Review Article
Austin J Nutri Food Sci. 2014;2(5): 1029.
Food Allergy: Prevalence and Food Technology Approaches for the Control of IgE-mediated Food Allergy
Ontiveros N4, Flores-Mendoza LK2, Canizalez-Román VA3 and Cabrera-Chavez F1*
1School of Nutrition Sciences. Autonomous University of Sinaloa, Mexico
2Mexican Social Security Institute (IMSS), CIBIOR, Immunology Department, Puebla, Mexico
3School of Medicine, Autonomous University of Sinaloa, Mexico
4Regional Program for PhD in Biotechnology, FCQB, Autonomous University of Sinaloa, Mexico
*Corresponding author: :Cabrera-Chavez F, School of Nutrition Sciences. Autonomous University of Sinaloa. Av. Cedros y Calle Sauces S/N, Fracc. Los Fresnos, 80019, Culiacán, Sinaloa, México
Received: February 20, 2014; Accepted: April 24, 2014; Published: April 29, 2014
Abstract
IgE–mediated food allergy (FA) is a reproducible adverse immune reaction to certain proteins in food matrices and the only available treatment is to avoid the allergen of interest. Establishing the accurate prevalence of IgE–mediated FA is challenging as a number of factors affect the estimates and relevance of the type of FA. Based on recent studies, clinical FA affects less than 5% of the population and has become a serious health concern. Thus, there is an increasing interest to find strategies to give a solution for dietary restrictions which affect the patients’ quality of life. In this frame, some food technologies are proposed as good tools to reduce the allergenic or sensitizing potential of food proteins. This review presents and discusses the current prevalence data of FA and addresses the main food technologies used to control IgE–mediated FA. Also, the implications of these food technologies on the functional properties of foods are discussed.
Keywords: Food allergy; Prevalence; Modification of allergens; Food processing
Abbreviation
FA: Food Allergy; IgE: Immunoglobulin E; IL–: Interleukin; IFN–γ: Interferon Gamma; Th1, Type 1 T Helper Cells; Th2: Type 2 T Helper Cells; FceRI: High–Affinity IgE Receptor; Mpa: Megapascal
Introduction and Background
Food allergy (FA) is defined as an adverse immune response that occurs reproducibly on exposure to a given food and is distinct from other adverse responses to food, such as pharmacologic reactions, toxin–mediated reactions, and food intolerance (an immunologically unrelated adverse reaction to food) [1–2]. FA can be mediated or not by IgE antibodies and includes, but it is not limited to, food–induced anaphylaxis, food protein–induced enterocolitis syndrome, and foodinduced eosinophilic gastrointestinal disorders [3]. Particularly, IgEmediated FA appears to be on the increase and has become a serious health concern in some countries such as U.S.A, Canada, Australia, China, and the United Kingdom [4–9]. On the basis of meta–analysis studies and systematic reviews, the overall prevalence of FA confirmed by oral food challenge tests (challenge–proven FA) is expected to be less than 5% [10,11], although a few population–based studies have found higher estimates (7.7% to 8.9%) [7,8]. Certainly, populationbasic studies of prevalence of FA can be influenced by some factors such as geographic location and study designs or methodologies. Therefore, prevalence data should be interpreted taking into account all those factors that could influence the estimates of prevalence and relevance of types of FA.
There are no currently accepted therapeutic approaches for FA and the only available treatment is to avoid the relevant allergen. However, accidental exposures to food allergens are common among individuals affected with FA [12,13] and restricted diets are usually costly and limit social activities [14]. An effective therapy that controls the allergic reaction by promoting immune tolerance to food allergens is expected to have a profound impact on the patient’s lifestyle and quality of life. In this context, specific therapies (e. g. oral and epicutaneous immunotherapy) are promising therapeutic approaches, but safety and efficacy of different dose–regiments are still main issues to be addressed specially using multiple food allergens simultaneously [15]. Other therapies, termed non–specific, have shown good results in preclinical and clinical studies [3] and could be particularly relevant in those cases where more than one food trigger the allergic reaction.
In this frame, food processing technologies such as heat treatment and enzymatic proteolysis are promising strategies for accelerating immune tolerance acquisition in individuals affected with cow’s milk [16–18] or raw egg allergies [19]. Notably, these allergenic processed foods are well tolerated by the majority of the individuals [7, 20,21]. Furthermore, other food processing methods have also shown potential to reduce the IgE–mediated allergic immune response in mouse models [22–24]. This includes high pressure conditions and irradiation of foods as well as chemical modification of food allergens.
In this review we aimed to present current prevalence data of FA as well as the main food processing technologies used for preventing or reducing the immune response to allergenic food proteins undergoing digestion and leading to IgE–mediated FA.
Analyses and Interpretation
Prevalence of food allergy
Except for those cases where the cause of a severe FA reaction can be clearly identified, FA diagnosis should be confirmed by food challenge tests, the “gold standard”, ideally performed as a doubleblind placebo–controlled food challenge test. However, just a few studies have based their prevalence data on this laborious and time consuming clinical practice. As an alternative, other studies of prevalence of FA are based on questionnaires⁄interviews (self reported FA) or at less extend on allergen–specific IgE serology tests and⁄or skin prick test (where a tiny amount of allergen is introduced into the skin and may elicit a localized allergic response) to estimate the prevalence of adverse reactions or sensitization to food.
