Review Article
Austin J Nutri Food Sci. 2014;2(5): 1030.
Vitamin D Status May Affect Resilience and Recovery from Mild Traumatic Brain Injury in Military Personnel
Laurel M Wentz*
Department of Nutrition Science, East Carolina University, USA
*Corresponding author: :Laurel M. Wentz, Department of Nutrition Science, RW-333 Rivers Building, Mail Stop 505, East Carolina University, Greenville, NC 27858-4353, USA
Received: March 19, 2014; Accepted: May 08, 2014; Published: May 09, 2014
Abstract
Experiencing mild traumatic brain injury (mTBI) may lead to chronic postconcussive symptoms, increasing the risk for post–traumatic stress disorder (PTSD) and suicide. Vitamin D deficiency is associated with cognitive decline, depression, and potentially PTSD through its relationship to testosterone production. Furthermore, vitamin D deficiency elevates systematic inflammation, meaning that poor vitamin D status at the time of blast may prolong inflammatory response to mTBI and exacerbate post–concussive symptoms. Since widespread vitamin D deficiency is observed across the U.S. population, poor vitamin D status is expected in service members. Given the high risk for mTBI in service members and suboptimal vitamin D levels observed in this population, treatment of vitamin D deficiency and elucidation of its mechanism in mTBI resilience and recovery merits exploration. Evidence in this review investigates possible protection of achieving optimal vitamin D levels for mTBI resiliency and recovery through its influence on inflammatory and hormonal biomarkers. Despite interest in using vitamin D as treatment for TBI, no human trials have tested the role of vitamin D in mTBI resiliency or recovery, nor have data been prospectively collected on the prevalence of vitamin D deficiency in service members. The neuroprotective effects of vitamin D warrant further investigation into the role of vitamin D in mTBI management.
Keywords: Vitamin D; Military; mTBI; Nutrition
Abbreviation
1,25–(OH) 2D: 1,25–Dihydroxyvitamin D; 25–(OH)D: 25–Hydroxyvitamin D; IGF–I: Insulin–like Growth Factor–I; IL–6: Interleukin–6; MAPK: Mitogen–Activated Protein Kinase; mTBI: Mild Traumatic Brain Injury; NF–κB: Nuclear Factor kappa Beta; PTSD: Post–Traumatic Stress Disorder; TNF–α: Tumor Necrosis Factor–alpha; VDR: Vitamin D Receptor
Introduction and Background
Optimizing vitamin D status may increase resiliency to mild traumatic brain injury (mTBI) and improve symptom recovery following injury by attenuating the inflammatory response and maintaining testosterone levels. The findings that vitamin D receptors are located in the hypothalamus, a brain region associated with learning, reason, and emotion, as well as the possible role of vitamin D in androgen synthesis and inhibiting inflammation, make vitamin D an appropriate nutrient for further investigation with regard to its role in improving functional outcomes of mTBI. The purpose of this review is to examine data supporting the medical hypothesis that optimal vitamin D status may improve mTBI resiliency and recovery through two different pathways. First, optimal vitamin D levels may limit sustained inflammation in response to injury. Second, optimal vitamin D status may maintain testosterone levels, which may reduce the risk of post–traumatic stress disorder (PTSD) associated with mTBI in service members.
mTBI Increases Risk for Post–Traumatic Stress Disorder and Suicide
Characterized by a disruption of normal brain functioning, TBI is estimated to afflict 10–20% of returning veterans and comprise a third of combat–related injuries [1]. The majority of these head injuries are classified as mTBI. However, accurate prevalence of mTBI in service members may be underreported given the challenges with making an objective diagnosis and the perseverance of warrior culture. Experiencing mTBI may lead to chronic post–concussive symptoms, increasing the risk for PTSD and suicide [2]. Postconcussive symptoms range from somatic (headache, dizziness,weakness, sensitivity to light⁄sound) to cognitive (poor attention, memory problems, balance problems) to more severe psychological and behavioral (irritability, depression, anxiety, personality changes) [3]. These symptoms may persist for more than a year after injury,interfering with service member’s combat power and effectiveness, while increasing morbidity across the force [4]. Furthermore, mTBI increases risk for chronic depression and PTSD, as nearly half of U.S. military personnel with an mTBI present with symptoms of PTSD [5]. However, both depression and PTSD are related to neuroendocrine dysfunction and alterations in the pituitary hormones, strengthening the case for clinically relevant biomarkers in mTBI management [6].
