Review Article
Austin J Nutri Food Sci. 2014;2(7): 1036.
Possibilities of Whey Utilisation
Rajka Božanic,Irena Barukcic*, Katarina Lisak, Jakopovic and Ljubica Tratnik
Department of Food Engineering, University of Zagreb, Croatia
*Corresponding author: :Irena Barukcic, Laboratory of Milk Technology and Dairy Products, Department of Food Engineering, Faculty of Food Technology and Biotechnology, University of Zagreb, Pierottijeva 6, 10000 Zagreb, Croatia
Received: February 03, 2014; Accepted: July 21, 2014; Published: July 25, 2014
Abstract
Over the past decades, many researchers have studied the economical possibilities of whey utilisation, primarily how to unwanted by-product converted into a valuable raw material. This paper gives an overview of whey utilisation possibilities. Traditionally, whey (sweet and acidic) is usually dried into powder; however, considering other processing options to improve the economic value such by-product, whey could be utilised, for example, in fermentation, production of soft drinks, production of Whey Protein Concentrate (WPC) and whey protein isolate (WPI), fractionation of certain protein components, such as isolation and purification α-lactalbumin (α-la) including specific peptides, and production lactose, lactic acid and bioethanol. This review provides most recent developments.
Keywords: Whey powder; Whey protein concentrate; Whey protein isolate; Lactose; Lactic acid; Bioethanol
Introduction
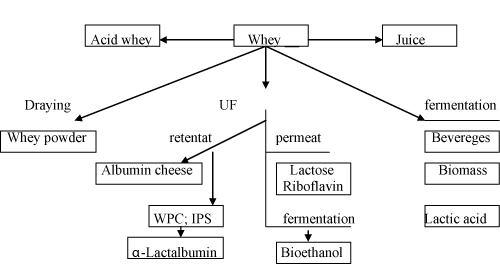
Whey is a by-product of cheese making (~96%) or a casein (~6%) production. Based on the casein coagulation method, acidic (acid action) or sweet whey (enzyme action) is produced. In general, from 100 L of milk utilised during the cheese manufacture, approximately 80-90 L of whey is produced. Depending on the variety of the cheese produced (e.g. hard or semi-hard), the average yield is 1 kg from 10 L of milk, where the balance (9 L) is whey. Hence, it is evident that daily production of whey can amount up to several millions of litres in large cheese plants. The world whey production is over 160 million tons per year (estimated as 9-fold of the cheese production), showing a 1-2% annual growth rate [1] (Figure 1). About 70% of whey is processed into different products, and about 30% of whey is still being utilized for pig feeding, spread on agricultural land as fertilizer or even dumped into the rivers or the sea [2]. Whey is being considered one of the most polluting food by/co-product streams since it has a biochemical oxygen demand (BOD) >35,000 ppm and the chemical oxygen demand (COD) >60,000 ppm) [3]. Tunick [4] estimated that 4000 L of whey could cause high environmental damage equivalent to that caused by faecal waste produced by 1900 humans.
Figure 1: Possibilities of whey utilisation.
Whey is well known product since cheese was first made, i.e. > 8000 years ago. In 17th and 18th century, whey was considered medically effective, but later became an unwanted by-product of the cheese industry. Traditionally, whey used to be disposed on agricultural land or dumped in rivers and the sea but, towards the mid of the 20th century, statutory laws in the cheese making countries were passed by many governments around the world forbidding its disposal in the environment. Today, the annual global increase of whey production is equivalent to 2%, which is parallel to annual increase in milk production [3]. As a consequence, many researchers have been studying alternative possibilities to utilize whey more economically, especially in the production of valuable raw mater materials rather than the manufacture of whey powder. Underpinning the scientific challenges, for example, how to capitalise on a product that contains low total solids content (6-7 g/110 g) to produce more profitable raw ingredients and, secondly, how to handle such highly perishable cheese by-product.
Compositional Quality of Whey
The chemical composition of whey varies in relation to method used for its production (acid whey or sweet whey). Whey usually contains about 50% of milk constituents, such as lactose (~70%; i.e. depending on the acidity of the whey), whey proteins (~14%), minerals and some fat. The main differences are in the calcium, phosphate, lactic acid and lactate contents, which are higher in acid whey than in sweet whey (Table 1). Colloidal calcium becomes more soluble in acid environment and, consequently, by the acid coagulation of casein, part of the calcium dissolves and passes into the whey. In contrast, sweet whey, except whey proteins, contains glycomacropeptides formed by the enzymatic hydrolysis of ?-casein. In addition, the proportion of whey protein is slightly lower in whey obtained from ultra filtered (UF) milk, and in whey obtained from milk heated at high temperature. The main reason is that part of the whey proteins is retained in curd of the cheese and not seeped into the whey, i.e. an approach in increase the yield of cheese [5]. In the conventional processes of cheese making, proteins which are insensitive to the action of enzymes and/or acids, pass into the whey. Consequently this group of proteins were termed as whey proteins [6].