Certainly, self–reported FA studies are influenced by other food related conditions leading to an overestimation of the prevalence of FA [25]. Although the assessment of “convincing” symptoms of immediate hypersensitivity (e.g., wheezing, trouble for breathing, skin with hives, vomiting, and diarrhea) could improve the performance of these studies [5, 26], the main value of self–reported FA prevalence is thought to serve as groundwork for further investigations based on objective diagnostic criteria (e.g., allergen–specific IgE, skin prick test, and food challenge tests). With regards to IgE levels in blood, clinical studies have proven that higher allergen–specific IgE levels indicate a greater probability of clinical FA [27,28] and this could be helpful to better estimate prevalence of FA [29].
A research study on FA funded by the European Commission (EuroPrevall program) carried out a meta–analysis that included 51 articles published in the period of January 1990 to December 2005 [11]. Under the basis of self–reported FA, combination of symptoms plus sensitization, and challenge–proven FA, the estimates of overall prevalence to specific foods were as follows, respectively: cow’s milk (3.5%, 0.6%, 0.9%), hen’s egg (1%, 0.9%, 0.3%), peanut (0.75%, 0.75%, not available), fish (0.6%, 0.2%, 0.3%), and Shellfish (1.1%, 0.6%, not available). Prevalence of self–reported FA to any food varied widely (from 3% to 35%) showing heterogeneity among studies. Additionally, the EuroPrevall working group also reported prevalence of plant food allergy in a systematic review that included 36 studies published in the time period of January 1990 to December 2006 [30]. Notably, 27 of the studies were originated from Europe. Overall estimates of prevalence of self–reported FA, skin prick test, and challenge–proven FA ranged as follows, respectively: fruits (0.03% to 11.5%, 0.03% to 4.2%, 0.1% to 4.3%), vegetables⁄legumes (0.01% to 13.7%, 0.01% to 2.7%, 0.1% to 1.4%), nuts (0% to 7.3%, 0.02% to 4.5%, 0.1% to 4.3%), wheat (0.2% to 1.3, 0.03% to 0.2%, 0% to 0.5%), soy (0.03% to 1.3%, 0.03% to 0.2%, 0% to 0.7%), and other food items (<1.3%, <1%, <0.1% one study only). Estimates of IgE sensitization to wheat and soy were <3.7% and <3% respectively.
A recent meta–analysis that included 30 studies published in the period of January 2000 to 30 September 2012 evaluated the prevalence of FA in Europe [10]. It was found a prevalence of perceived FA to any food of 6.8% in children and 5.0% in adults. The overall prevalence of sensitization was 10.1% and 2.7% on the basis of blood levels of allergen–specific IgE or skin prick tests respectively. When this analysis was performed including symptoms (clinical FA), the prevalence of sensitized FA was 2.7% (5 studies) and 1.5% (4 studies) respectively. With regards to challenge–proven FA (12 studies), its prevalence was 0.99% in children and 0.89% in adults.
In a systematic review published in 2010 the prevalence of FA was summarized as affecting more than 1% or 2% but less than 10%of the US population [2]. A recent study from the National Health and Nutrition Examination Survey reported that the prevalence of self–reported FA in U.S.A was 6.5% in children and 9.7% in adults[31]. The main allergens reported in this study were milk, peanut and shellfish. Alternatively, based on the study by Liu et al., [29] the prevalence of sensitization to food or clinical FA (considering allergen–specific IgE levels and age–based criteria) was summarized as 16.8% and 2.5% respectively. In this US nationally representativecohort study, blood levels of allergen–specific IgE to peanut, cow’s milk, egg white, and shrimp were assessed.
Other recent studies have reported estimates of prevalence of FA in Canada, Australia, Asia, and Latina America. A nationwide telephone survey reported that the prevalence of self–reported FA in Canadian population was 7.1% in children and 8.3% in adults [32]. Milk, shellfish, fruits⁄vegetables, tree nut, and peanuts were the most reported allergens. In a challenge–proven FA study representative of the Melbourne (Australia) population, Osborne et al. [7] reported that the prevalence to peanut, raw egg, and sesame was 3%, 8.9%, and 0.8% respectively. In the same study, the estimates of sensitization to cow’s milk and shellfish assessed by skin prick test were of 5.6% and 0.9% respectively.
In Asia, some studies found that the prevalence of self–reported FA and challenge–proven FA ranged from 4.8% to 16.7% and 1.1% to 3.8% respectively [8,33–36]. Different from others geographic locations, fish seems to be the most reported allergen in Asian population and this has been attributed to the abundance of seafood in this region [37]. With regards to Latin America, Marrugo et al. [38] found an overall prevalence of self–reported FA of 14.9% in a cohort of 3099 individuals from Cartagena Colombia aged 1–83 years. Fruit⁄vegetables, seafood, and meats were the most reported allergens. In another self–reported FA study, Hoyos–Bachiloglu et al. [39] found an overall prevalence of 5.5% in a cohort of 455 Chilean school–aged children. In this study, typical symptoms of immediate hypersensitivity allergic reactions were assessed in a second questionnaire. Therefore, walnut, peanut, egg, chocolate, avocado, and banana were the most reported allergens.
Taking into account the background presented above, we consider that estimates of prevalence of FA are influenced by several factors such as, dietary exposures, differences between populations (age, race⁄ ethnicity), study designs or methodologies, among others. This make challenging to determine the prevalence of FA with certainly. Also we believe that the prevalence data of FA are widely variable among populations and therefore an overall prevalence of challenge–proven FA of less than 5% could be considered an appropriated estimate.
