The oil remained uniformly liquid at refrigeration temperature; this makes the oil suitable for salad making [7]. Tiger nut was found to be a good substitute for some other (plant) milk sources. The nuts are valued for their highly nutritious starch content, dietary fibre, carbohydrate (mono, di and polysaccharides) [7]. The nut was reported to be rich in sucrose (17.4 to 20.0%), fat (25.50%), and protein (8%) [8]. The nut is also very rich in mineral content (Sodium, Calcium, Potassium, Magnesium, Zinc and traces of Copper [9]. Research studies have shown that 100g Tiger–nuts contain 386 kcal (1635 kJ), 7% proteins, 36% fats (oils), 31% starch, 21% glucose, and 26% fiber of which 14% is non–soluble and 12% soluble [4]. Tiger–nuts are regarded as a digestive tonic having a heating and drying effect in digestive system and alleviating flatulence. They also promote urine production. The nuts are said to be stimulant and tonic and also used in the treatment of indigestion, colic diarrhoea, dysentery and excessive thirst [10].
Soybean (Glycine max M) with 40% protein and 20% fat assumes the most predominant position in solving the nutritional imbalances prevailing. It not only provides quality macronutrients but also various other micronutrients, which are required to fight against malnutrition. Soybean is rich in protein content and can furnish protein supply to bridge up the protein deficiency gap at low–cost than any other crop [11]. Among the numerous soy food items, soymilk (extract of soybean) had been the first product ever prepared and consumed by human since long ago. Soymilk not only provides protein but also is a source of carbohydrate, lipid, vitamins and minerals [12]. Soymilk is an alternate of dairy animal milk due to its cheaper high–quality protein. Soymilk is a healthy drink and is important for people who are allergic to cow milk protein and lactose. In spite of its nutritional merits, it has not gained much popularity mainly due to its beany flavor and astringency [13].
In view of the scarce milk supply in various countries and the ever increasing gap between the requirement and population, efforts have been made over the years to develop alternative milk–like products from vegetable sources [14]. Soybeans, peanuts and cowpea have been accorded high attention in the investigations on milk substitutes. However, hardly any attention has been given to the use of locally available tiger–nut as such or in combination with milk to produce a palatable ready–to–serve bottled beverage, like ‘Horchata de chufas’ as done in South Europe especially in Spain [15]. Tigernut–Soy Milk (TSME) is a blended, processed commodity and is a source of high quality energy, protein, minerals, and vitamins. The implication of using the two different milk sources in the diet is the high contents of protein and fat. The total energy value of the milk is from the fat content hence, higher fat content is an indication of more total energies available [16].
The objectives of this work therefore were to evaluate the microbial status of tiger nut–Soy milk extract and to determine the effect of storage on the quality of tiger nut–soy milk extract.
Materials and methods
Fresh Tiger nuts and soybean seeds were purchased from the local traders in Eke–onunwa market in Owerri, Imo State, Nigeria. The equipment and chemicals used were obtained from Nigerian Institute of Science Laboratory Technology (NISLT), Samonda, Ibadan, Oyo State and Anthony Van–Leeuwenhoek Research Centre, Nekede, Owerri, Imo State.
Sample preparation: Fresh tubers of tigernuts and soybean seeds were sorted; foreign materials, bad⁄cracked nuts and seeds which may affect the taste and quality of the milk extract were removed, washed and rinsed with portable water and used to produce milk.
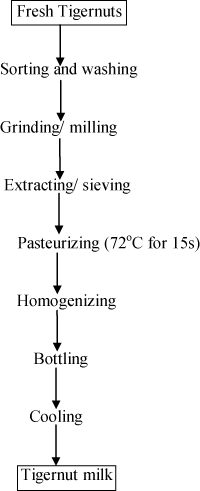
Tigernut milk extract: 1kg of the fresh tigernuts was blended several times into slurry with water (6L) in a Q–link auto–clean blender. The slurry was pressed using muslin cloth to extract the milk. The extract was pasteurized at 72°C for 15s. It was homogenized using improvised equipment; Q–link auto–clean blender, bottled when hot and rapidly cooled. The flow chart for tigernut milk extract (TME) production is shown in (Figure 1).