Whey
Total solids
Lactose
Proteins
Calcium
Phosphates
Lactate
Chlorides
Sweet
63.0 - 70.0
46.0 - 52.0
6.0 - 10.0
0.4 - 0.6
1.0 - 3.0
2.0
1.1
Acid
63.0 - 70.0
44.0 - 46.0
6.0 - 8.0
6.0 - 8.0
2.0 - 4.5
6.4
1.1
Table 1: Composition (g/L) of sweet and acid whey (Jelen [2]).
Whey proteins are nutritionally the most valuable components in whey. They are composed of thermo sensitive fractions, such as β-lactoglobulin (β-Lg), α-lactalbumin (α-La), blood serum albumin and immunoglobulin as well as thermo stable proteose-peptone. Whey proteins have a compact globular structure that accounts for their solubility (unlike caseins that exist as a micellar suspension, with a relatively uniform distribution of non-polar, polar and charged groups). These proteins have amino acid profiles quite different from caseins: they have a smaller fraction of Glu and Pro, but a greater fraction of sulphur-containing amino acid residues (i.e. Cys and Met). These proteins are dephosphorylated, easily denatured by heat, insensitive to Ca2+, and susceptible to intra molecular bond formation via disulfide bridges between Cys sulfhydryl groups. Nutritionally whey proteins fractions are most valuable proteins because they contain high concentration of essential amino acids (especially lysine, cysteine and methionine) and high concentration of cystine. Because of the desirable amino acid composition, whey proteins have higher biological value when compared to casein, and other proteins of animal origin, including egg, which were considered for a long time as a referent proteins. Utilization of proteins in the body is closely related to the ratio of cysteine/methionine, which is about 10 times higher in whey proteins than in casein. Thermally denatured α-la is almost completely absorbed in the digestive system compared to case in which one is absorbed only 75% [7]. Daily requirements for the most essential amino acids may be obtained by consuming ~1.5 L of whey or 0.5 L of milk [8]. Whey also can be pool of bioactive peptides. Bioactive peptides have been defined as specific protein fragments that have a positive impact on body functions or conditions and may ultimately influence health. Upon oral administration, bioactive peptides, may affect the major body systems—namely, the cardiovascular, digestive, immune and nervous systems. The beneficial health effects may be classified as antimicrobial, antioxidative, antithrombotic, antihypertensive, antimicrobial or immunomodulatory.
The protein content in acid and sweet whey is very similar; however, the amount of free amino acids can vary and depends on a degree of casein hydrolysis during the manufacture of cheese (acid or sweet). Thus, the amount of free amino acids in sweet whey is about 4 times higher, and in acid whey even 10 times higher than in milk [6].
\In addition, whey proteins have excellent functional properties, such as good solubility, viscosity, gelling and emulsifying properties, and their concentrates are widely used in the food industry. Since whey proteins are easier to digest than casein, they are used for purposes such as the manufacture of infant formulas or to increase the nutritional value of dairy and other food products. Also, immunoglobulin and other glycoprotein's (lactoferrin, transferrin) and enzymes (lysozyme, lactoperoxidase) are very important factors that contribute to human immunoactive system. They exert antimicrobial properties, and may reduce or inhibit allergic reactions [9].
However, the largest constituent of whey is lactose (~70% based on dray matter basis). Incidentally, most milk carbohydrates pass into the whey after cheese making of which 90% is lactose including some glucose, galactose, oligosaccharides and amino sugars [6]. Lactose is a very important source of energy, and has multiple roles. Some of the beneficial effects of lactose are:
- stimulation of intestinal peristalsis, facilitate calcium and phosphorus absorption [10].
- establishment of a mildly acid reaction in the intestines and thereby preventing the growth and multiplication of harmful bacteria,
- ensures optimal magnesium levels and improves digestion of fat and other nutrients in the human body,
- articipate in the development of dental plaque,
- Heat treatment of whey converts lactose into lactulose, which is one of the growth promoters of the bifidobacteria [9,11].
Also, during the manufacture of cheese or casein some water-soluble vitamins permeate from milk into the whey, but their amounts are very variable and greatly dependant on whey handling. The most important of these vitamins are riboflavin, folic acid and cobalamine. However, the latter two vitamins are bonded to whey proteins, what is the reason why they pass into the whey during cheese making. It is of interest to note that whey contains higher amounts of vitamin B2 than milk due to the activity of some starter cultures (i.e. lactic acid bacteria - LAB) during the manufacture of cheese. Due to relatively high levels of riboflavin, whey has a characteristic yellowish-green colour [6,8].