Basic pathogenesis of IgE mediated FA
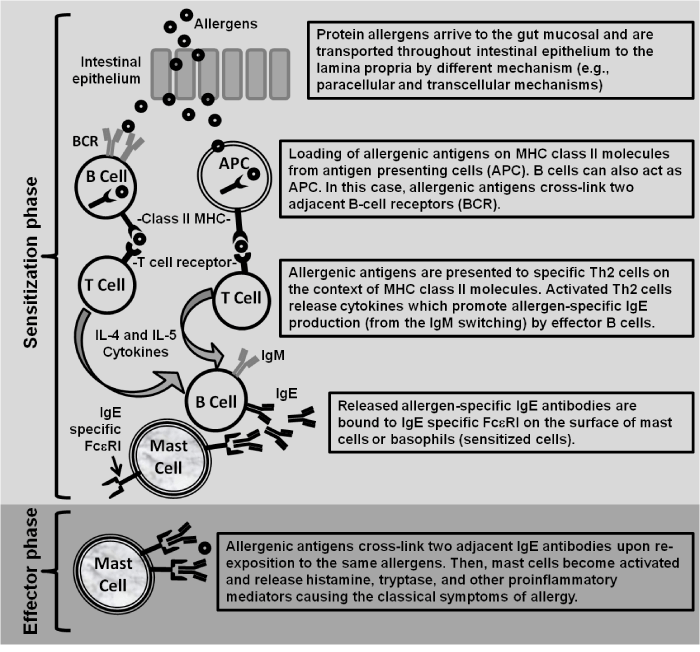
By passing oral tolerance and generating allergen–specific IgE antibodies with subsequent sensitization of mast cells or basophils are central events to trigger the allergic immune response. These basic events are typically called sensitization and effector phases [40,41] (Figure 1). The former involves antigen presenting cells, T cells, Th2 cytokines such as interleukin (IL)–4, IL–5 and IL–13, cross–linking of allergens with B–cell receptors, and IgE production. Sensitization occur after antigen presenting cells such as dendritic and B cells have recognized allergenic segments or “epitopes” in the protein component of food or ingredients within food. Then, allergen antigens are loaded on MHC class II molecules and antigen presentation to T cells may occur. This interaction activates allergen–specific T cells which produce Th2 cytokines and promotes the production of IgE antibodies by allergen–specific B cells [41]. IgE antibodies may bind to IgE receptor FceRI on the membrane of mast cells and basophils generating sensitized cells.
Figure 1: Sensitization and effector phases involved in the development of IgE-mediated food allergy.
Allergic sensitization could take place if the primary contact with the allergen does not occur via the oral route due to the gut associated lymphoid tissue is naturally tolerogenic [3]. In this context, according to studies carried out in mouse models, the skin could be an important route of sensitization [42,43]. However, tolerance can be induced via skin exposure [44] and some allergens do not generate sensitization through the skin without exogenous adjuvant [45]. In these cases, the intrinsic properties of food allergens to promote activity on the innate immune system could play a role to induce adjuvant–independent sensitization via the skin [3].
Alternatively, on the basis of some studies [46–49], it has been suggested that proteins undergoing digestion do not have the capability to sensitize and may promote tolerance. On the other hand, digestion stable proteins are strongly associated with IgE secretion and sensitization [50]. Furthermore, it has been hypothesized that the transport of food proteins and peptides across the gastrointestinal barrier is needed to induce sensitization or to elicit an allergic reaction [51]. The transport of proteins throughout the intestinal epithelium may occur either via the para–cellular route or via trans–cellular routes [51]. In general, sensitization might occur by different routes and the nature of the allergen is determinant to establish it. The effector phase begins when the same allergen that gave rise to sensitization crosslinks two adjacent IgE on sensitized mast cells or basophils. Then,these activated cells release proinflammatory mediators or cytokines causing the clinical manifestations of allergy [41].
Molecular characterization of epitopes
The current knowledge is not enough to categorize any food protein as ‘absolutely non–allergenic’. For instance, amaranth (Amaranthus spp.) proteins have been widely accepted as ‘nonallergenic’ food component, however, recently Kasera et al. [52] have presented the first case of food allergy induced by Amaranthus paniculatus. In this frame, the molecular characterization of epítopes of food allergens, firstly involves the identification of the protein molecules in which are contained. However, as to the allergenic foods contain more than one allergenic epitope it is necessary to know the whole protein components and to discriminate its allergenic and non–allergenic components [53]. The common steps to reach this aim have been as follow: 1) Observation of individuals exhibiting allergic symptoms after certain food consumption; 2) Collection of sera from individuals with allergy triggered by the same food; 3) Identification of proteins recognized by the IgE antibodies in the sera samples from allergic patients. This last point is crucial to identify the epitopes that cross–link with B–cell receptors and thus, it is also important for characterization of allergens and for better understanding of the complex allergic reactions.
Among the tools for analysis of allergens, immune–informatics (a subdivision of bioinformatics) is a useful instrument to find epitopes from proteins and to model their characteristics. The first attempts to predict characteristics from epitopes were based on the development of hydrophilicity scales related to antigenic profile [54–55]. Then, other molecular characteristics (e. g., secondary structure, amino acids propensities) have been considered in several algorithms used in computational analysis methods. The excellent review by salimi et al. [56] shows a growing number of immune–informatics instruments which makes epitope prediction easier and more efficient. Finally, the immune–informatics tools are also useful to predict the implications of allergens’ modifications (via technological methods) on the immune allergic response [57].
Modification of allergens
Allergenic proteins may be modified at molecular level during food processing and then, their capability to interact with allergenspecific immune cells could be altered. These modifications (mainly hysicochemical changes) could modify the digestibility of proteins and also may change the mechanism in which the allergenic proteins are transported from side to side into the gut mucosal. With regards to the interaction with immune mediators, common points are the avoiding of the interaction between allergen epitopes and specific IgE antibodies and⁄or the avoiding of the allergenic antigen presentation by antigen presenting cells. However, modified allergens should be tested to determine their allergenic and sensitizing potential as food processing can modify directly the allergenic proteins, masking or unmasking specific epitopes [58,59].