Figure 1: Flow chart for Tigernut milk extract (TME) production.
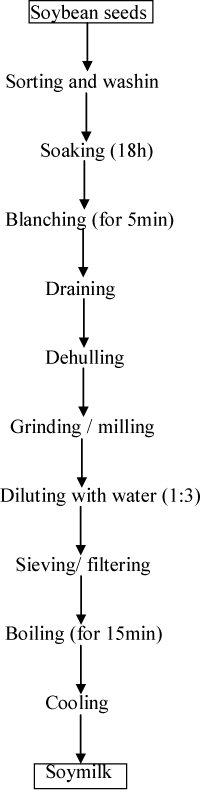
Soybean milk extract: 1kg of soybeans was soaked overnight for 18h in a 3L of warm portable water to give a bean: water ratio of 1:3. The beans were then drained, rinsed with treated water and blanched for 5min in boiling water. The blanched beans were drained, dehulled and ground with 750ml of treated water in a Q–link autoclean blender. The resulting slurry was filtered through a muslin cloth and the extract (milk) obtained was boiled for 15min. The flow chart for soymilk extract (SME) production is shown in (Figure 2).
Figure 2: Flow chart for soy milk extract (SME) production.
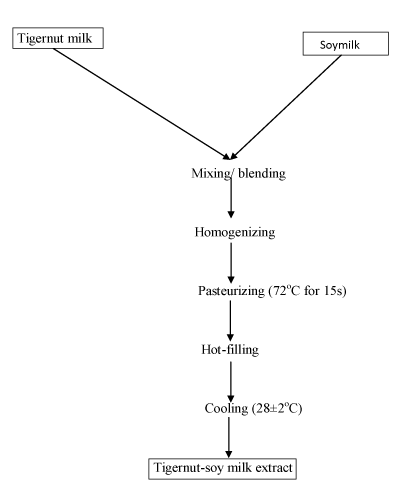
Tigernut–Soy milk extract (TSME) preparation: Tigernut milk extract (TME) and soymilk extract (SME) were mixed at varying proportions (TM:SM); 100:0, 90:10, 80:20, 70:30, 60:40 and 50:50 to obtain the final product (TSME). This was done using a food blender operated at full speed for 10min. The resulting blends were homogenized using improvised equipment; Q–link auto–clean blender and pasteurized at 72°C for 15s, hot–filled into sterile bottles (leaving 1cm head space), cooled to room temperature (28±2°C) and then stored in a refrigerator at 4°C until analyzed. The flow chart for tigernut–soymilk extract production is shown in (Figure 3).
Figure 3: Flow chart for the production of Tigernut-Soy Milk Extract.
The samples were duplicated, one portion stored at 4°C and the other stored at 28±2°C for 14days. A ten–fold serial dilution was done for the microbial analysis.
Microbial Analysis
Preparation of Diluent and Media: Diluent (peptone water) and media (Nutrient Agar, MacConkey Agar and Potato Dextrose Agar) were prepared according to manufacturer's specification [17].
Microbiological Analysis of Samples: One milliliter (1ml) of each sample was serially transferred into nine milliliters (9ml) of the sterile diluent (peptone water) with a sterile pipette and shaken vigorously. Serial dilution was continued until 106 dilution was obtained [17–19].
Aliquot portion (0.1ml) of the 106 and 105 dilutions were inoculated onto freshly prepared, surface–dried nutrient agar (NA) and MacConkey agar (MCA) respectively. The same quantity (0.1ml) of the 104 dilution was inoculated onto potato dextrose agar (PDA). The inoculi were spread with a sterile (hockey stick–like) glass spreader to obtain even distribution of isolates after incubation. Nutrient agar and MacConkey agar plates were incubated for 24–48h at 37°C, while potato dextrose agar plate was incubated at ambient temperature (28± 02°C) for 3–5 days [17].
