Review Article
Austin J Nutri Food Sci. 2014;2(8): 1044.
Dysbiosis, Probiotics, Synbiotics and Human Health
Saikh MAA*
Department of Pharmaceutical Sciences, Shiats (Deemedto-be-University), India
*Corresponding author: : Saikh MAA, Department of Pharmaceutical Sciences, Shiats (Deemed-to-be-University), India
Received: August 14, 2014; Accepted: October 09, 2014; Published: October 09, 2014
Abstract
The manuscript aims to project handy information on intricacies and interwoven of the ecosystem of gut-dysbiosis, and probiotics and synbiotics, with human health. Information collected from diverse source including databases. Presented information might insight and paves the way for better management of human health with probiotics and synbiotics therapy, in diverse domain.
Keywords: Dysbiosis; Effect; Health; Probiotics; Symbiotic; Therapy
Abbreviations
GIT: Gastrointestinal Tract; SCFA: Short-Chain Fatty Acid; DC: Dendritic Cell; MHE: Minimal Hepatic Encephalopathy; LAB: Lactic Acid Bacteria; AchR: Acetylcholine Receptor; NF-kB: Nuclear Factor Kappa-light-chain-enhancer of activated B-cells; TNF: Tumor Necrosis Factor; Th-cell: T helper cell; Tr-cell: T regulatory cell; and NK-cell: Natural Killer Cell
Introduction
The human GIT colonizes thousands species of microbes and enriched with many molecules, be using as nutrients by microbes. It possesses potentiality to be colonizing by microbes, to highest diversity. In fact, billions of bacteria inhabit the human digestive system, referred to as the GIT-microflora. They contribute over a kilo of body weight. Biotic species-/strain-diversity of the GIT-microflora is over 1000, which are beneficial (non-pathogenic) and pathogenic (potentially harmful) [1-5].
The mucosa of the GIT is continuously exposing to an environment that enriched in foreign substances, food particles, and microbial antigens. Within GIT, the large intestine or colon is the site preferred by microbes for colonization. Thus, the gut-microbiota play a key role in efficient nutrient absorption thus has a crucial role in disease and maintaining human health. Through metabolic process, the intestinal microbes metabolizes diverse nutritional substrates to terminating metabolites like organic acids, vitamins, SCFAs, etc [4].
Consensus on roles of the intestinal-microbiota in human health and disease is expanding rapidly. The gut-microbiota appears to be contributing to nearly every aspect of the host's growth and development. The gut-health in is a key sector for maintaining overall health, of people whose gut-microbiota plays key role in metabolism and nutrient absorption. Consensus is that tremendous arrays of diseases and dysfunctions associated with an imbalance in composition, numbers, or habitat of the gut-microbiota. Hence, dynamic balance between the host gut and its microbiota is prerequisite [1-8].
Perturbation of the gut-microflora/ecosystem may leads to different gut/functional-bowel disorders, certain illness, or chronic diseases like autoimmune diseases, colon cancers, gastric ulcers, cardiovascular disease, and obesity. Circumstances like diet, medication, stress, age and inhabiting conditions perturb this balance. This perturbation, alteration or imbalances of the microflora are outcomes gut-dysbiosis. This state events the colonization of pathogenic species, manifested, consequences diseases and disorders, aforesaid [3-9].
Restoration of the gut-microbiota may difficult to be accomplishing. However, therapies of dietary supplement, especially probiotics/prebiotics/synbiotics, are able to manipulate composition of the gut-microbiota in infants and adults [10-11]. The therapy comprises a large heterogeneous group of bacteria, the normal inhabitants of the human GIT. These enables in maintaining a balance between the beneficial and harmful bacteria. Related promising results had been establishing in a large number of well-designed studies, clinical one [1-3].
Probiotics (the beneficial/friendly bacteria) and prebiotics (the indigestible food ingredients) maintain intestinal health and deliver specific health benefits. In symbiotic [combination of probiotics and prebiotics], a synergistic chemopreventive actions is exerted with combinations of the two. Here the probiotics uses the prebiotics as a food source that enables in extending their survival within the intestine than would be possible otherwise [1-3,12-15].
Nowadays consumers are interested in intake of food that needs to be healthy and or alleviates illness. Since their introduction, probiotics, prebiotics, and synbiotics have been attracted much attention for ameliorating health. These are becoming increasingly popular, evidenced from rapidly expanding research support and an ever-widening choice of products. Their products presented commercially in diverse forms like foods, dietary supplements, and clinical therapeutics (oral or non-oral delivery). Scientific communities nowadays engaged in searching for different prebiotics as well as effective synbiotics combinations, for maintaining the beneficial microbiota of human GIT. Knowledge of gut-microflora and its interactions may lead to development of the dietary strategies that serve to sustain or even improve the healthier gut-microflora [1- 5,10-15].
There is therefore needs to have an exhaustive handy reference on the functions of the gut-microbiota, occurrence of gut-dysbiosis, intricate and interwoven the ecosystem of gut-dysbiosis with human health, probiotics and synbiotics, and future perspectives. In addition presenting is how gut-microbiota triggers development of disease in gut-dysbiosis state and synbiotics alleviating it.