Thermal modification
Several studies have described the effects of thermal treatment of allergens on the allergic immune response. The work by Martos et al., [60] concludes that heat treatment of ovalbumin and ovomucoid can reduces the allergenicity of these proteins in part due to alterations on their digestive properties. Also Golias et al., [61] found a reduction of allergic immune response to ovalbumin in a murine model. The authors conclude that thermal processing of ovalbumin caused minor irreversible changes in its secondary structure modifying its digestibility and affecting the epitope formation. In both studies a Th1–skewing effect induced by the thermal treatment was observed.
When sensitized mice were fed with heated ovalbumin, minimal T cell proliferation was developed in the Peyer’s Patches or mesenteric lymph nodes (related to animals consuming native ovalbumin) [60]. These results suggest that the absorption of heated ovalbumin at intestinal level could be affected as these proteins in its native form are normally able to generate effectors cells. In addition, the thermal treatment of allergens has been used for desensitization in murine models. For instance, sensitized animals orally–treated with heated ovomucoid were not able to develop anaphylactic symptoms [62]. Furthermore, serum levels of IL–13, IL–10 and IFN–γ (Interferongamma) were reduced, remaining this effect until two weeks after the treatment. This result was not attributed to a Th1–skewing effect, but a suppression of Th1⁄Th2 responses was observed.
The study by Nowak–Wegrzyn et al., [21] reveals that extensively heated cow’s milk is well tolerated by most patients affected with IgEmediated cow’s milk allergy. There are two types of patients affected with FA: those with transient milk allergy that produce IgE antibodies primarily directed to conformational epitopes and those individuals with persistent allergy that also produce IgE antibodies against linear epítopes (which do not disappear under thermal treatments) [46,63]. Thus, an increased IgE affinity and IgE epitope diversity have been related to the severity of cow’s milk allergy [64]. In agreement to some authors, heated foods such as cow’s milk [17,21] and raw egg [7,19–20] can be well tolerated by patients with allergy against to those foods. Moreover, its dietary inclusion appears to be favorable for development of tolerance. However, the prescription of heated products requires an individual clinical study which should be carried out by allergists⁄immunologists.
On the other hand, the reduction of the allergenic potential of thermal–treated allergens is mainly attributed to the structural modification of allergens. Thus it is necessary to characterize firstly the thermal properties of the allergen, i.e., it is basic to determine the required heat to reach irreversible changes in the allergenic protein.
Hydrolysis treatment
Generally, partial or exhaustive hydrolysis of proteins reduces their allergenic potential, but not necessary abolish it. This has been specially studied in hypoallergenic milk formulas for infants (surrogates of cow’s milk). To considerate a milk formula as hypoallergenic, it should be tolerated by more than 90% of the population with the specific allergy [65]. In the case of cow’s milk hypoallergenic formulas, the exhaustive hydrolysates are tolerated by approximately 95% of allergic individuals. However the partial hydrolyzed whey formulas can trigger allergic reactions in 33% to 50% of the cow’s milk allergic cases and are not considered hypoallergenic[66]. The reduction of the allergenic potential of hydrolyzed proteinsis attributed to the breaking of linear and⁄or conformationalepitopes. To achieve this point, the type of hydrolysis is decisive as some proteins are prone or resistant to digestion depending on the enzyme used. Also hydrolysis conditions are relevant. For instance, it has been shown that the hydrolysis of β–lactoglobulin by trypsin,chymotrypsin, and pepsin reduces its allergenicity and when the enzymatic reaction involves both enzymatic hydrolysis and heat treatments this reduction is more pronounced [67].
Fermentation is other food processing approach which involves indirectly hydrolysis of proteins. Depending on the bacterial strain used for fermentation, the allergenicity of different proteins can be reduced. For instance, Lactobacillus delbrueckii. Bulgaricus CRL 656 re able to degrade β–lactoglobulin and its epitopes [68] avoiding the interaction with allergen–specific IgE from children affected with cow’s milk allergy. Similarly, the proteolysis achieved during the fermentation of cow’s milk by Lactobacillus fermentum IFO3956and Lactobacillus helveticus A75 reduces the capability of interaction between specific IgE and aS1–casein and β–casein [69–70].
Beyond the hydrolysis for destruction of epitopes, this reaction can generate peptides with immunomodulatory properties. According to Pan et al.[71] cow’s milk protein hydrolysates have the capability to reduce the allergic immune response to ovalbumin in sensitizedmice. Low serum levels of allergen–specific IgE and IL–4, as well as high levels of transforming growth factor–β (an inhibitory cytokine secreted by regulatory T cells) were found in mice treated with the milk hydrolysates. It is believed that protein–bioactive components in extracts from diverse sources may have potential for applications in immune–pharmacology.
Although food processing approaches based on hydrolysis of proteins can certainly be used to obtain hypoallergenic proteins, their application in foods is sometimes limited. From the point of view of the food science and technology, the use of hypoallergenic hydrolysates in several food products is not viable due to the proteolysis affects the functionality of proteins. For instance, during cheese processing, the results in the destabilization of protein micelles and the increased amount of small–soluble peptides and free aminoacids compromise the conventional cheese yield [72]. Similarly, fermentation processes are also limited to food products that require microbial action (e.g. fermented bread) [73].