Enumeration of Microbial Population: Total plate counts for the nutrient and MacConkey Agar were done by counting colonies at the reverse side of the culture plates. Total colony count was expressed in colony forming units per millilitre (cfu⁄ml) (Harrigan and McCance, 1990). Plate counts for PDA plates was done using colony counter for the yeasts and hand lens for moulds [18].
Characterization and identification of Microbial Isolates: Microbial isolates (bacteria, yeasts and moulds) were characterized based on colonial, microscopic and biochemical characteristics to know the genera using the method as described by [17,18]. Thus, the identities of the isolates were determined using a reference manual by [20–22].
Results and Discussion
Microbial Status and Effect of Storage on the Microbiological Quality of TSME: A total of 7 different genera of microorganisms were isolated from the milk extract samples as shown in (Table 1). These included Bacillus spp, Staphylococcus aureus, Saccharomyces spp, Penicillium spp, Aspergillus flavus, Mucor spp and Rhizopus spp.
A
B
C
D
E
F
Bacillus subtilis
Refrigerated
+
+
+
+
+
+
Ambient-stored
+
+
+
+
+
+
Bacillus cereus
Refrigerated
-
-
-
-
-
-
Ambient-stored
+
+
-
+
+
+
Staphylococcus aureus
Refrigerated
-
-
-
-
+
+
Ambient-stored
+
-
+
+
+
+
Penicillium notatum
Refrigerated
-
-
-
+
-
-
Ambient-stored
-
-
+
-
-
+
Aspergillus flavus
Refrigerated
-
+
+
-
-
-
Ambient-stored
-
-
+
-
-
-
Rhizopus spp
Refrigerated
+
+
+
+
+
-
Ambient-stored
+
+
+
+
-
-
Saccharomyces spp
Refrigerated
+
+
+
+
+
+
Ambient-stored
+
+
+
+
+
+
Mucor spp
Refrigerated
+
+
+
-
+
+
Ambient-stored
+
+
+
+
+
+
Table 1: Scientific name, common name and sources of mushroom samples.
All ambient–stored milk samples had high numbers of microflora in them with cfu⁄ml ranging from 1.0 x 102 to 2.69 x104. Results of total counts indicated that according to the current guidelines for microbiological quality of milk and dairy products (FAO⁄WHO, 2002a,b), only the refrigerated samples were satisfactory.
A wide variety of microorganisms, some of which can bring about the spoilage of the milk samples on prolonged and⁄ or unprotected storage (e.g., Staphylococcus aureus, Bacillus spp, Penicillium spp, and Aspergillus flavus) were isolated from the samples which could be due to the source of the raw materials (purchased from the open market under conditions that allow the organisms in⁄on them to thrive). The exteriors of harvested grains, legumes, nuts and other food substrates retain some of the natural micro flora they had while growing on the field in addition to contamination from soil, insects, and other sources [23].
Processing (pasteurization) removed some of the microorganisms but then, there was a possibility of re–contamination during packaging and handling. Microorganisms were also detected in samples stored at 4°C. It was very significant to note that spore–forming bacteria (Bacillus) and moulds were found associated with the milk samples on storage. Bacteria re–contaminating pasteurized milk originate primarily from water and air in the filling equipment or immediate surroundings and can be resident for prolonged periods of time [24].
Bacillus are spore–forming bacteria that are commonly found in soil, water (through soil–water contamination) and on vegetables [23]. The presence of these bacteria and moulds in food samples in this work may be unavoidable because the spores of some strains of these organisms are resistant to pasteurization temperature. Furthermore, the oxygen level during processing may be sufficiently low to permit the growth of microbes.
High bacterial and fungal counts may be attributed to the fact that the samples were held at temperatures lower than 46°C for more than 4 hours. Previous studies had revealed that viable counts on foods prepared in advance and kept at ambient temperatures (20 to 46°C) for a long period of time (4 hours or more) reached critical levels [25,26].