Human Gut-Microbiota
The term "microbiota," "microflora," or "normal-flora" had been using to designate the vast host of microbes coexisting with the host [3,16-18]. An estimate is that the human-microbiota contains as many as 1014 microbial cells, a number 10 times greater than the number of cells present in human bodies [19-21]. Said microbiota comprises bacteria, unicellular eukaryotes, and other organisms, in large numbers. Every surface of the human body starting from skin surface to genitourinary tract, oral cavity, respiratory tract, ear, and GIT colonized heavily with diverse species of bacteria [18,22-24]. Amongst them, the gut is most heavily colonized organ that houses a huge microbial ecosystem, having biotic species-/strain-diversity over 1000. The colon alone contains over 70% of the human-microbiota, as estimated. The gut dominated by several bacterial phyla including Bacteroidetes, Firmicutes, and Actinobacteria [3,7-8,19,21,25].
The GIT is a sterile environment at birth. Colonization of it begins during the delivery process, from the maternal genital-flora and/or environment. Later on, it colonizes from the surroundings or environment [26]. Factors like microbiota of the maternal-genital-tract, obstetric techniques (vaginal or caesarean delivery), sanitary conditions, and type of feeding have an immediate effect on the level and frequency at which various species colonizes the infant's gut [3,25].
The gut initially colonizes with the facultative anaerobes like Escherichia coli and Streptococcus spp. These first colonizers metabolize any traces of oxygen in the gut, thereby transforming the environment towards strong anaerobic conditions. The feeding profile of the infant determines largely the subsequently colonizing microbes. Weaning of baby to solid foods changes the composition and complexity of the gut-microbiota, and final phase of microbiota acquisition. Dietary changes in the adulthood are significantly responsible for the composition and complexity of intestinal-microbiota. The gut-microbiota of adult has majorly non-sporing anaerobes. Most numerically predominant are species of Bacteroides and Bifidobacterium, Clostridium, Eubacterium, Fusobacterium, Lactobacillus, and various gram-positive cocci. Bacteria numerically non-predominant include Enterococcus spp., Enterobacteriaceae, and methanogens and dissimilatory sulfate-reducing bacteria [3,7-8,25].
Role in functions
Gut-microorganisms are able readily to degrade available substrates, derived may be from the diet or endogenous secretions. Substrates available majorly for colonic fermentation are starches and soluble dietary fibers. Substrates of other carbohydrate, available in lower concentrations, are oligosaccharides and portions of non-absorbable sugars and sugar alcohols. Proteins and amino acids can be the substrates for effective growth of colonic microbes. Other substrates that may contribute are bacterial secretions, lysis-products, sloughed epithelial cells, mucins, etc. Diverse microbial enzymes degrade these materials. Gut-microbes eventually able to ferment these intermediates to terminate-products like carbon dioxide, histamine, organic acids, and other products, neutral, acidic, and basic. Said fermentation processes progress through a series of energy-yielding reactions that do not use oxygen in the respiratory chains [7-8,25].
Basing upon the metabolic activities of gut-microbiota and their fermentation terminate-products, these can be categorizing either beneficial or pathogenic. Their health promoting effects are improved digestion and absorption, pathogen growth inhibition, immunostimulation, vitamin synthesis, and cholesterol and flatulence reduction. Harmful effects are toxin and carcinogen production, diarrhea/constipation, intestinal putrefaction/infections, liver damage, etc [7-8,25].
Role in histological functions
The gut-microbiota ensures function of intestine and intestinal structure, as possesses role in cell and tissue development. Colonic microbes secrete butyrate, a SCFA, which reinforces defense barrier in the colon. This butyrate regulates cell differentiation and growth, inhibits cell transformation, promotes cell reversal from a neoplastic to a nonneoplastic phenotype, and induces secretion of mucin, antimicrobial peptides, and other factors [3,27-28]. Mucin secretion and degradation balances the intestinal mucus layer that obstructs pro-inflammatory compounds and antigen uptake [29]. In addition, development of the microvasculature of intestinal villi depends on indigenous microbes [30]. All these signify the importance of the gut-microbes in developing morphology and structure of the gut [3,8].
Role in metabolic and protective functions
The colonic bacterium synthesizes amino acids, produce group B vitamins, and biotransforms bile. Enzymatic biotransformation of bile is important for the metabolism of glucose and cholesterol [31]. These microbiomes provide needful biochemical pathways for the fermentation or metabolism of non-digestible substrates like fibers and endogenous mucus. Said metabolism promotes their growth and production of SCFAs and gases [32]. SCFAs produced majorly are acetate, propionate, and butyrate. Other terminating metabolites are lactate, ethanol, succinate, formate, caproate, isobutyrate, 2-methyl-butyrate, valerate, and isovalerate [3,8].
Many of the beneficial gut-organisms produce antimicrobial compounds, and compete for nutrients and attachment sites, in the gut-lining, preventing colonization by pathogens. They demote production of peptidoglycans and lipopolysaccharides, all can be detrimental to the host [3,14-15,33-35].
Development of B cells, Tr-cell, and Th-cells (1, 2, and 17) is dependent on the signals from the gut-microbiota [36-40]. Butyrate, have been shown to inhibit NF-kB in patients with ulcerative colitis thus exerting immunomodulatory effects [41-42]. They also inhibit DNA synthesis, stimulate apoptosis, and may play significantly in preventing cancer of the GIT [8,25].