Owing to the high nutritional value and the high amounts produced, researchers have been studying for the last 50 years opportunities to maximize whey utilization, focusing especially on its high-quality proteins. As mentioned elsewhere, whey could be utilized in the production of different types of powders, protein isolates, riboflavin, lactic acid, whey cheese and other products, and details of the manufacturing practices will follow.
Whey Powder (WP)
In general, the main industrial processing of whey is drying (i.e. WP production), which is 70% of the annual production of whey. Small tonnage of WPC and to a lesser extent WPI are produced every year, and the remaining permeate can be used to produce lactic acid, bioethanol or lactose.
The main advantage of drying is that the process does not produce residues that should be treated separately, but the main disadvantages are: - (a) the high capital investment required to purchase the equipment, (b) the high energy consumed during production, and (c) the relatively low selling price WP compared to WPC. Production of WP involves evaporation, lactose crystallization and drying, which is performed after evaporation and before drying. Although lactose crystallization is not required in the drying process and, if it is not implemented, a solid mass of bulk powder is formed suitable only for animal feed. Amorphous lactose is sticky, creates drying problems and causes hygroscopicity of the product, which has the tendency to clump because the powder reacts readily with moisture in the atmosphere, mostly due to the present amorphous lactose, protein and salt. The powder has the tendency to clump depending on the degree of lactose crystallization as well as the number, size, distribution of crystals and residual free moisture. Such undesirable characteristic of lactose is minimized by transforming it into a crystalline form of α-hydrate. Though it is not possible to achieve 100% lactose crystallization, but the primary objective is to transform as much as possible of lactose to α-mono-hydrated form (90-95%).
The manufacturing stage of WP include removal and any fat, heat treatment, evaporation (40-60 g/100 g total solids - TS), followed by lactose crystallization and spray drying. Traditionally, roller drying was the normal practice, but it is not widely used because of the inferior in quality of the powder (the product becomes dark colour due to Maillard's reaction between proteins and lactose resulting in poor solubility of the powder). A target specification of WP is ~95 g/100 g TS (Table 2).
Whey
Whey powder
Sweet
Acid
Sweet
Acid
Total solids
6.4
6.5
96.0
96.0
Water
93.7
93.5
3.6
4.0
Fat
0.5
0.1
0.8
0.6
Proteins
0.8
0.8
13.1
12.5
Lactose
4.9
4.9
75.0
67.4
Ash
0.5
0.8
7.3
11.8
Lactic acid
0.1
0.4
0.2
4.2
Table 2: Whey and whey powder composition (w/w) (Caric [12]).
Whey powder is mainly used for the production of animal feed, because it is an inexpensive source of high quality proteins and carbohydrates. However, because of the high nutritional value of whey powder, it could be used in the food industry as an additive in the production of many products (Table 3) including the confectionery industry, bakery, dairy products, baby food, meat products and production of beverages, soups, sauces, toppings, and cream. Properties of food products that may be affected by using WP are very diverse, such as: (a) improving sensory properties, (b) enhancing physical characteristics, such as the ability to create foam, stability of fruit and acid, and (c) using it as adsorbent and as a carrier of fats and oils. For example, in ice-cream production, whey powder is used successfully, in part, to replace the more expensive skimmed milk powder (SMP). Replacement up to 21% has no effect on the taste, consistency and melting point of ice cream [12], or the sensory properties of yoghurt [13].
Product
% whey total whey solids
Characteristic that are improved
Pastry
3 (on flour weight)
Aroma, texture, shorter fermentation, increased durability
Ice-cream
2.7
Aroma, acid and fruit stability
Sweets
10
Aroma, texture, water binding
Glazes, sugar dressings
6
Air incorporation
Jams
4
Aroma
Melted cheese
10
texture, aroma
Table 3: Use of whey powder in food products (Caric [12]).
However, whey powder contains a fairly high proportion of mineral matter (8-10 g/100 g) and for some products it is more desirable to use demineralised whey otherwise the salty taste becomes evident. Demineralisation of whey before drying is generally performed by ion exchange electro dialysis or nanofiltration [14]. (Table 4) shows the average chemical composition of the major products of whey powder.