Staphylococcus aureus is a common environmental bacterium and could have been introduced after processing through crosscontamination. Staphylococcus aureus is known to produce an enter otoxin of importance in food–borne illness. The processing utensils might have been kept on the open table, where they can be readily contaminated with food–poisoning organisms from raw food and from environment [27].
The presence of these organisms in the samples at ambient temperature indicated improper storage condition of foods.
The total coli form counts for refrigerated samples and ambientstored samples were carried out. No growth was found on refrigerated samples. Similarly, no growth was found on ambient–stored samples. This suggested the absence of coli forms in the samples, thus water used for production process was portable (not faecally contaminated)
The total heterotrophic bacterial count in (Tables 2a and 2b) showed that bacterial growth was highest in FR (2.62x102 cfu⁄ml) and lowest in DR (1.4x102 cfu⁄ml) for refrigerated samples at zero day, was highest in FR (2.8x102 cfu⁄ml) and lowest in CR (1.21x102 cfu⁄ml) for refrigerated samples at 7th day and was highest in CR (7.2x102 cfu⁄ml) and lowest in CR (1.74x102 cfu⁄ml) in refrigerated samples at 14th day. No growths were detected in AR, BR and CR at zero day; AR and BR at 7th day and 14th day. The counts in ambient–stored samples ranged from 1.0x102 to 2.35x105cfu⁄ml. This indicated that contamination existed in the product samples with decreasing tigernut milk substitution. Soy milk is imitation milk similar in composition with animal milk. Its rich nutrient and moderate pH makes it an excellent culture medium for the growth of microorganisms especially bacteria. Hence, the more the soymilk was available, the higher the bacterial contamination. Milk products are, however, easily perishable because contaminating bacteria may multiply rapidly and render it unfit for human consumption [28]. Bacterial growth was retarded to some level by refrigeration, although refrigeration was not feasible throughout due to economic⁄technical reasons. After zero days, the level of contamination was critical to the microbiological status of the ambient–stored milk samples; this could be attributed to the absence of a chemical preservative to keep the product shelf–stable and the pH⁄ temperature of the medium which was favorable to microbial proliferation.
Sample
Storage period (day)
0 7 14
AR
NG
NG
NG
BR
NG
NG
NG
CR
NG
1.21x102
1.74x102
DR
1.4x102
1.76x102
3.6x102
ER
2.4x102
2.6x102
5.8x102
FR
2.62x102
2.8x102
7.2x102
Table 2a: Antioxidant activities of mushroom samples extracted by different solvents.
Sample
Storage period (day)
0 7 14
AA
1.0x102
6.1x102
1.31x105
BA
2.1x102
9.6x102
1.69x105
CA
4.9x102
1.28x103
1.72x105
DA
6.8x102
4.9x103
2.01x105
EA
2.8x102
8.4x103
2.12x105
FA
3.04x103
2.51x104
2.35x105
Table 2b: Antioxidant activities of mushroom samples extracted by different solvents.
The total heterotrophic fungal count recorded in (Table 3a and 3b) showed that the mould and yeast contaminants in the samples ranged from 1.2x102 to 7.8x102cfu⁄ml, 1.81x102 to 9.6x102cfu⁄ml and 1.71x102 to 9.8x102cfu⁄ml for refrigerated samples at zero, 7th and 14th day respectively while counts for ambient–stored samples at zero, 7th and 14th day were 1.61x102 to 9.9x102cfu⁄ml, 2.7x102 to 9.3x103cfu⁄ml and 1.16x104 to 2.69x104cfu⁄ml, respectively.
Sample
Storage period (day)
0 7 14
AR
7.8x102
9.6x102
9.8x102
BR
7.7x102
8.3x102
9.2x102
CR
6.9x102
8.7x102
8.92x102
DR
3.17x102
5.9x102
6.3x102
ER
1.48x102
1.68x102
1.71x102
FR
1.21x102
1.81x102
1.92x102
Table 3a: Antioxidant activities of mushroom samples extracted by different solvents.