Immune system development is governing also by the nature of the indigenous gut-microflora, immature at birth, develops gradually upon exposure to the gut-microbiota [3,6,43]. The innate immune system allows the host in sensing a concrete microbial environment, prerequisite to promote the release of signaling molecules (cytokines and chemokines) for initiating an immune response [25]. These concepts illustrate a dynamic relationship between the immune system and the microbiota [4]. The gut-mucosa averts menaces by signaling to the innate immune system through toll-like receptors. These receptors recognize and bind to specific microbial macromolecules like lipopolysaccharides, flagellin, peptidoglycans, and N-formylated peptides. Activation of these receptors initiates NF-kB pathways, mitogen-activated protein kinase, and caspase-dependent signaling cascades. These consequences are in production and release of protective peptides, cytokines, chemokines, and phagocytes. Thus resulting can be a protective response to non-pathogenic bacteria, an inflammatory response to pathogens, or a trigger of apoptosis [3,8].
Microbial metabolism takes place in the cecum and colon, the site where SCFAs are absorbed. SCFAs have a protective effect on the intestinal epithelium [32]. Fermentation of carbohydrate and production of SCFAs significantly stimulates absorption of magnesium, salts, water, calcium, and phosphorus [25]. As most of the butyrate completely metabolized, the colonic bacterium prefers it as the sole source of energy. Acetates serve as a substrate in cholesterol biosynthesis. Likewise, the gut-microbiota performs diverse and essential metabolic activities in the hosts [3,8].
Role in hepatic encephalopathy
Endotoxemia causes inflammation leading to cirrhosis of the liver. MHE is a complication of cirrhosis characterized by neurological manifestations where neurotoxic substances accumulate in the bloodstream. The exact pathogenesis of MHE is unclear but hypothesized that gut-derived-nitrogenous substance, specifically ammonia derived primarily from enteric bacteria, plays central role [3,8,44-45].
Role in diabetes and obesity
Patients with diabetes mellitus have different gut-microbiota comparing non-diabetic adults. Diabetic individuals had a lower number of Faecalibacterium prausnitzii with an increase in inflammatory markers. This concept establishes correlations between the gut-microbiota composition, and inflammation happenings and metabolic alterations, in obese individuals. The gut-microbiota maintains metabolic equilibrium of the host whilst obesity is associated with large changes in the abundances of diverse bacteria from different taxa [3,46-50]. In addition, they increase production of angiopoetin-related protein-4, a lipoprotein lipase inhibitor that inhibits the uptake of fatty acids from circulating triglyceride-rich lipoproteins in muscle and white adipose tissues [3,8,51].
Role in brain and behavior
The mechanistic influence of gut-microbiota on the brain and behavior is still unclear but an explanation could involve immune-mediated neural or humeral mechanisms. Presence of catecholamine biosynthetic pathways in probiotics indicates the possibility that cell-to-cell signaling in vertebrates may be due to late horizontal gene transfer from bacteria [52]. There were also elevated levels of TNF-α in the Central Nervous System [7-8,53].
DCs embedded in the gut-wall are in close proximity to sheaths of neurons. The function of DCs is modulating by the sensory neuropeptide, calcitonin-gene-related peptide [54]. DCs might signal about gut-microbiota to the brain through the vagus nerve [55]. This nerve has principal role in signaling between GIT and brain. The vagal response can be stimulating by endotoxins and inflammatory cytokines like interleukin-1β and TNF-α [56]. Vagal response also demotes proinflammatory cytokine release from intestinal macrophages [3,7-8,56-57].
Dysbiosis
Factors like pH of the gut contents, nutrient availability, redox potential within the tissue, age and health of the host, bacterial adhesion and cooperation, mucin secretions containing immunoglobulins, bacterial antagonism, and transit time may affect the diversity and quantity of microbiota present in the segments of the GIT [3,6,25,58].
The signal of the intestinal microbes determines normal physiology of the host. The gut-lumen consist gastric acid, digestive enzymes, and immunoglobulin-A. These constituting the first line of defense are lethal to invading and ingested pathogens. The indigenous gut-microbe degrades intra-luminal antigens and inhibits the pathogens from adherence and colonization. They are necessary also for induction of B-cells and T-cells (regulatory and helper) [59]. Any imbalance or perturbation in gut-microbial ecosystem could outcome deregulation of its microbiota (dysbiosis) [4]. Dysbiosis often associated with various disease states ranging from the most common inflammatory bowel disease [60,61] and irritable bowel syndrome [4,9,62-66] to the more unexpected activation of chronic HIV infection and atopy [3,67-70]. It seems associated with other diseases, particularly prevalent in the 21st century. These consensuses complex/dynamic relationship between human health and the human microbiome and might pave the way for better management of health [3,4].
Dysbiosis Alleviation
Microbiomics has spurred a dramatic increase in scientific, industrial, and public interest in probiotics and prebiotics as potential agents for managing and controlling gut-microflora. Genomics and bioinformatics, as tools may let in establishing mechanistic relationships among gut-microbiota, health status, and effects of drugs in the individual. Hope this will provide perspectives for individualized gut-microbiota management [3].
Therefore, important is restoring the bacterial homeostasis, may have been disturbed by any or several factors. One of the ways to alter the gut-microbiota favorably is by using of select intestinal-microbiota. Consensus is that select microbiota may involved in diverse clinical states like preventing or treating various GIT disorders, promoting gastrointestinal health, produce vitamins and minerals, contribute to protein homeostasis, and preventing metabolic syndrome. However, their precise role is unclear and additional research is prerequisite to determine their causal or associative relationship [3,6,25,58].