High pressure treatment
High pressure treatment of foods can lead to structural changes of proteins such as denaturation and formation of aggregates [74]. Then, this approach may also alter the allergenic potential of food proteins. When the pressure is relatively high (~ 200 MPa), the tertiary structures of proteins are destabilized [57]. López–Expósito et al.,[22] evaluated the allergenicity of enzymatically hydrolyzed β–lactoglobulin under two different pressure conditions. The β–lactoglobulin hydrolysates obtained under high–pressure showed a reduced allergenic potential when tested in sensitized mice. The absence of anaphylactic symptoms was attributed to the formation of high hydrostatic pressure hydrolysates of β–lactoglobulin which are immunologically inert. Other studies have also reported that enzymatic hydrolysis under high pressure can reduce the antigenic potential of whey proteins [75,76]. Certainly, proteins can become unfolded under high pressure conditions [77,78]. Therefore, the immunogenic hydrophobic regions of the protein (inaccessible in the native form) are then exposed making them more susceptible to enzymatic hydrolysis [79].
Irradiation of food allergens
According to Lee et al., [80,81] gamma–irradiation is recommended for the production of egg processed foods with reduced allergenic potential. Gamma irradiation causes protein denaturation since radiolysis generates hydroxyl, hydrogen, and hydroperoxyl radicals. In addition, gamma irradiation of proteins alters the intermolecular interactions causing fragmentation or aggregation and even the formation of disulfide bonds. In a murine model Seo et al., [23] found that irradiated ovalbumine significantly suppressed the release of Th2 cytokines (IL–4 and IL–5) and induced production of Th1 cytokines (IL–12 and IFN–γ). This type of modification has also been applied to other food allergens obtaining different results [80,82]. However, the use of this food technology could cause an alteration on the functionality of proteins in food matrices (e.g. reduction of their solubility) [82].
Chemical modification of amino acids residues to block epítopes
Other kind of modification of allergens is the derivatization or modification of amino acids residues in the epítopes. This principle has been used to evade the immune response in celiac disease [83]. Although this disorder is not an IgE–mediated FA, it involves the processing and presentation of food antigens. Thus, the transglutaminase enzyme was used to bind free amino acids into the immunogenic sequences to block the immune response. Alternatively, the conjugation with reducing sugars (glycation) or oligosaccharides is another way for masking allergens in foods [25,84,85]. Independently of the type of chemical reagents or enzymatic reactions, all of those modifications should provide a steric bulk to block allergenic epítopes.
Conclusion
In this review we have highlighted the current knowledge about the prevalence of FA and some food technology approaches used to control IgE–mediated FA. We conclude that the accurate estimation of prevalence of IgE–mediated FA is challenging as several factors affect the estimates, and therefore, the available data are enough to support an overall prevalence of challenge–proven FA of less than 5%. Furthermore, individuals affected with FA must restrict their diets in an attempt to avoid the allergen of interest. In this frame, the food science and technology offer tools to reduce or abolish the allergenicity of some food components. Heat treatment and hydrolysis appear to be the most promising approaches as have been tested not only in animal models but also in allergic patients. However, in the case of thermal treatment, there are scarce studies about the precise thermal conditions to control the IgE–mediated allergic immune response triggered by specific foods or allergenic proteins. With regards to hydrolysis, this approach might have restrictions to be applied in several food products as proteolysis cause a more severe loss on the functionality of proteins than thermal processing or other technologies which do not involve breaking of proteins. Finally, we believe that independently of the food processing technologies usedto control the IgE–mediated allergic immune response, the involved concepts must be in line with food producers’ technologies and scientific knowledge, and notably, be safe for allergic patients.
Acknowledgements
This review was done as a part of a project financially supported by Universidad Autónoma de Sinaloa through the grant PROFAPI2013⁄026 (Programa de Fomento y Apoyo a Proyectos de Investigación) given to Francisco Cabrera–Chávez.
References
- NIAID-Sponsored Expert Panel, Boyce JA, Assa'ad A, Burks AW, Jones SM, Sampson HA, et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol. 2010; 126: S1-58.
- Chafen JJ, Newberry SJ, Riedl MA, Bravata DM, Maglione M. Diagnosing and managing common food allergies: a systematic review. JAMA. 2010; 303: 1848-1856.
- Berin MC, Sicherer S . Food allergy: mechanisms and therapeutics. Curr Opin Immunol. 2011; 23: 794-800.
- Gupta RS1, Springston EE, Warrier MR, Smith B, Kumar R. The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics. 2011; 128: e9-17.
- Sicherer SH, Mu&nTilde;oz-Furlong A, Godbold JH, Sampson HA. US prevalence of self-reported peanut, tree nut, and sesame allergy: 11-year follow-up. J Allergy Clin Immunol. 2010; 125: 1322-1326.
- Ben-Shoshan M, Harrington DW, Soller L, Fragapane J, Joseph L. A population-based study on peanut, tree nut, fish, shellfish, and sesame allergy prevalence in Canada. J Allergy Clin Immunol. 2010; 125: 1327-1335.
- Osborne NJ, Koplin JJ, Martin PE, Gurrin LC, Lowe AJ. Prevalence of challenge-proven IgE-mediated food allergy using population-based sampling and predetermined challenge criteria in infants. J Allergy Clin Immunol. 2011; 127: 668-676.
- Hu Y, Chen J, Li H. Comparison of food allergy prevalence among Chinese infants in Chongqing, 2009 versus 1999. Pediatr Int. 2010; 52: 820-824.
- Kotz D, Simpson CR, Sheikh A. Incidence, prevalence, and trends of general practitioner-recorded diagnosis of peanut allergy in England, 2001 to 2005. J Allergy Clin Immunol. 2011; 127: 623-630.
- Nwaru B, Hickstein L, Panesar SS, Muraro A, Werfel T, Cardona V, et al. The epidemiology of food allergy in Europe: a systematic review and meta-analysis. Allergy. 2014; 69: 62-75.