Sample
Storage period (day)
0 7 14
AA
9.9x102
1.16x103
2.01x104
BA
9.2x102
9.6x103
1.21x104
CA
7.2x102
8.1x102
1.96x104
DA
4.2x102
1.26x103
2.41x104
EA
2.6x102
1.01x103
1.16x104
FA
1.61x102
2.7x102
2.69x104
Table 3b: Antioxidant activities of mushroom samples extracted by different solvents.
The level of contamination was not critical to the microbiological status of the refrigerated milk samples after production. Presence of yeasts in the tigernut milk was expected as yeasts are inherent in tigernuts, although the level of yeast cells (cfu⁄g) in the raw material was not determined.
The presence of these microbes may not be harmful to consumers as some of them assist in the enzymatic breakdown of food and some synthesize useful vitamins, but on prolonged storage these microbes can bring about the microbial spoilage of the beverage. [29] had earlier documented that Bacillus aureus is among the microorganisms responsible for the spoilage of tofu (a soymilk product). This also is in agreement with the report of [30] who noted that non–pathogenic genera of microorganisms such as Streptococcus, Lactobacillus and Bacillus aureus survived pasteurization and eventually spoilt milk product.
The levels of microbes present, generally, were below the limit of acceptance which is 2.0 x 105 cfu⁄ml for dairy milk by Codex Alimentations Commission [31,32].
Conclusion
The microbial status of the samples after storage revealed that the different storage conditions (refrigeration and ambient–storage) affected the quality of beverages differently; suggesting that the beverage should be consumed immediately after processing as no chemical preservative was added or kept at temperature (refrigeration– 4°C) that will rather extend the shelf–life or improve storage stability considering its composition and to avoid food borne–illnesses when consumed by the masses.
Recommendation
It will be necessary, however, to determine the effects of storage in different packaging materials at refrigeration and ambient temperatures on the microbiological status of tigernut–soy milk extract to ascertain its shelf–life. It is also suggested that tigernut–soy milk extract should not be kept at ambient temperature soon after processing.
References
- The spectators, Importance of tiger nuts @ www.peacefmonline.com. Accessed January 2010.
- Anthony Uyekpen Osagie, Offiong Udo Ekam. Victor Ogieva Igodan. Nutritional quality of plant foods. Post- harvest research unit, University of Benin, Nigeria. 1998; 246-249.
- Eteshola E, Oraedu ACI. Fatty acids composition of Tigernut tubers (Cyperus esculentus), Baobab seeds (Adansonia digitata L.) and their mixtures. J. American Oil Chemists Society. 1996; 73: 255-257.
- Muhammad NO, Bamishaiye EI, Bamishaiye OM, Usman LA, Salawu MO, Nafiu MO, et al. Physicochemical Properties and Fatty Acid Composition of Cyperus esculentus (Tiger Nut) Tuber Oil. Bioresearch Bulletin. 2011; 5: 51-54.
- Martinez V. Scientific analysis of effects of tiger nut on heart diseases and related aspects. In: Tiger Nut and Health. 2003; 1-2.
- Bamgbose AM, Eruvbetine D, Dada W. Utilization of tigernut (Cyperus rotundus, L.) meal in diets for cockerel starters. Bioresour Technol. 2003; 89: 245-248.
- Umerie SC and Enebeli JN. Malt caramel from the nuts of Cyperus esculentus. J. Bio. Resource Tech. 1997; 57: 215-216.
- Kordylas JM. Processing and Preservation of Tropical and subtropical food. J. Agri. Food Tech. 1990; 13: 28-40.
- Ayo A Omode, Olalekan S Fatoki, Olaogun KA, Physicochemial properties of some under-exploited and non-conventional oil seed. J. Agri. Food Chem. 1995; 14: 2850-2853.
- David F. Human nutritional dietary. Food Encyclopedia. 1986; 35-36.