Protein
Lactose
Minerals
Whey powder
12.5
73.5
8.5
Demineralized (70%) whey powder
13.7
75.7
3.5
Demineralized (90%) whey powder
15.0
83.0
1.0
Ultra filtered permeate powder
1.0
90.0
9.0
Whey protein concentrate
65.0-80.0
4.0-21.0
3.0-5.0
Whey protein isolate
88.0-92.0
<1
2.0-3.5
Table 4: Average composition (%) of major products from whey powder (Jelen [2]).
α-lactalbumin (α-La) and β-lactoglobulin (β-Lg)
α-La is the second major whey protein after β-Lg. It comprises ca. 20% of all proteins in bovine whey, and 3.5% of the total protein content of whole milk, and is nutritionally the most valuable protein due to its high content of essential amino acids, especially tryptophan, cysteine, and lysine. Thus, it is mainly used as a nutraceutical for therapeutic purposes. Amino acid composition of bovine α-La coincides closely (72%) with the human α-La, and it makes an ideal protein for infant formulas [15]. Therefore, isolation of α-La and development of new products suitable for sensitive population is of great interest for the industry. A good reason for the development of new and cheaper method for the isolation α-La is its high commercial price, which is approximately € 67,000 for one kilogram of product (Sigma Aldrich, USA).
Some researchers have isolated α-La on an industrial scale, which are mostly based on membrane processes. Separation can be achieved by using Microfiltration (MF) or UF technologies; recommended membrane porosity of 50 or 100 kDa, where α-La permeates through the membrane, whilst β-Lg is retained in the retentate [16]. For the isolation of α-La from whey, cascade of two-stage UF membrane module is used by combining 30 and 100 kDa [17]. However, α-La is more heat stable than β-Lg and, selective exposure of thermally stable α-La to a mildly acidic conditions (pH <5), β-Lg precipitates as these fractions have different isoelectric points [18]. Also, separation of α-La and β-Lg can be performed by employing a combination of UF and anion exchange chromatography under very mild conditions [19], followed by separation using ion exchange chromatography, which is based on the different affinity of the protein to either mobile or stationary phase [20]. Drawbacks of these methods are a very low degree of purification and yield, significant denaturation of the proteins, and the high cost required when scaled-up to an industrial level.
Recent studies have shown the great potential of enzymatic hydrolysis of the proteins to produce bioactive peptides, which cause minimal denaturation as well as significant cost reduction. Many researchers have concluded that trypsin has selective enzymatic activity [16, 21,22]. Trypsin hydrolyses β-Lg, while α-La remains more or less in the native form. [23] Reported for the first time that the enzyme α-chymotrypsin was used for the isolation of α-La by selective hydrolysis of WPI, whilst the same authors discovered a similarity in the mechanism and sequence of whey protein hydrolysis with α-chymotrypsin when compared to trypsin. During the enzymatic hydrolysis, the hydrolytic sequence was as follows: first, β-Lg A was hydrolysed followed by β-Lg B and finally α-La. The best condition for α-La isolation was at 25°C regardless of the pH value, and the worst condition was at 50°C. Process efficiency was improved when hydrolysis was performed with 5% of WPI on protein basis when compared to 10% WPI, and in both cases there were small amounts of residual β-Lg. For example, the best conditions for α-chymotrypsin hydrolysis of 5% of WPI were at 25°C, pH 8.5, and 1% enzyme where complete hydrolysis of β-Lg was achieved and 81% of α-La remained intact [24].
Proper enzyme selection and hydrolysis conditions can affect the selectivity and kinetics of enzyme reactions and, thus, the desired WPI fraction. Also, this approach provides the opportunity to develop products depleted of β-Lg for humans of allergy sensitivity. However, the most important aspect of such hydrolysis process are considerably lower costs and readily applicable in industrial-scale operation.
Albumin or Whey Cheese(s)
The oldest product deriving from whey is known as albumin or whey cheese. Whey cheeses are solid, semi-solid, or soft products which are principally obtained either through the concentration of whey and the moulding of the concentrated product (1) or through the coagulation of whey by heat with or without the addition of acid (2) [25]. In each case, due to the low content of the total whey solids, whey is mainly pre-concentrated prior to the further concentration of whey or coagulation of the whey proteins. The process may also include the addition of milk, cream, or other raw materials of milk origin before or after concentration or coagulation [25]. By using the concentration method, the lactose is also concentrated beside the whey proteins. Retention of a large amount of lactose in the albumin cheese provides distinctive characteristics of the product, such as yellowish to brown colour and sweet/cooked or caramelized flavour. Whey cheese made by the heat precipitation method of the whey proteins or combination mixtures of whey, milk or cream, with or without the addition of acid, contains less lactose and the colour is white to yellowish [25].