Vitamin D in mTBI Management
Optimal vitamin D levels may provide resilience to mTBI by modifying the inflammatory response. In response to biomechanical forces, mTBI stimulates a cascade of metabolic events including axonal injury, damaged cellular membranes, disrupted ion exchange, as well as reduced cerebral blood flow, inflammation, and cellular death [7]. While the acute release of inflammatory cytokines appears to be neuroprotective by promoting tissue repair, prolonged inflammation contributes to oxidative stress along with neurotoxicity and cellular death. Thus, the Institute of Medicine TBI Report [1] suggests that that this secondary inflammatory response contributes to more severe and longer duration symptoms following concussion and is an appropriate target for treatment strategies. As a key regulator of inflammation, vitamin D may provide resilience to mTBI by limiting the inflammatory response following impact. NHANES data estimate that one third of the U.S. population is deficient in vitamin D [8]. Analysis of stored serum samples from service members found similar rates of vitamin D deficiency and correlated low vitamin D status with increased risk of suicide [9]. Vitamin D regulates gene transcription, and its receptors and metabolizing enzymes have been identified in the brain, establishing support for a role of vitamin D in maintaining homeostasis of a healthy central nervous system [10].
Vitamins D Affects Androgens Synthesis
contributes to oxidative stress along with neurotoxicity and cellular death. Thus, the Institute of Medicine TBI Report [1] suggests that that this secondary inflammatory response contributes to more severe and longer duration symptoms following concussion and is an appropriate target for treatment strategies. As a key regulator of inflammation, vitamin D may provide resilience to mTBI by limiting the inflammatory response following impact. NHANES data estimate that one third of the U.S. population is deficient in vitamin D [8]. Analysis of stored serum samples from service members found similar rates of vitamin D deficiency and correlated low vitamin D status with increased risk of suicide [9]. Vitamin D regulates gene transcription, and its receptors and metabolizing enzymes have been identified in the brain, establishing support for a role of vitamin D in maintaining homeostasis of a healthy central nervous system [10].
This review analyzes the role of vitamin D status in resilience and recovery from mTBI, highlighting critical gaps in knowledge on nutrition in mTBI research. The literature summarized here suggests that vitamin D status has a measureable effect on resiliency to mTBI and supports a mechanism by which vitamin D may attenuate mTBI inflammatory response. Furthermore, this review will evaluate evidence that indicates maintaining optimal vitamin D status improves recovery from mTBI and reduces symptoms associated with post–concussive impairment.
Analyses and Interpretation
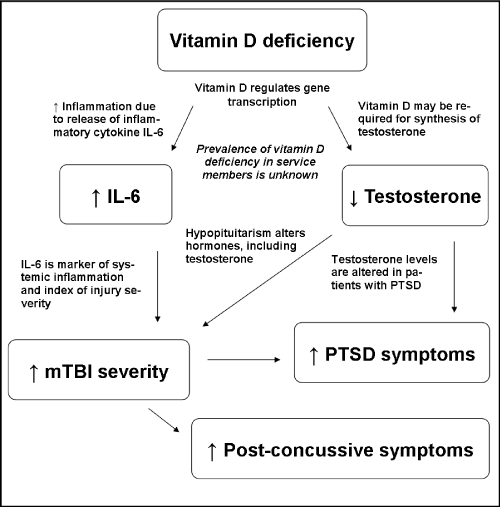
The Institute of Medicine Nutrition and TBI report [1] recommends the study of nutritional interventions for the prevention, resilience, and treatment of TBI with specific recommendations to study pre– and post–injury nutrition assessments along with potential biomarkers. Brain injuries are a serious national health concern given their potential to initiate lifelong cognitive and physical ailments. If nutrition and hormonal deficiencies can explain the etiology for any of the cognitive or physical symptoms associated with mTBI, then screening and correcting these deficiencies has the potential to maximize resiliency of experienced leaders and limit loss of assets (Figure 1). Thus, establishing a relationship of vitamin D status to mTBI resilience and severity proposes a new paradigm for prevention and treatment of brain injuries.