- Rona RJ, Keil T, Summers C, Gislason D, Zuidmeer L . The prevalence of food allergy: a meta-analysis. J Allergy Clin Immunol. 2007; 120: 638-646.
- Fleischer DM, Perry TT, Atkins D, Wood RA, Burks AW. Allergic reactions to foods in preschool-aged children in a prospective observational food allergy study. Pediatrics. 2012; 130: e25-32.
- Canani RB, Nocerino R, Terrin G, Leone L, Troncone R. Hospital admissions for food-induced anaphylaxis in Italian children. Clin Exp Allergy. 2012; 42: 1813-1814.
- Avery NJ, King RM, Knight S, Hourihane JO. Assessment of quality of life in children with peanut allergy. Pediatr Allergy Immunol. 2003; 14: 378-382.
- Bégin P, Winterroth LC, Dominguez T, Wilson SP, Bacal L . Safety and feasibility of oral immunotherapy to multiple allergens for food allergy. Allergy Asthma Clin Immunol. 2014; 10: 1.
- Berni Canani R, Nocerino R, Terrin G, Coruzzo A, Cosenza L. Effect of Lactobacillus GG on tolerance acquisition in infants with cow's milk allergy: a randomized trial. J Allergy Clin Immunol. 2012; 129: 580-582, 582.
- Kim JS, Nowak-Węgrzyn A, Sicherer SH, Noone S, Moshier EL. Dietary baked milk accelerates the resolution of cow's milk allergy in children. J Allergy Clin Immunol. 2011; 128: 125-131.
- Berni Canani R, Nocerino R, Terrin G, Frediani T, Lucarelli S. Formula selection for management of children with cow's milk allergy influences the rate of acquisition of tolerance: a prospective multicenter study. J Pediatr. 2013; 163: 771-777.
- Konstantinou GN, Giavi S, Kalobatsou A, Vassilopoulou E, Douladiris N. Consumption of heat-treated egg by children allergic or sensitized to egg can affect the natural course of egg allergy: hypothesis-generating observations. J Allergy Clin Immunol. 2008; 122: 414-415.
- Eigenmann PA. Anaphylactic reactions to raw eggs after negative challenges with cooked eggs. J Allergy Clin Immunol. 2000; 105: 587-588.
- Nowak-Wegrzyn A, Bloom KA, Sicherer SH, Shreffler WG, Noone S. Tolerance to extensively heated milk in children with cow's milk allergy. J Allergy Clin Immunol. 2008; 122: 342-347, 347.
- López-Expósito I, Chicón R, Belloque J, López-Fandi&nTilde;o R, Berin MC. In vivo methods for testing allergenicity show that high hydrostatic pressure hydrolysates of β-lactoglobulin are immunologically inert. J Dairy Sci. 2012; 95: 541-548.
- Seo JH, Lee JW, Kim JH, Byun EB, Lee SY, Il-Jun Kang, et al. Reduction of allergenicity of irradiated ovalbumin in ovalbumin-allergic mice. Radiation Physics and Chemistry. 2007; 76: 1855-1857.
- Woods RK, Stoney RM, Raven J, Walters EH, Abramson M. Reported adverse food reactions overestimate true food allergy in the community. Eur J Clin Nutr. 2002; 56: 31-36.
- Nakamura S, Suzuki Y, Ishikawa E, Yakushi T, Jing H, Miyamoto T, et al. Reduction of in vitro allergenicity of buckwheat Fag e 1 through the Maillard-type glycosylation with polysaccharides. Food Chem. 2008; 109: 538-545.
- Sicherer SH, Burks AW, Sampson HA. Clinical features of acute allergic reactions to peanut and tree nuts in children. Pediatrics. 1998; 102: e6.
- Sampson HA, Ho DG. Relationship between food-specific IgE concentrations and the risk of positive food challenges in children and adolescents. J Allergy Clin Immunol. 1997; 100: 444-451.
- Sampson HA. Utility of food-specific IgE concentrations in predicting symptomatic food allergy. J Allergy Clin Immunol. 2001; 107: 891-896.
- Liu AH, Jaramillo R, Sicherer SH, Wood RA, Bock SA. National prevalence and risk factors for food allergy and relationship to asthma: results from the National Health and Nutrition Examination Survey 2005-2006. J Allergy Clin Immunol. 2010; 126: 798-806.
- Zuidmeer L, Goldhahn K, Rona RJ, Gislason D, Madsen C . The prevalence of plant food allergies: a systematic review. J Allergy Clin Immunol. 2008; 121: 1210-1218.
- McGowan EC, Keet CA . Prevalence of self-reported food allergy in the National Health and Nutrition Examination Survey (NHANES) 2007-2010. J Allergy Clin Immunol. 2013; 132: 1216-1219.
- Soller L, Ben-Shoshan M, Harrington DW, Fragapane J, Joseph L . Overall prevalence of self-reported food allergy in Canada. J Allergy Clin Immunol. 2012; 130: 986-988.
- Chen J, Hu Y, Allen KJ, Ho MH, Li H. The prevalence of food allergy in infants in Chongqing, China. Pediatr Allergy Immunol. 2011; 22: 356-360.
- Ho MH, Lee SL, Wong WH, Ip P, Lau YL. Prevalence of self-reported food allergy in Hong Kong children and teens--a population survey. Asian Pac J Allergy Immunol. 2012; 30: 275-284.
- Lao-araya M, Trakultivakorn M. Prevalence of food allergy among preschool children in northern Thailand. Pediatr Int. 2012; 54: 238-243.
- Wu TC, Tsai TC, Huang CF, Chang FY, Lin CC. Prevalence of food allergy in Taiwan: a questionnaire-based survey. Intern Med J. 2012; 42: 1310-1315.