LAB and non-LAB have shown to positively influence health. Common and well-known beneficial bacteria belong to the genus Lactobacillus and Bifidobacterium. These can be introducing into the gut and/or encouraged to multiply through ingestion of appropriate probiotic strain(s) or by making provision of growth substrates, known as prebiotics or soluble fibers. Hence, restoring the balance by using these bacteria or materials for preventing and treating disease should be advantageous. Prebiotics along with probiotics and synbiotics can favorably influence microbial interactions with the immune system and gut-epithelium. These had been studied and using in diverse sickness [3,25].
Synbiotics
Several members of the gut-microbiota produce vitamins and minerals and provide them to the host, maintain protein homeostasis, and inhibit adhesion and displacement of pathogens. In addition, they compete for nutrients and some of the attachment sites same as of pathogens, acidification of the gut-environment and production of antimicrobial compounds inhibiting the growth of pathogens, and the production of toxic compounds such as ammonia and amines. Germ-free animals are susceptible to infections and require 30% more energy in their diet and supplementation with vitamins (K and B), mandatory to maintain their body weight. Exposure to the gut-microbiota is prerequisite for developing the innate immune system [5,25].
Oral ingestion of beneficial living intestinal microbes increases the amount of health-promoting microbes in the gut. Certain limitations, including nutrient availability and the ability of beneficial gut-microbes to survive in the physiochemical protective barriers of the host in order to reach the lower GIT must be overcome before an ecological niche can be become established. Accomplishments of said limitations mean the selected gut-microbiota may have a number of remarkable postulated health effects on acute and chronic disease in humans [5,10-11,25].
Pharmaceuticals been unable to decrease global morbidity and mortality (associating acute and or chronic diseases) is growing awareness for search and exploiting potential of alternative agents, as preventative and therapeutic. Amongst them probiotics, prebiotics, and synbiotics ought to have direct and or indirect effects on the pathogenesis and disease progression. Nowadays these are proposing as novel therapeutic option. Progression in the concepts is, first probiotics followed by prebiotics and then synbiotics [1,2].
These facts evolutes are the emerging concepts of probiotics, prebiotics and synbiotics, to modulate the targeted gut-microflora/ ecology. Using of living organisms in the diet to increase amount of health-promoting bacteria in the gut, postulates probiotic approach. The selective promotion of these bacteria by the intake of certain non-digestible carbohydrates postulates prebiotics. Both probiotics and prebiotics can fortify the lactate-producing microbes of the human or animal gut [5,10-11,25].
Synbiotics are combination of probiotics and prebiotics useful in alleviating disease and ailments. Synergistic chemopreventive actions exerted with combinations of the two, which together be called synbiotics. It may define as "the nutritional supplements that are combinations of probiotic bacteria and prebiotic food ingredients" [1,10-11,71-75].
Synbiotics therapies enables in manipulating composition of gut-microbiota and reducing the levels of pathogenic microorganisms. In synbiotics, the probiotics uses the prebiotics as a food source, which enables them to survive for extended period within the intestine than would otherwise be possible. Thus enables in improving the viability of probiotics and delivering projected health benefits. These in consequence decreases intestinal inflammation, alleviates allergy and diarrhea, produce antimicrobials, enhances and regulates immune function, elicit antitumorigenic or anticarcinogenic activity, binds to potential food carcinogens and toxins, and demotes bacterial enzymes which hydrolyse precarcinogenic compounds, such as beta-glucuronidase [1,71-75].
True probiotic, without its prebiotic food, does not survive well in the digestive system, the main reason for using a synbiotics. In absence of necessary food source, the probiotics will have a greater intolerance for oxygen, low pH, and temperature. The prebiotics provides a great place for probiotics to thrive thus conserving the population of these beneficial bacteria [1-5]. Examples of synbiotics provided with Table 1.
Synbiotics
Probiotics
Prebiotics
Lactobacilli
Lactitol
Lactobacilli
Inulin
Lactobacilli
Fructooligosaccharides or inulin
Lactobacillus rhamnosus GG
Inulin
Bifidobacteria
Galactooligosaccharides
Bifidobacteria
Fructooligosaccharides
Bifidobacteria and Lactobacilli
Fructooligosaccharides or inulin
Table 1: Examples of synbiotics [1,71-75].
Prebiotics
Prebiotics are the indigestible food ingredients stimulate the growth and or activity of probiotics in the digestive system in ways claimed to be beneficial to health. However these having multi-functional nature. They are using also to modify textural properties, including creaminess and mouth feel in a wide range of food and beverage products [1-5, 71-75].
Definition of prebiotics still debated and under evolution. In year, 2007 Roberfroid defined it "a prebiotic is a selectively fermented ingredient that allows specific changes, both in the composition and/ or activity in the gastrointestinal-microflora that confers benefits upon host well-being and health" [1-5,71-75].
These have potential effects on calcium and other mineral absorption, metabolism, bone composition and architecture, immune system stimulation, pathogen exclusion, hypertension, cancer, cholesterol lowering, bowel pH, inflammatory bowel disorders, and intestinal regularity. Proclaim on bringing down risk of colon cancer emanates from increased production of SCFAs [1-5,71-75].
Several food ingredients had been using as prebiotics, which may be short-chain, long-chain, and full-spectrum prebiotics [1-5]. Their classification and examples provided with Table 2.
Prebiotics
Synbiotics
Remark
Approved
Inulin, lactulose, actitol
Fructooligosaccharides.
Isomaltooligosaccharides, lactosucrose, cereals fibers, xylooligosaccharides, and so on.