Figure 1: Vitamin D has a role in regulation of inflammatory response and testosterone synthesis associated with post-concussive symptoms and PTSD.
Vitamin D deficiency
No data have been published on the prevalence of vitamin D deficiency in service members at high risk for mTBI. Analysis of archived serum from male and female active duty personnel found that 35% of samples had serum 25–hydroxyvitmain D (25(OH) D) levels less than 20 ng⁄ml [9]. However, significant amounts of research support sufficient vitamin D as 25(OH) D levels between 30–100 ng⁄ml based on maximizing dietary calcium absorption and normalizing parathyroid hormone levels [16]. Fifty–seven percent of female recruits entering basic training had serum 25(OH)D levels less 30 ng⁄ml, increasing to 75% of recruits with low vitamin D after 8 weeks despite outdoor training [17]. These vitamin D levels reported in service members are similar to those measured across the U.S population [8]. Thus, it is expected that a significant proportion of military personnel have insufficient or deficient levels of vitamin D, which may increase their risk for mTBI.
Vitamin D neuroprotection
Mechanisms for the neuroprotective effects of vitamin D relate to its regulation of inflammatory cytokines as well as possible roles in reducing oxidative stress, regulating neural calcium concentration, and enhancing nerve conduction. Circulating 25(OH) D crosses the blood–brain barrier into the central nervous system [10]. Vitamin D receptors (VDR) and metabolizing enzymes have been identified in the brain, including the hypothalamus, which is a region associated with learning, reason, and emotion. As a regulator of gene transcription, vitamin D targets neural genes that affect a variety of behavioral and biochemical processes. Animal models have shown that vitamin D deficiency slows learning and increases anxiety, while human studies have correlated low vitamin D levels with depression [18] and accelerated cognitive decline [19]. In service members who had previously deployed, vitamin D deficiency was found to increase the risk of suicide [9]. Furthermore, vitamin D levels decrease during outdoor military training, likely related to protective clothing and equipment required for service [17]. Elevated levels of inflammatory cytokines have been correlated with both depression and vitamin D deficiency, suggesting that vitamin D suppresses excess neural inflammation and maintains cognitive health [20]. This idea is supported by neuroinflammatory models showing greater requirement for the active form of vitamin D, 1,25–hydroxyvitamin D (1,25(OH)2D) in response to inflammation [10]. Therefore, vitamin D deficiency may limit regulation of the inflammatory response, consequently exacerbating injury and increasing risk of concussive symptoms. To date, no human studies have looked at vitamin D status in human subjects prior to mTBI.
Vitamin D affects mTBI severity
Vitamin D deficiency at the time of injury may exacerbate postconcussive symptoms, postponing their resolution and keeping a service member from returning to duty. Vitamin D regulates gene transcription of inflammatory cytokines and limits their expression, a role supported by the association of vitamin D deficiency with various inflammatory disorders [10]. In an animal TBI model, vitamin D deficient rats had elevated levels of IL–6 and other inflammatory markers at baseline and post–injury compared to vitamin D sufficient animals [21]. Post–injury treatment with vitamin D was inferior to maintaining sufficient levels pre–injury. Acutely protective, prolonged inflammation is linked to poor injury outcomes, which has been indicated by higher serum IL–6 levels measured within 24 hours of injury correlating with more severe brain injury [22]. As an index of injury severity and a prognostic marker of outcome, IL–6 is the most accurate marker of systemic inflammation and traumainjury due to its regulation of the acute hepatic response [23], and levels have been shown to remain elevated for up to 12 months following fractures [24]. Vitamin D deficiency restricts regulation of cytokine production, thereby allowing sustained inflammatory response to injury [25,26]. This prolonged inflammatory response to mTBI increases injury severity and presents an appropriate target for minimizing concussive symptoms.