Furthermore, when using the heating method for the manufacture of whey cheese, certain manufacturers heat fresh sweet whey immediately after the cheese making. However, the majority uses 1-3 days old whey, so that the present starter culture is able to produce more acid. Alternatively, the desired acidification level in sweet whey is achieved by adding acid whey (10%), acetic or citric acid. Sometimes a small amount of milk is added to the whey prior to heating, in order to obtain better extraction of the residual proteins, as well as to achieve better quality and higher yield of whey cheese. Heat treatment of the whey and milk mixture at 70°C causes the proteins to coagulate (i.e. formation of white albumin and globulin flakes). The coagulum formed that way floats to the surface of the heated whey whereby a certain amount of foam appears, which needs to be removed. As heating is increased to 90-95°C for 10-20 min, the precipitated curd begins to "break", where it is removed from cheese caldron (tub or vat) and transferred either into the perforated plastic moulds to draining for the next 4-6 h or into the cloth bags for 6-8 h. Salting can be conducted during the heating stage or by adding salt to the curd after the draining stage [26].
Traditional home-made whey cheese is mainly produced from sheep's milk whey due to the significantly higher total whey solids content (i.e. whey proteins when compared to cow's milk whey). Sometimes goat's milk whey is also used for purposes of whey cheese production. However, the chemical composition of these types of whey cheeses differ from the industrial product because of the type of whey used (sheep, goat and cow) and the type of additive employed (salt, vinegar, acid whey) during the processing.
Lactose
Lactose is the main component of whey, and constitutes ~70% of the total whey solids so whey is regarded as a good substrate for the production of lactose. Thereby, lactose is isolated from de-proteinized whey (e.g. whey permeate obtained by UF) by applying different processes such as the concentration of whey by evaporation; crystallization of lactose from the concentrated whey and separation of the obtained crystals by centrifuges or decanters [27]. In this manner raw or refined lactose is produced. The technological operations as well as the type of the used equipment are very similar to those employed for the manufacture of sucrose from sugar cane.
After the evaporation stage, concentrated and supersaturated warm whey is obtained, which is then submitted to a crystallization process [28]. Lactose crystallization is very complex process, and it implies the diffusion of lactose molecules to the surface of a crystal. Afterwards the crystals are formed and simultaneously release the crystallization energy, which is then being remised into the liquid. The crystallization process occurs spontaneously either due to the super saturation of whey or due to the addition of nuclei for the purpose of crystallization induction whereby the latter aspect is more common. The aim of the crystallization process is to obtain large number of crystals similar in proportions (average diameter of 0.2 mm), which facilitate good separation. The crystallization nuclei are added prior to the entrance or directly into the crystallization tanks. That way the occurrence of the crystallization process during evaporation is prevented. The rate of the crystallization process depends on several factors such as the available crystallization area, the purity and the viscosity of the whey permeate, the degree of the super saturation, the processing temperature, and the intensity of the mechanical treatment as well as their mutual interactions, which most certainly influence the kinetics of the reaction.
The unrefined crystalline lactose (also known as α-lactose monohydrate) is separated either by continuous centrifuges or by decanters. In both cases, two devices in parallel are used. When continuous centrifuges are being applied, crystals are only separated but, in the case of decanters application, crystals are rinsed with fresh clean water; therefore, separation and rinsing are being conducted at the same time. The rinsing water contains a certain amount of dissolved lactose but, in comparison with the evaporated whey used as a source of crystalline lactose (the molasses), it has a higher degree of purity. Therefore, the rinsing water is drawn back into the crystallization process. The molasses (TS 38-48 g/100 g of which 30 g/100 g is lactose) can also be processed again, but firstly it has to be diluted by the addition of whey or water until the content of the solids content is reduced to 15 g/100 g. The unrefined lactose produced this way contains 10-14 g/100 g water and approximately 99% lactose in the total whey solids.
If the lactose is not being refined, it is submitted to drying in the vibrating dryers at 70°C until the water level is reduced to about 0.1- 0.5 g/100 g, and the dried powder is known as 'crude' lactose. The duration of the drying process should not be too short otherwise a thin layer of amorphous lactose is formed at the surface of α-hydrate crystals. Such phenomena can cause the appearance of clots during storage period of the product. The rest of the technological operations, such as milling, sieving and/or packaging, are very similar to those for the manufacture of SMP.