Galactooligosaccharides.
Raffinose
Soy oligosaccharides.
Under conflict
Mannan oligosaccharides, and so on.
Also immunosaccharides.
Under trial
Glucooligosaccharides, isomaltooligosaccharides, soybean oligosaccharides, polydextrose, xylooligosaccharides, lactosucrose, and so on.
Preliminary and promising data exists but lacks in sufficient evidence.
Miscellaneous
Germinated barley foodstuffs, gentiooligosaccharides, gluconic acid, lactose, glutamine, hemicellulose-rich substrate, oligodextrans, lactoferrin-derived peptide, mannan oligosaccharides, oligosaccharides from melibiose, pectic oligosaccharides, N-acetylchitooligosaccharides, resistant starch and its derivatives, and so on.
Under study.
Table 2: Classification and examples of prebiotics [1,71-75].
Short-chain prebiotics contain 2-8 links per saccharide molecule. They be fermented more quickly in the right side of the colon, where provide nourishment to the bacteria. The long-chain one contains 9-64 links per saccharide molecule. These ferments tardily and is nourishing predominantly on left side of colon. Full-spectrum prebiotics have 2-64 links per molecule and nourish bacteria throughout the colon. Oligofructose is short chain prebiotics, inulin is long-chain prebiotics, and oligofructose-enriched inulin is full-spectrum prebiotics [1-5,71-75].
Majority of research carried out with full-spectrum prebiotics. Inulins are using also to replace fat and sugar in various foods and beverages. Fructooligosaccharides, galactooligosaccharides, polydextrose and xylooligosaccharides mostly exploited oligofructose. Besides them, tagatose and lactulose is used. Inulin and trans-galactooligosaccharide fully met prebiotics definition as of Roberfroid. Fructooligosaccharides and lactulose classified as prebiotics, by others [1-5,71-75].
Probiotics
Many of the beneficial gut-microbes resulting can be an antimicrobials production, an inflammatory response to pathogenic organisms, a trigger of apoptosis, prevention of pathogenesis, or development and homeostasis of the immune system. These postulated as probiotic approach, an alternative approach aiming to promote health-promoting microbes in the gut [25].
Lilly and Stillwell firstly coin the term "probiotics" in 1965 while its definition has evolved through the years [1]. Table 3 provides definitions of probiotics.
Definition
Ref. No.
''Live microorganisms that beneficially affect the host's health by improving its microbial balance.''
[1,14-15,144]
"Live microorganisms and cells of viable microorganisms, when a health benefit has been demonstrated."
[1,14-15,145]
"Viable microorganisms, sufficient amounts of which reach the intestine in an active state and thus exert positive health effects."
[1,14-15,73]
''Live microorganisms which when administered in adequate amounts confer a health benefit on the host.''
[1,14-15,146]
Table 3: Definitions of probiotics.
Microorganisms that are normal inhabitants of the human GIT had been using as probiotics. This comprises a large number of microbes of diverse genera and species comprising mainly bacteria [LAB and non-LAB] and yeast. LABs are Gram-positive and facultative anaerobe comprising species of Enterococcus, Lactobacillus, Lactococcus, Leuconostoc, Streptococcus, etc. Bifidobacterium are non- LAB, Gram-positive and strict anaerobe that proliferates between pH ranges of 4.5-8.5. Other non-LAB are species of Saccharomyces, Escherichia coli, Bacillus, etc [1]. Classification and examples of probiotics presented with Table 4.
Class
Species
Strains
Lactic Acid Bacteria
Lactobacillus
L. acidophilus, L. acidophilus SBT-2062, L. acidophilus LA-5, L. acidophilus ATCC 43121, L. acidophilus NCFM,L. acidophilus DSM 20079, L. amylovorus, L. amylovorus DSM19280,L. casei, L. casei CRL-431, L. casei F19, L. casei DN 014001, L. casei DN-114001, L. casei Shirota, L. crispatus, L. delbruekii, L. gallinarum, L. gasseri, L. helveticus, L. helveticus CP790, L. johnsonii, L. johnsonii La1 (Lj1), L. paracasei, L. plantarum, L. plantarum 299v (Lp299v), L. plantarum mtcc 5422, L. reuteri, L. reuteri ATCC 55730, L. rhamnosus, L. rhamnosus R011, L. rhamnosus LB21, L. rhamnosus 271, L. rhamnosus GG (ATCC 53013), L. salivarius UCC118, and so on.
Strepcoccus
S. thermophilus, S. thermophilus 1131, S. salivarius subsp. thermophilus, S. diaacetylactis, and so on.
Miscellaneous
Enterococcus LAB SF68, E. faecalis, E. faecium, Lactococcus lactis L1A, Leucoccus mesenteroides, and so on.
Non-Lactic Acid Bacteria
Bifidobacterium
B. adolescentis, B. adolescentis 15703T, B. animalis, B. animalis DN 173010, B. animalis subsp. lactis Bb-12, B. bifidum, B. breve, B. breve Yakult, B. infantis, B. infantis 35624, B. lactis Bi-07, B. lactis HN019, B. longum, B. longum BB536, B. longum SBT2928,and so on.
Saccharomyces
S. boulardii, S. cerevisiae, S. cerevisiae (boulardii) lyo, and so on.
Sporolactobacillus
S. inulinus, S. inulinus BCRC 14647, and so on.
Miscellaneous
Bacillus cereus, B. coagulans, Escherichia coli, E. coli Nissle 1917, Propionibacterium freudenreichii, Homeostatic soil organisms, and so on.