- Lee AJ, Thalayasingam M, Lee BW. Food allergy in Asia: how does it compare? Asia Pac Allergy. 2013; 3: 3-14.
- Marrugo J, Hernández L, Villalba V. Prevalence of self-reported food allergy in Cartagena (Colombia) population. Allergol Immunopathol (Madr). 2008; 36: 320-324.
- Hoyos-Bachiloglu R, Ivanovic-Zuvic D, Alvarez J, Linn K, Thöne N, de Los Ángeles Paul M, et al. Prevalence of parent-reported immediate hypersensitivity food allergy in Chilean school-aged children. Allergol Immunopathol (Madr). 2014; doi:10.1016/j.aller.2013.09.006.
- He S, Zhang H, Zeng X, Yang P. Self-amplification mechanisms of mast cell activation: a new look in allergy. Curr Mol Med. 2012; 12: 1329-1339.
- He SH, Zhang HY, Zeng XN, Chen D, Yang PC. Mast cells and basophils are essential for allergies: mechanisms of allergic inflammation and a proposed procedure for diagnosis. Acta Pharmacol Sin. 2013; 34: 1270-1283.
- Brown SJ, Asai Y, Cordell HJ, Campbell LE, Zhao Y. Loss-of-function variants in the filaggrin gene are a significant risk factor for peanut allergy. J Allergy Clin Immunol. 2011; 127: 661-667.
- Oyoshi MK, Murphy GF, Geha RS. Filaggrin-deficient mice exhibit TH17-dominated skin inflammation and permissiveness to epicutaneous sensitization with protein antigen. J Allergy Clin Immunol. 2009; 124: 485-493, 493.
- Mondoulet L, Dioszeghy V, Vanoirbeek JA, Nemery B, Dupont C. Epicutaneous immunotherapy using a new epicutaneous delivery system in mice sensitized to peanuts. Int Arch Allergy Immunol. 2011; 154: 299-309.
- Dunkin D, Berin MC, Mayer L. Allergic sensitization can be induced via multiple physiologic routes in an adjuvant-dependent manner. J Allergy Clin Immunol. 2011; 128: 1251-1258.
- Vila L, Beyer K, Järvinen KM, Chatchatee P, Bardina L. Role of conformational and linear epitopes in the achievement of tolerance in cow's milk allergy. Clin Exp Allergy. 2001; 31: 1599-1606.
- Untersmayr E, Schöll I, Swoboda I, Beil WJ, Förster-Waldl E. Antacid medication inhibits digestion of dietary proteins and causes food allergy: a fish allergy model in BALB/c mice. J Allergy Clin Immunol. 2003; 112: 616-623.
- Schöll I, Untersmayr E, Bakos N, Roth-Walter F, Gleiss A. Antiulcer drugs promote oral sensitization and hypersensitivity to hazelnut allergens in BALB/c mice and humans. Am J Clin Nutr. 2005; 81: 154-160.
- Barone KS, Reilly MR, Flanagan MP, Michael JG. Abrogation of oral tolerance by feeding encapsulated antigen. Cell Immunol. 2000; 199: 65-72.
- Untersmayr E, Jensen-Jarolim E. Mechanisms of type I food allergy. Pharmacol Ther. 2006; 112: 787-798.
- Reitsma M, Westerhout J, Wichers HJ, Wortelboer HM, Verhoeckx KC. Protein transport across the small intestine in food allergy. Mol Nutr Food Res. 2014; 58: 194-205.
- Kasera R, Niphadkar PV, Saran A, Mathur C, Singh AB. First case report of anaphylaxis caused by Rajgira seed flour (Amaranthus paniculatus) from India: a clinico- immunologic evaluation. Asian Pac J Allergy Immunol. 2013; 31: 79-83.
- Ciardiello MA, Tamburrini M, Liso M, Crescenzo R, Rafaiani C, Adriano Mari. Food allergen profiling: A big challenge. Food Res Int. 2013; 54: 1033-1041.
- Hopp TP, Woods KR. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981; 78: 3824-3828.
- Parker JM, Guo D, Hodges RS. New hydrophilicity scale derived from high-performance liquid chromatography peptide retention data: correlation of predicted surface residues with antigenicity and X-ray-derived accessible sites. Biochemistry. 1986; 25: 5425-5432.
- Salimi N, Fleri W, Peters B, Sette A. Design and utilization of epitope-based databases and predictive tools. Immunogenetics. 2010; 62: 185-196.
- Somkuti J, Smeller L. High pressure effects on allergen food proteins. Biophys Chem. 2013; 183: 19-29.
- Davis PJ, Williams SC. Protein modification by thermal processing. Allergy. 1998; 53: 102-105.
- Davis PJ, Smales CM, James DC. How can thermal processing modify the antigenicity of proteins? Allergy. 2001; 56 Suppl 67: 56-60.
- Martos G, Lopez-Exposito I, Bencharitiwong R, Berin MC, Nowak-Węgrzyn A. Mechanisms underlying differential food allergy response to heated egg. J Allergy Clin Immunol. 2011; 127: 990-997.
- Golias J, Schwarzer M, Wallner M, Kverka M, Kozakova H. Heat-induced structural changes affect OVA-antigen processing and reduce allergic response in mouse model of food allergy. PLoS One. 2012; 7: e37156.
- Leonard SA, Martos G, Wang W, Nowak-Węgrzyn A, Berin MC. Oral immunotherapy induces local protective mechanisms in the gastrointestinal mucosa. J Allergy Clin Immunol. 2012; 129: 1579-1587.