- Rehman S, Hussain S, Nawaz H, Ahmad MM, Huma N, Virk WA. Preparation and quality evaluation of lathyrus sativus L-bovine milk blend. Pak. J. Nutr. 2007; 6: 134-137.
- Chien JT, HE Snyder. Detection and control of soymilk astringency, J. Food Sci. 1983; 48: 438-440.
- Cheman YB, Wei LS, Nelson AI. Acid inactivation of soybean lipoxygenase with retention of protein solubility. J. Food Sci. 1989; 54: 963-967.
- Singh T and Bains GS. Grain extract-milk beverage, processing and physico-chemical characteristics. J. Food Sci. 1988; 53: 1387-1390.
- Mordi JI, Ozumba AU, Elemo GN, Olatunji O. Physicochemical and Sensory Evaluation of Nigerian Tiger-Nut Extract Beverage. Biosci. Res. Commun. 2010; 22: 203- 207.
- Belewu MA, Belewu KY. Comparative Physico- Evaluation of Tiger-nut, Soybean and Coconut Milk Sources. Int. J. Agric. Biol. 2007; 9: 785-787.
- Cheesbrough M. District Laboratory Practice in Tropical Countries, Part 2; Cambridge University Press, UK. 2006; 62 - 69.
- Harrigan WF, McCance ME. Laboratory Methods in Food and Diary Microbiology. 8th edn. London: Academic Press Inc. 1990; 286 - 303.
- Pelczar MJ, Chan ECS. Laboratory Exercise in Microbiology, Black Dot. Inc, New York, USA. 1977.
- Beishir I. Microbiology in Practice. Self-instructions Laboratory Course. 4th edn. New York: Harper and Row Publishers. 1987; 46 - 111, 120 - 130, 138 - 272.
- Buchannan RE, Gibbon NE. Bergeys Manual of Determinative Bacteriology, Williams and Wilkens Co. Baltimore, USA. 1974.
- Barnett HI, Hunter BB. Illustrated Genera of imperfect Fungi. 4th edn. New York: Macmillan Publishing Company, USA. 1987; 106 - 130.
- Edema MO, Omemu AM. Microbiology and Food Hygiene in Public Food services. Proceeding of the International Conference on Science and National Development. Oct. 25-28, 2004; 25 -29.
- Eneroth A, Ahrne S, Molin G. Contamination routes of Gram-negative spoilage bacteria in the production of pasteurized milk, evaluated by randomly amplified polymorphic DNA (RAPD). International Dairy Journal. 2000; 10: 325-331.
- Ali AA, Spencer NJ. Hazard analysis and critical control point evaluation of school food programs in Bahrain. J Food Prot. 1996; 59: 282-286.
- Mosupye FM, Von Holy A. Microbiological quality and safety of ready-to-eat street-vended foods in Johannesburg, South Africa. J Food Prot. 1999; 62: 1278-1284.
- Adrian R Eley. Microbial food poisoning. 2nd edn. United Kingdom: Chapmall and Hall. 1996; 191.
- Ukwuru MU, Ibeneme CL, Agbo GI. New Product Development from Tigernut (Cyperus esculentus) and Their Sensory, Proximate and Microbiological Evaluation. Pak. J. Nutr. 2011; 10: 101-105.
- Pelczar MJ (Jr), Chan ECS, Krieg NR. Microbiology 5th edn. New York: McGraw-Hill Book Co, USA. 1986; 37 - 50, 133 - 146.
- Ogbulie JN, Uwaezuoke JC, Ogieche SI. Introductory Microbiology Practical. 2nd edn. Nigeria: Concave publishers, Owerri. 2001; 63-64.
- FAO/WHO. Milk and milk products. Joint FAO/WHO Food standards programme. CODEX Alimentarius Commossion. 2002; 42.
- FAO/WHO. Joint FAO/WHO Expert Report on diet, nutrition and the prevention of chronic diseases, cited in Food and Nutrition Bulletin. 2002; 24: 255-256.