TBI–Related hypopituitarism
TBI of any severity has the potential to alter endocrine function, prolonging post–concussive symptoms for years after injury [27]. Gonadotropin and growth hormone synthesis are the most commonly altered pathways following mTBI, correlating to deficiencies in testosterone and IGF–I and responsible for symptoms such as fatigue, weight gain, low blood pressure, low libido, and loss of muscle mass [28]. Untreated pituitary hormone deficiencies delay injury recovery and may progress into more severe psychiatric problems characteristic of PTSD. Hypopituitarism has been found in 42% of male veterans who had at least one mTBI related to blast exposure, compared to no evidence of pituitary dysfunction in veterans who had deployed but never sustained mTBI [14]. Vitamin D levels have been associated with both total and free testosterone [29], whereas vitamin D supplementation raised total and free testosterone in vitamin D deficient men, suggesting vitamin D has a role in hypogonadism and may play a role in synthesis of pituitary hormones [30].
Post–concussive symptoms
Initial symptoms following combat mTBI include headache, amnesia, confusion, behavioral changes, balance problems, vertigo, dizziness, vision changes, and nausea⁄vomiting. While these symptoms typically resolve within 7–10 days in most mTBI cases [31], prolonged cognitive and physical abnormalities may persist, leading to long term health ailments such as memory loss, depression and increased risk for PTSD [2]. These symptoms have also been associated with vitamin D deficiency [20] and endocrine dysfunction [14], supporting a case for measuring hormonal levels in individuals who have sustained mTBI.
Conclusion and Future Directions
By definition, mTBI is a complex path physiological process rather than a true pathological injury, resulting from juxtaposed acceleration and deceleration forces to the brain [31]. Its clinical complexity and symptom diversity present unique challenges in injury management. Given the high prevalence of vitamin D deficiency complimented by ongoing debate regarding optimal status, vitamin D levels may prove a target for medical personnel to assess and monitor for mTBI management. Many of the chronic symptoms associated with mTBI such as depression, balance problems, and cognitive decline are also associated with vitamin D deficiency. Furthermore, these symptomsare associated with endocrine dysfunction and deficiencies in pituitary hormones such as testosterone and growth hormone, which may be regulated by vitamin D. An important distinction is treating vitamin D deficiency rather than indiscriminate supplementation in potentially vitamin D sufficient subjects.
Taken together, these data suggest that vitamin D deficiency is linked to altered cognitive function and post–concussive symptoms, owing to its role in regulating inflammatory cytokines and hormone synthesis. To date, one human trial has treated severe TBI patients with 5µg⁄kg (>10,000 IU) vitamin D per day for the first 5 days after injury in combination with progesterone [32]. The vitamin D plus progesterone treatment group experienced greater recovery compared to progesterone alone or placebo, supporting a role of vitamin D in accelerating brain repair. However, this research did not assess serum vitamin D levels and thus had no data on pre– or post–injury vitamin D status. The Institute of Medicine TBI Report [1] emphasized that nutrients status (pre– and post– injury) may have important relationships to mTBI outcomes such as mTBI resiliency and progression of symptoms, with a call to identify clinically relevant biomarkers.