- Järvinen KM, Chatchatee P, Bardina L, Beyer K, Sampson HA. IgE and IgG binding epitopes on alpha-lactalbumin and beta-lactoglobulin in cow's milk allergy. Int Arch Allergy Immunol. 2001; 126: 111-118.
- Wang J, Lin J, Bardina L, Goldis M, Nowak-Wegrzyn A. Correlation of IgE/IgG4 milk epitopes and affinity of milk-specific IgE antibodies with different phenotypes of clinical milk allergy. J Allergy Clin Immunol. 2010; 125: 695-702, 702.
- Koletzko S, Niggemann B, Arato A, Dias JA, Heuschkel R. Diagnostic approach and management of cow's-milk protein allergy in infants and children: ESPGHAN GI Committee practical guidelines. J Pediatr Gastroenterol Nutr. 2012; 55: 221-229.
- Bahna SL. Hypoallergenic formulas: optimal choices for treatment versus prevention. Ann Allergy Asthma Immunol. 2008; 101: 453-459.
- Micinski J, Kowalski IM, Zwierzchowski G, Szarek J, Pierozynski B, Elwira Zablockaa. Characteristics of cow's milk proteins including allergenic properties and methods for its reduction. Polish annals of medicine. 2012; 20: 69-76.
- Pescuma M, Hébert EM, Rabesona H, Drouet M, Choiset Y. Proteolytic action of Lactobacillus delbrueckii subsp. bulgaricus CRL 656 reduces antigenic response to bovine β-lactoglobulin. Food Chem. 2011; 127: 487-492.
- El-Ghaish S, Rabesona H, Choiset Y, Sitohy M, Haertlé T. Proteolysis by Lactobacillus fermentum IFO3956 isolated from Egyptian milk products decreases immuno-reactivity of αS1-casein. J Dairy Res. 2011; .
- Ahmadova A, El-Ghaish S, Choiset Y, Rabesona H, Drouet M, Chobert JM, et al. Modification of IgE binding to β- and aS1-Caseins by Proteolytic Activity of Lactobacillus Helveticus A75. J Food Biochem. 2013; 37: 491-500.
- Pan DD, Wu Z, Liu J, Cao XY, Zeng XQ. Immunomodulatory and hypoallergenic properties of milk protein hydrolysates in ICR mice. J Dairy Sci. 2013; 96: 4958-4964.
- Chromik C, Partschefeld C, Jaros D, Henle T, Rohm H. Adjustment of vat milk treatment to optimize whey protein transfer into semi-hard cheese: A case study. J Food Eng. 2010, 100: 496-503.
- El-Ghaish S, Ahmadova A, Hadji-Sfaxi I, El Mecherfi KE, Bazukyan I, Yvan Choiset, et al. Potential use of lactic acid bacteria for reduction of allergenicity and for longer conservation of fermented foods. Trends Food Sci Technol. 2011, 22: 509-516.
- Iametti S, Transidico P, Bonomi F, Vecchio G, Pittia P, Pierpaolo Rovere, et al. Molecular modifications of β-lactoglobulin upon exposure to high pressure. J Agric Food Chem. 1997; 45: 23-29.
- Chicón R, López-Fandi&nTilde;o R, Alonso E, Belloque J. Proteolytic Pattern, Antigenicity, and Serum Immunoglobulin E Binding of β-Lactoglobulin Hydrolysates Obtained by Pepsin and High-Pressure Treatments. Journal of Dairy Science. 2008; 91: 928-938.
- Chicón R, Belloque J, Alonso E, López-Fandi&nTilde;o R. Immunoreactivity and digestibility of high-pressure-treated whey proteins. International Dairy Journal. 2008; 18: 367-376.
- Silva JL, Foguel D, Royer CA. Pressure provides new insights into protein folding, dynamics and structure. Trends Biochem Sci. 2001; 26: 612-618.
- Winter R, Lopes D, Grudzielanek S, Vogtt K. Towards an Understanding of the Temperature/ Pressure Configurational and Free-Energy Landscape of Biomolecules. Journal of Non-Equilibrium Thermodynamics. 2007; 32: 41-97.
- Bonomi F, Fiocchi A, Frøkiaer H, Gaiaschi A, Iametti S. Reduction of immunoreactivity of bovine beta-lactoglobulin upon combined physical and proteolytic treatment. J Dairy Res. 2003; 70: 51-59.
- Lee JW, Kim JH, Yook HS, Kang KO, Lee SY. Effects of gamma radiation on the allergenic and antigenic properties of milk proteins. J Food Prot. 2001; 64: 272-276.
- Lee JW, Seo JH, Kim JH, Lee SY, Kim KS, Myung.-Woo Byun, et al. Changes of the antigenic and allergenic properties of a hen's egg albumin in a cake with gamma-irradiated egg white. Radiation Physics and Chemistry. 2005; 72: 645-650.
- Kaddouri H, Mimoun S, El-Mecherfi KE, Chekroun A, Kheroua O. Impact of gamma-radiation on antigenic properties of cow's milk beta-lactoglobulin. J Food Prot. 2008; 71: 1270-1272.
- Gianfrani C, Siciliano RA, Facchiano AM, Camarca A, Mazzeo MF. Transamidation of wheat flour inhibits the response to gliadin of intestinal T cells in celiac disease. Gastroenterology. 2007; 133: 780-789.
- Hattori M, Miyakawa S, Ohama Y, Kawamura H, Yoshida T. Reduced immunogenicity of beta-lactoglobulin by conjugation with acidic oligosaccharides. J Agric Food Chem. 2004; 52: 4546-4553.
- van de Lagemaat J, Silván JM, Moreno FJ, Olano A, del Castillo MD. In vitro glycation and antigenicity of soy proteins. Food Res Int. 2007; 40: 153-160.