Table 4: Classification and examples of probiotics [1-5,14-15,33-35,72,76,103,145-148].
Mechanism of action of probiotics
Three requisite for eliciting prebiotic effect are:
- Resistance to gastric acidity, hydrolysis by mammalian enzymes, and gastrointestinal absorption,
- Fermentation by intestinal-microbiota, and
- Selective stimulation of the growth and/or activity of intestinal bacteria associated with health and well-being.
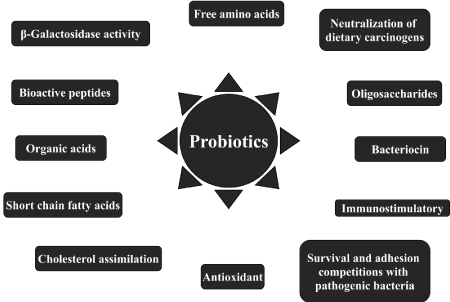
Type of effect(s) a probiotics strain elicits depends on its metabolic properties, the molecules presented at its surface or on the components secreted. Distinguished diverse levels of microbe-host interaction are microbe-gut epithelium, microbe-immune system, and microbe-microbe. The individual or combination of such interaction inherited by probiotic strain outcomes strain specific probiotic action; consequence is its effective application in prevention and/or treatment of a certain disease [1-5,14-15,25,33- 35]. Mechanism of action of probiotics presented with Figure-1.
Figure 1: Figure presents mechanism of action of probiotics.
Clinical applications of probiotics and synbiotics
In immune system: Probiotics modulate functions of the immune system at both systemic and mucosal level. Enhanced specific and nonspecific immune responses was believed to be mediated through activation of macrophages, promotion of cytokines and immunoglobulins levels, and/or promotion of NK-cell activity [77-80].
Probiotics strains can signal innate immune system through pattern-recognition or toll-like receptors, resulting in activation of various intracellular signaling pathways. The active signaling components for these includes enzymes, secreted factors, surface-layer proteins, isolated DNA, bacterial formulated peptides such as N-formyl-methionyl-leucyl-phenylalanine, lipopolysaccharide, and peptidoglycan cell wall constituents [25].
In addition, they alter mucosal immune function by enhancing antibody production, increasing phagocyte and NK-cell activity, and inducing regulatory DCs and CD4+Foxp3+T-cells. Some species generate a DCs phenotype characterized by release of increased levels of interleukin-10, and small amounts of interleukin-12 and TNF-α. An increased interleukin-10 production may induce the generation of Tr-cell. Some lactobacilli generate a DCs phenotype characterized by increased costimulatory marker expression with low yield of proinflammatory cytokines. Overall, probiotics tend to induce an immunoregulatory phenotype of DCs rather than an aggressive immune response [25].
In myasthenia gravis administration of probiotics strain(s) decreases AchR-reactive lymphocyte proliferation, anti-AchR reactive immunoglobulin-G levels and inflammatory cytokine levels such as interferon gamma, TNF-α, interleukin-6 and interleukin-17. Said down-regulation of inflammatory mediators in AchR-reactive lymphocytes is mediating by the generation of regulatory DCs that express increased levels of interleukin-10, transforming growth factor-β, arginase-1 and aldh1a2. These regulatory DCs effectively convert CD4+T-cells into CD4+Foxp3+Tr-cells [58].
Familial Mediterranean fever is an autoinflammatory disorder, is dysbiosis linked first genetic disease. Here the gene for pyrin an important regulator of innate immunity mutated. Hypothesis is that the functionality of pyrin affects the ability of commensals to breach the gut-barrier, resulting in characteristically high systemic reactivity towards probiotics [3,81-82].
In stress: Probiotics bacterium alleviates exposure/osmotic/ oxidative stress and environmental stresses. They retain a broad arsenal of molecular mechanisms to combat stress, often-lethal environmental stresses. Probiotics influences psychological states by producing neurochemicals, identical to that of mammalian systems [52]. These helps also in alleviating stress by releasing essential amino acids and tryptophan, and producing nitric oxide. In human the released neurochemicals reduces chronic psychological stress and stress-induced visceral pain, attenuate psychological distress and fatigue syndrome, and alleviate depressive symptoms in fatigued adults, under stress [3,83-86]. Tryptophan produces serotonin, a calming neurotransmitter. Some of them reduce stress-induced bacterial translocation to mesenteric lymph nodes thereby reduce stress induced bacterial adherence, within the lumen. Some of the probiotics strain reduces increased colonic permeability induced by both partial restraint stress and maternal deprivation [2].
In allergy, inflammation, and bowel disease/syndrome: Atopic diseases arise from aberrant immune responses to environmental allergens leading to allergic inflammation [87]. Probiotics combat allergy/inflammation by enhancement of endogenous barrier mechanisms of the gut thereby preventing adhesion of pathogens and inhibiting their invasion into the body [88], alleviation of food allergy associated intestinal inflammation [89-90], and up-regulation of anti-inflammatory cytokines like interleukin-10, in atopic children [91]. Probiotics may act in the allergic responses, resulting from improper functioning of immune system, mediating through Th-cells-2, producing interleukin-4, -5, -9, and -13 [3,76]. Additional postulated mechanisms are inflammatory response associated gastrointestinal-permeability modulation through regulating or modulating the immune system in the GIT [92-93], and reinforcement of mucosal degradation of antigens through enhanced breakdown of macromolecules [94]. Overall probiotics shifts immune response towards an anti-inflammatory state with altered colonic immune-states [78,95].