This review proposes a new paradigm for mTBI management by focusing on pre–injury nutrition status to produce a measurable improvement on the resiliency to both mTBI and PTSD. Rather than identifying methods to selectively inhibit the physiological inflammatory response stimulated by injury, which is likely critical to tissue repair, we identify potential nutritional targets to optimize pre–injury status. We suggest that maintaining optimal vitamin D status improves mTBI resilience by regulating inflammatory cytokine expression and improves mTBI recovery by suppressing the prolonged secondary inflammation associated with lingering symptoms. This medical hypothesis should be tested in human interventions. Service members at risk of mTBI would be recruited and screened for vitamin D status. Those will low vitamin D would be assigned to a vitamin D supplement or placebo, while those with sufficient status would be followed without intervention. Subjects should be followed throughout deployment or other high–risk activity to assess mTBI diagnosis, evaluation, and treatment outcomes. Upon return, all subjects would be repeat serum vitamin D measurements to determine their association with mTBI incidence and recovery as well as inflammatory and hormonal biomarkers.
Vitamin D supplementation is a non–invasive treatment and outweighs the potential risks of performing combat operations with vitamin D deficiency. Treating vitamin D deficiency has the potential to improve resilience to mTBI and improve chronic symptoms as well as impact cognitive health and risk of suicide.
Acknowledgements
The editorial guidance of Drs. Margie Gallagher and Zachary Domire is greatly appreciated. Also, the assistance of ECU’s Operation Reentry North Carolina and United States Army 3rd Special Forces Group (Airborne) has been invaluable.
References
- Institute of Medicine. Nutrition and traumatic brain injury: Improving acute and subacute health outcomes in military personnel. Washington, DC: National Academies Press. 2011.
- Bryan CJ, Clemans TA. Repetitive traumatic brain injury, psychological symptoms, and suicide risk in a clinical sample of deployed military personnel. JAMA Psychiatry. 2013; 70: 686-691.
- Marshall KR, Holland SL, Meyer KS, Martin EM, Wilmore M, Grimes JB. Mild traumatic brain injury screening, diagnosis, and treatment. Mil Med. 2012; 177: 67-75.
- Lange RT, Brickell T, French LM, Ivins B, Bhagwat A, Pancholi S, et al. Risk factors for postconcussion symptom reporting after traumatic brain injury in U.S. military service members. J Neurotrauma. 2013; 30: 237-246.
- Hoge CW, McGurk D, Thomas JL, Cox AL, Engel CC, Castro CA. Mild traumatic brain injury in U.S. Soldiers returning from Iraq. N Engl J Med. 2008; 358: 453-463.
- Wilk JE, Herrell RK, Wynn GH, Riviere LA, Hoge CW. Mild traumatic brain injury (concussion), posttraumatic stress disorder, and depression in U.S. soldiers involved in combat deployments: association with postdeployment symptoms. Psychosom Med. 2012; 74: 249-257.
- Patterson ZR, Holahan MR. Understanding the neuroinflammatory response following concussion to develop treatment strategies. Front Cell Neurosci. 2012; 6: 58.
- Ganji V, Zhang X, Tangpricha V. Serum 25-hydroxyvitamin D concentrations and prevalence estimates of hypovitaminosis D in the U.S. population based on assay-adjusted data. J Nutr. 2012; 142: 498-507.
- Umhau JC, George DT, Heaney RP, Lewis MD, Ursano RJ, Heilig M, et al. Low vitamin D status and suicide: A case-control study of active duty military service members. PLoS One. 2013; 8: e51543.
- Smolders J, Moen SM, Damoiseaux J, Huitinga I, Holmøy T. Vitamin D in the healthy and inflamed central nervous system: access and function. J Neurol Sci. 2011; 311: 37-43.
- Karlovic D, Serretti A, Marcinko D, Martinac M, Silic A, Katinic K. Serum testosterone concentration in combat-related chronic posttraumatic stress disorder. Neuropsychobiology. 2012; 65: 90-95.
- Mulchahey JJ, Ekhator NN, Zhang H, Kasckow JW, Baker DG, Geracioti TD. Cerebrospinal fluid and plasma testosterone levels in post-traumatic stress disorder and tobacco dependence. Psychoneuroendocrinology. 2001; 26: 273-285.
- Blomberg Jensen M. Vitamin D metabolism, sex hormones, and male reproductive function. Reproduction. 2012; 144: 135-152.