The refined lactose intended, which is intended for pharmaceutical uses (minimum lactose content 99.6 g/100 g and protein-free), is most likely being produced in the same production unit as the unrefined lactose. The manufacturing stages are the same until the separation and rinsing of lactose crystals. These operations are followed by refining, which implies that the lactose is dissolved, the processing is at higher temperatures including the addition of different chemicals, filtering, evaporation, crystallization and separation of crystals. Afterwards, the remaining processing stages are the same as in case of unrefined lactose production. During the refining process, 1% of active carbon is added in order to absorb the unwanted colouring agents and other impurities. Thereby often the addition of acids (e.g. HCl) is needed for purposes like acidity adjustment, dissolving the present salts or denaturation of proteins (if there was no prior operation of protein removal) as well as to enhance the effect of the active carbon. Also agents for alleviation of filtration (e.g. 0.1% of Kieselguhr) or bleaching agents (~0.22% of Na-bisulphite) are added. Since the content of the refining tank is heated, the neutralization by addition of an alkaline agent until reaching pH 5.4-5.8 is needed. Afterwards the content is submitted to intense boiling for several minutes whereby the present proteins flocculate while carbon and insoluble salts precipitate. The formed sediments are separated by filtration and a clear lactose solution is obtained. The lactose solution is then undergoes drying until the concentration of 65-70 g/100 g of total whey solids is reached.
Edible and refined lactose are mostly utilized in the pharmaceutical and food industry. Applications in the food industry include production of infant formulae (since human milk contains more lactose than bovine) or baby foods as well as for production of modified and reconstituted dairy products. In addition, lactose enhances consistency and shelf-life of confectionary products; it improves flavour, appearance and baking properties of pastry. Furthermore, lactose can also be used as a carrier of volatile aroma compounds, for adsorption of the unwanted aromas or as a sweetening agent in certain foods to enhance product aroma. Lactose is also commonly used in the production of potato crisps, pudding, sauces, salad dressings, etc. The main advantages of lactose application in the food industry are the relatively low sweetness, the ability to enhance the characteristic flavour of products and to stabilize proteins, as well as the ability to induce colour changes where appropriate (i.e. bakery and confectionary products) due to the Maillard reaction.
Lactic Acid Production
Lactic acid and its derivatives are widely used in the food, cosmetic, pharmaceutical, leather, textile and chemical industries. Recently, it has also been used as a raw material in the production of biodegradable plastic. Lactic acid can also be used instead of citric or tartaric acid in the production of non-alcoholic beverages, sour candies, medicinal products, etc. Worldwide, 80 000 tonnes of lactic acid are produced every year. Lactic acid can be produced either by microbial fermentation or synthetically by hydrolysis of lactonitrile [29], whereby recently there is prevalence for microbial fermentation application due to environmental reasons and limited amount of petrochemical resources. When considering lactic acid production, there is the tendency for using low-cost raw materials, and whey (UF permeate) contains a relatively high content of lactose (5-6 g/100 g) is proven to be a cheap and a good source of raw material.
Different strains of rod shaped LAB (Lactobacillus helveticus, Lactobacillus delbrueckii subsp. bulgaricus, Lactobacillus acidophilus, Lactobacillus casei, etc.) have been used for the production of lactic acid from whey. Due to a high conversion rate, Lb. helveticus is the generally preferred strain since it can produce almost twice the amount of lactic acid in comparison with other common LAB. The efficiency of lactobacilli fermentations can be enhanced by supplementation of the whey permeates with yeast extracts or yeast protein auto lysates (peptones) [29]. In addition to the use of LAB, Kluyveromyces marxianus var. marxianus has been used successfully used for lactic acid production. Recent reports suggest that mixed cultures of LAB may be more effective to enhance lactic acid production due to synergistic effects that exist among these organisms [30]. Investigated whey fermentation using different mono- and mixed cultures. The highest conversion rate to lactic acid (19.8 g/L) was achieved when using Kluyveromyces marxianus var. marxianus in combination with homofermentative LAB strains (Lb. delbrueckii subsp. bulgaricus and Lb. helveticus). Application of immobilised microbial cell systems significantly enhances the production of lactic acid from an economic point of view. Immobilisation technology enables reutilisation of the used cells, continuous operation and higher cell densities in bioreactors as well as better purification of the end product [29].
Whey can also be used as a substrate for production of different microorganism or their metabolites [31]. Investigated the possibilities of LAB cultures production with appropriate characteristics for use in the bakery industry. As substrates for microbial growth, deproteinized whey and modified MRS agar were used and compared. Afterwards, the growth and fermenting ability of certain LAB (Leuconostoc mesenteroides subsp. mesenteroides L3, Lactobacillus brevis L62 and Lactobacillus plantarum L730 in fermentation substrates and sour dough were investigated in terms of lactic and acetic acids production. Although the measured activity of the LAB strains was somewhat lower in case of whey, the results have shown that deproteinized whey can be used for production of microbial biomass (yield 1.7 g/L) and lactic acid (9.15 mg/mL) were achieved when using Lb. plantarum L730 strain. Sensory evaluation showed that supplementation of bread with starters prepared by cultures produced on deproteinized whey as a substrate resulted in an improved taste and odour, better dough elasticity and better shelf-life in comparison with bread produced in a classical way (with the addition of yeast monoculture).