LAB may improve intestinal peristalsis and relieve constipation possibly through reducing gut-pH, modulating absorption and secretion, intestinal permeability, or modulating gut-physiology through mediating interaction of host's intestinal-microbiota and gastrointestinal tissues [96-102].
In barrier function: Probiotics enhance barrier function by modulating epithelial tight-junction proteins, promoting intestinal mucus production, enhancing mucosal immunoglobulin, releasing bioactive factors, inducing cellular heat-shock proteins, and preventing epithelial apoptosis. Some promote b-defensin production from epithelial and Paneth cells, and mRNA; and protein secretion manner, through regulation of the NF-kB- and activator-protein-1- dependent pathways. They can prevent cytokine and oxidant induced epithelial damage by promoting cell survival. Soluble factors (p75 and p40) released from probiotics strain prevent epithelial cell apoptosis by activating anti-apoptotic Akt in a phosphatidylinositol-3'-kinase-dependent manner and demoting the pro-apoptotic p38/ mitogen-activated protein kinase [25].
In obesity: The prebiotics prevent the overexpression of several host genes that related to adiposity and by increasing production of angiopoetin-related protein-4 [3,51].
In pathogenesis: Probiotics suppress the growth and invasion of pathogens involving diverse mechanisms. Important are they alter the gene expression of resident gut-microbes, competes pathogens for intestinal epithelium, displace pathogens that have already attached, competitively exclude pathogens, and cataboloze undigested polysaccharides to SCFAs, thus reducing pH and inhibiting pathogen growth [25,103-106].
A postulation is probiotics alleviates viral infections by strengthening the tight-junctions between enterocytes, producing potential antiviral substances, stimulating host-cell immune system, and competing with pathogenic viruses for binding sites on epithelial cells. Some probiotics deconjugate and absorb bile acids thereby decreases colonic fluid secretion or motility in diarrhea through reduction in intracolonic bile acids amount [107-110].
In toxin detoxification: Probiotics species detoxifies mycotoxins and aflatoxin (may metabolize to carcinogenic, mutagenic, teratogenic and immunosuppressive metabolite, causing acute and long-term toxicity) by binding or complexing with them, followed by elimination with feces [111-118]. Certain probiotics species removes microcystins (a cyanotoxin that is hepatotoxins or neurotoxins) by binding to it. Thus, they prevent cyanotoxin associated acute and chronic toxicities, and hepatotoxicity and tumor-promoting activity [119-122].
In autism probiotics species retards colonization by autism-triggering microorganism(s) or demote overgrowth of neurotoxin-producing bacteria like Clostridium tetani [123]. By this, they debilitate progressive autism including neurotoxin production, involving number of mechanisms. Important are autoantibody production that detoxifies neuron-associated proteins and or toxic metabolites and diminish microbial production of toxic metabolites having neurological effects [3,124].
In hepatic encephalopathy: Diverse modes of action are following by probiotics to combats MHE. They act by decreasing bacterial urease activity, intestinal permeability, inflammation, uptake of other toxins, and other modes of action. Some species reduce load of microbe, producing urease [44,125]. Their certain species can produce a ligand for the benzodiazepine receptor that may contribute to the encephalopathy [3,45].
In hypocholesterolaemia and cardioprotection: Lactobacilli with bile-salt hydrolase activity and its metabolic byproducts retard cardiovascular disease, specifically atherosclerosis, by choline catabolism. Other cardiovascular effects are prevention and therapy of various ischemic heart syndromes, reduces myocardial infarction, and demoting risk factors level for cardiovascular disease like serum levels of leptin and fibrinogen and cholesterol (low-density-lipoprotein) [3,126-129].
Probiotic strains combat hypercholesterolemia by either assimilating cholesterol (low-density-lipoprotein) or conjugating to them. They also inhibit angiotensin-converting enzyme through peptide generation, upon hydrolyzing casein, resulting low blood pressure [130-132].
In cancer: Probiotics might either detoxifies/binds ingested/ generated carcinogens or suppress growth of bacteria, converting procarcinogens into carcinogens [135-134]. Thus decreases absorption of mutagens or carcinogens [136]. In addition, they express RAS-p21 onto-protein, inhibit tumor-cells proliferation, or produce metabolic products, improving cell apoptosis. They stimulate immune system to defend proliferation of cancer cell through increased activity of tumor ornithine decarboxylase, immunoglobulin-A, interleukin-10, T-cells, NK-cell and macrophages [78,137-139].
In renal health and lactose intolerance: Synbiotics and probiotics may reduce levels of creatinine, dimethylamine, nitro-dimethylamine, and urea-nitrogen in blood. Preventing accumulation of these toxins postulated for improved quality of life [3,140-141]. Lactic acid liberated by LAB increases lactase activity in lactase-deficient individuals [142-143].
Conclusion
Extensive and exhaustive study of intricacies and interwoven of the ecosystem of gut-dysbiosis, and probiotics and synbiotics, with human health might pave the way for better management of health. Future will evidence utility of probiotics and symbiotic therapy in diverse domain.
References
- Saikh MAA. Microencapsulate probiotics. Germany: LAP Lambert Academic Publishing. 2014.