- Wilkinson CW, Pagulayan KF, Petrie EC, Mayer CL, Colasurdo EA, Shofer JB, Hart KL. High prevalence of chronic pituitary and target-organ hormone abnormalities after blast-related mild traumatic brain injury. Front Neurol. 2012; 3: 11.
- Henning PC, Park BS, Kim JS. Physiological decrements during sustained military operational stress. Mil Med. 2011; 176: 991-997.
- Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011; 96: 1911-1930.
- Andersen NE, Karl JP, Cable SJ, Williams KW, Rood JC, Young AJ, et al. Vitamin D status in female military personnel during combat training. J Int Soc Sports Nutr. 2010; 7: 38.
- Ganji V, Milone C, Cody MM, McCarty F, Wang YT. Serum vitamin D concentrations are related to depression in young adult US population: the Third National Health and Nutrition Examination Survey. Int Arch Med. 2010; 3: 29.
- Llewellyn DJ, Lang IA, Langa KM, Muniz-Terrera G, Phillips CL, Cherubini A, et al. Vitamin D and risk of cognitive decline in elderly persons. Arch Intern Med. 2010; 170: 1135-1141.
- McCann JC, Ames BN. Is there convincing biological or behavioral evidence linking vitamin D deficiency to brain dysfunction? FASEB J. 2008; 22: 982-1001.
- Cekic M, Cutler SM, VanLandingham JW, Stein DG. Vitamin D deficiency reduces the benefits of progesterone treatment after brain injury in aged rats. Neurobiol Aging. 2011; 32: 864-874.
- Woodcock T, Morganti-Kossmann MC. The role of markers of inflammation in traumatic brain injury. Front Neurol. 2013; 4: 18.
- Cekic M, Stein DG. Traumatic brain injury and aging: is a combination of progesterone and vitamin D hormone a simple solution to a complex problem? Neurotherapeutics. 2010; 7: 81-90.
- Miller RR, Hicks GE, Shardell MD, Cappola AR, Hawkes WG, Yu-Yahiro JA, et al. Association of serum vitamin D levels with inflammatory response following hip fracture: the Baltimore Hip Studies. J Gerontol A Biol Sci Med Sci. 2007; 62: 1402-1406.
- Zhang Y, Leung DY, Richers BN, Liu Y, Remigio LK, Riches DW, et al. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J Immunol. 2012; 188: 2127-2135.
- Chen Y, Liu W, Sun T, Huang Y, Wang Y, Deb DK, et al. 25-Dihydroxyvitamin D promotes negative feedback regulation of TLR signaling via targeting microRNA-155-SOCS1 in macrophages. J Immunol. 2013; 190: 3687-3695.
- Guerrero AF, Alfonso A. Traumatic brain injury-related hypopituitarism: a review and recommendations for screening combat veterans. Mil Med. 2010; 175: 574-580.
- Defense Centers of Excellence Clinical Recommendation. Indications and conditions for neuroendocrine dysfunction screening post mild traumatic brain injury. 2012.
- Nimptsch K, Platz EA, Willett WC, Giovannucci E. Association between plasma 25-OH vitamin D and testosterone levels in men. Clin Endocrinol (Oxf). 2012; 77: 106-112.
- Pilz S, Frisch S, Koertke H, Kuhn J, Dreier J, Obermayer-Pietsch B, et al. Effect of vitamin D supplementation on testosterone levels in men. Horm Metab Res. 2011; 43: 223-225.
- McCrory P, Meeuwisse WH, Aubry M, Cantu B, Dvorák J, Echemendia RJ, et al. Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012. Br J Sports Med. 2013; 47: 250-258.
- Aminmansour B, Nikbakht H, Ghorbani A, Rezvani M, Rahmini P, Torkashvand M, et al. Comparison of the administration of progesterone versus progesterone and vitamin D in improvement of outcomes in patients with traumatic brain injury: A randomized clinical trial with placebo group. Adv Biomed Res. 2012; 1: 58.