Bioethanol
As mentioned elsewhere, lactose is one of the main constituents of whey permeate when using membrane technology for the production of WPC, which is an excellent substrates for bioethanol production as it is cheap and widely available in large volumes. This product is mainly used as a biofuel additive to gasoline, and most commonly produced from sugar cane or sugar beet, different crops or from cellulosic resources (wooden hydrolysates, agricultural by-products).
The production process of bioethanol can be divided into three main stages:- (a) preliminary processing of the substrate, i.e. preparation of the raw material, (b) alcoholic fermentation, and (c) separation of the end-product (distillation, rectification and dehydration of bioethanol).
For purposes of alcoholic fermentation yeasts (i.e. Saccharomyces cerevisiae) are usually used since they have a fast fermentation capacity and tolerate high concentrations of ethanol (up to 20% v/v). Since S. cerevisiae cannot ferment lactose, whey has to be enzymatically hydrolysized prior to the alcoholic fermentation. The hydrolysis step is not required if Kluyveromyces spp. are employed as they have the ability to catabolise lactose. In the commercial production of bioethanol from whey, K. marxianus var. marxianus and Kluyveromyces fragilis var. marxianus are commonly used [1,32,33] and, over the past few decades, whey permeate has been used for bioethanol production in Ireland, New Zealand, Denmark and in the United States of America (USA) [1,34].
Conclusion and Future Trend
The attitude towards whey and its utilization has changed over the years from being a by-product to value added raw material. Environmental issues have forced governments to legislate regarding the disposal of whey and, as a consequence, scientific challenges resulted in developing different technologies for the utilization of what used to be categorised as 'waste' from cheese making into an important and economical raw material for the manufacture many ingredients/products for the food industry. Although large volumes of whey still need to be processed, production of bioethanol may become more viable and more profitable by improving the technology and reducing the cost of manufacture.
References
- Guimar&aTilde;es PM, Teixeira JA, Domingues L. Fermentation of lactose to bio-ethanol by yeasts as part of integrated solutions for the valorisation of cheese whey. Biotechnol Adv. 2010; 28: 375-384.
- Jelen P. Utilization and Products. Whey Processing. In: Encyclopedia of Diary Sciences, 2nd edn. Fuquay JF, editor. Academic Press - An Imprint of Elsevier. 2011; 4: 731-738.
- Smithers GW. Whey and whey proteins - From "gutter to gold". International Dairy Journal. 2008; 18: 695-704.
- Tunick MH. Whey protein production and utilization: A brief history. In: Whey Processing, Functionality and Health Benefits. Onwulata CI and Huth PJ, edn. IFT & Blackwell Publishing and Institute of Food Technologists, Ames, Iowa, SAD. 2008; 1-15.
- Jelen P. Whey Processing. In: Encyclopedia of Diary Sciences. 2nd edn. Fuquay JF editor. Academic Press - An Imprint of Elsevier. 2003; 4: 2740.
- Tratnik LJ, Božanic R. Mlijeko i mlijecni proizvodi. Bašic Z, editor. Hrvatska mljekarska udruga. Zagreb. 2012
- Jelicic I, Božanic R, Tratnik LJ, Lisak K. Possibilities of application of non-traditional processing methods in the dairy industry. Mljekarstvo. 2010a; 60: 113-126.
- Popovic-Vranješ A, Vujicic I. Tehnologija surutke, Poljoprivredni fakultet Novi Sad, Novi Sad. 1997.
- Tratnik LJ. Uloga sirutke u proizvodnji funkcionalne mlijecne hrane. Mljekarstvo. 2003; 53: 325-352.
- Kwak HS, Lee WJ, Lee MR. Revisiting lactose as an enhancer of calcium absorption. International Dairy Journal. 2012; 22: 147-151.
- Seki N, Saito H. Lactose as a source for lactulose and other functional lactose derivatives. International Dairy Journal. 2012; 22: 110-115.
- Caric M. Technology and milk products, dried and concentrated. Doncev N, editor. IDP "Naucna knjiga" Beograd. 1990.
- Legac A. Utjecaj dodatka obranog mlijeka i sirutke u prahu na viskoznost i senzorska svojstva tekuceg jogurta. Graduate thesis. Faculty of Food Technology and Biotechnology University of Zagreb. 2012.
- Gernigon G, Schuck P, Jeantet R, Burling H. Demineralisation. Whey Processing. In: Encyclopedia of Diary Sciences, 2nd edn. Fuquay JF editor. Academic Press - An Imprint of Elsevier. 2011; 4: 738-743.
- Monaci L, Tregoat V, Hengel AJ, Anklam E. Milk allergens, their characteristic and their detection in food: A review. European Food Research and Technology. 2006; 223: 149-179.
- Konrad G, Kleinschmidt T. A new method for isolation of native?- lactalbumin from sweet whey. International Dairy Journal. 2008; 18: 47-54.
- Cheang B, Zydney AL. A two-stage ultrafiltration process for fractionation of whey protein isolate. Journal of Membrane Science. 2004; 231: 159-167.
- Alomirah H F and Alli I. Separation and characterization of β-lactoglobulin and a-lactalbumin from whey and whey protein preparations. International Dairy Journal. 2004; 14: 411-419.
- Kristiansen KR, Otte J, Ipsen R, Qvist KB. Large-scale preparation of β-lactoglobulin A and B by ultrafiltration and ion-exchange chromatography. International Dairy Journal. 1998; 8: 113-118.
- Outinen M, Tossavaineen O, Syvaoja EL. Chromatographic fractionation of a-lactalbumin and β-lactoglobulin with polystyrenic strongly basic anion exchange resins. LWT - Food Science and Technology. 1996; 29: 340-343.
- Custodio MF, Goulart AJ, Marques DP, Giordano RC, Giordano RLC, Monti R. Hydrolysis of cheese whey proteins with trypsin, chymotrypsin and carboxypeptidase. Alimentaç&aTilde;o e Nutriç&aTilde;o. 2005; 16: 105-109.
- Cheison SC, Leeb E, Tero-Sierra J, Kulozik U. Influence of hydrolysis temperature and pH on the selective hydrolysis of whey proteins and potential recovery of native alpha-lactalbumin. International Dairy Journal. 2011; 21: 166-171.
- Lisak K, Toro-Sierra JT, Kulozik U, Božanic R, Cheison SC. Chymotrypsin selectively digests β-lactoglobulin in whey protein isolate away from enzyme optimal conditions: potential for native a-lactalbumin purification. Journal of Dairy Research. 2013; 80: 14-20.
- Lisak K, Toro-Sierra JT, Kulozik U, Božanic R, Cheison SC. Influence of temperature, pH, enzyme and substrate concentration on the chymotrypsin hydrollysis of whey proteins for recovery of native alpha-lactalbumin. 7th International Congress of Food Technologists, Biotechnologists and Nuiritionists, Opatija, Croatia, Book of Abstracts. 2011; 46.
- Codex Alimentarius. Milk and milk products. 2nd edn. World Health Organization; Food and Agriculture Organization of the United Nations, Rome. 2011.
- Antunac N, Hudik S, Mikulec N, Maletic M, Horvat I, Radeljevic B, et al. Production and chemical structure and Pag curd. Mljekarstvo. 2011; 61: 326-335.
- Muir DD. Lactose: Properties, production, applications. In: Encyclopedia of Diary Sciences, 2nd edn. Fuquay JF, editoe. Academic Press - An Imprint of Elsevier. 2003; 3: 1525-1527.
- Bylund G. Dairy processing handbook. Tetra Pak, Processing Systems AB, Lund, Sweden. 1995.
- Panesar PS, Kennedy JF, Gandhi DN, Bunko K. Bioutilisation of whey for lactic acid production. Food Chemistry. 2007; 105: 1-14.
- Plessas S, Bosnea L, Psarianos C, Koutinas AA, Marchant R, Banat IM. Lactic acid production by mixed cultures of Kluyveromyces marxianus, Lactobacillus delbrueckii ssp. bulgaricus and Lactobacillus helveticus. Bioresour Technol. 2008; 99: 5951-5955.
- Mrvcic J, Stanzer D, Božic D, Stehlik-Tomas V. Sirutka - sirovina za proizvodnju starter kultura za pekarstvo. Mljekarstvo. 2008; 58: 117-130.
- Siso MIG. The biotechnological utilization of cheese whey - a review. Bioresource Technology. 1996; 57: 1-11.
- Pesta G, Meyer-Pittroff R, Russ W. Utilization of whey. In: Utilization of By products and Treatment of Waste in the Food Industry. Oreopoulou V, Russ W editors. Springer, New York. 2006; 1-11.
- Gapes JR, Gapes RF. Relevance & economics of biodiesel/biofuels industry. Vision 20/20, IPENZ Annual Conference, Auckland, New Zealand. 2007.