- Sekhon BS, Jairath S. Prebiotics, probiotics and synbiotics: an overview. J Pharm Educ Res. 2010; 1: 13-36.
- Vyas U, Ranganathan N. Probiotics, prebiotics, and synbiotics: gut and beyond. Gastroenterol Res Pract. 2012; 2012: 872716.
- Spiller R. Review article: probiotics and prebiotics in irritable bowel syndrome. Aliment Pharmacol Ther. 2008; 28: 385-396.
- Bandyopadhyay B, Mandal NC. Probiotics, Prebiotics and Synbiotics - In health improvement by modulating gut microbiota: the concept revisited. Int J Curr Microbiol App Sci. 2014; 3: 410-420.
- Clemente JC, Ursell LK, Parfrey LW, Knight R. The impact of the gut microbiota on human health: an integrative view. Cell. 2012; 148: 1258-1270.
- Guinane CM, Cotter PD. Role of the gut microbiota in health and chronic gastrointestinal disease: understanding a hidden metabolic organ. Therap Adv Gastroenterol. 2013; 6: 295-308.
- Sekirov I, Russell SL, Antunes LC, Finlay BB. Gut microbiota in health and disease. Physiol Rev. 2010; 90: 859-904.
- Macfarlane S, Cleary S, Bahrami B, Reynolds N, Macfarlane GT. Synbiotic consumption changes the metabolism and composition of the gut microbiota in older people and modifies inflammatory processes: a randomised, double-blind, placebo-controlled crossover study. Aliment Pharmacol Ther. 2013; 38: 804-816.
- Bosscher D, Breynaert A, Pieters L, Hermans N. Food-based strategies to modulate the composition of the intestinal microbiota and their associated health effects. J Physiol Pharmacol. 2009; 60 Suppl 6: 5-11.
- Roberfroid M, Gibson GR, Hoyles L, McCartney AL, Rastall R, Rowland I, et al. Prebiotic effects: metabolic and health benefits. Br J Nutr. 2010; 104 Suppl 2: S1-63.
- Macfarlane S, Macfarlane GT, Cummings JH. Review article: prebiotics in the gastrointestinal tract. Aliment Pharmacol Ther. 2006; 24: 701-714.
- Sawalha S. Functional Food. Austin J Nutri Food Sci. 2014; 2: 2.
- Saikh MAA. Development of product containing microencapsulated probiotics: an update on issues. J Drug Deliv Ther. 2013; 3: 121-131.
- Saikh MAA. Prospective action plan for developing product containing microencapsulated probiotics. Int Res J Pharm. 2013; 4: 232-236.
- Kunz C, Kuntz S, Rudloff S. Intestinal flora. Adv Exp Med Biol. 2009; 639: 67-79.
- Morelli L. Postnatal development of intestinal microflora as influenced by infant nutrition. J Nutr. 2008; 138: 1791S-1795S.
- Neish AS. Microbes in gastrointestinal health and disease. Gastroenterology. 2009; 136: 65-80.
- Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006; 124: 837-848.
- Savage DC. Associations of indigenous microorganisms with gastrointestinal mucosal epithelia. Am J Clin Nutr. 1970; 23: 1495-1501.
- Whitman WB, Coleman DC, Wiebe WJ. Prokaryotes: the unseen majority. Proc Natl Acad Sci U S A. 1998; 95: 6578-6583.
- Chiller K, Selkin BA, Murakawa GJ. Skin microflora and bacterial infections of the skin. J Investig Dermatol Symp Proc. 2001; 6: 170-174.
- Hull MW, Chow AW. Indigenous microflora and innate immunity of the head and neck. Infect Dis Clin North Am. 2007; 21: 265-282, v.
- Verstraelen H. Cutting edge: the vaginal microflora and bacterial vaginosis. Verh K Acad Geneeskd Belg. 2008; 70: 147-174.
- Wallace TC, Guarner F, Madsen K, Cabana MD, Gibson G, Hentges E, et al. Human gut microbiota and its relationship to health and disease. Nutr Rev. 2011; 69: 392-403.
- Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A. 2010; 107: 11971-11975.
- Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer RJ. Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther. 2008; 27: 104-119.
- Salminen S, Bouley C, Boutron-Ruault MC, Cummings JH, Franck A, Gibson GR, et al. Functional food science and gastrointestinal physiology and function. Br J Nutr. 1998; 80 Suppl 1: S147-171.
- Kleessen B, Blaut M. Modulation of gut mucosal biofilms. Br J Nutr. 2005; 93 Suppl 1: S35-40.
- Stappenbeck TS, Hooper LV, Gordon JI. Developmental regulation of intestinal angiogenesis by indigenous microbes via Paneth cells. Proc Natl Acad Sci U S A. 2002; 99: 15451-15455.
- Lefebvre P, Cariou B, Lien F, Kuipers F, Staels B. Role of bile acids and bile acid receptors in metabolic regulation. Physiol Rev. 2009; 89: 147-191.
- Wong JM, de Souza R, Kendall CW, Emam A, Jenkins DJ. Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol. 2006; 40: 235-243.
- Alli SM. Preparation and characterization of a coacervate extended-release microparticulate delivery system for Lactobacillus rhamnosus. Int J Nanomedicine. 2011; 6: 1699-1707.
- Alli SM. Formulation and evaluation of Bacillus coagulans-loaded hypromellose mucoadhesive microspheres. Int J Nanomedicine. 2011; 6: 619-629.
- Alli SM, Ali SM, Samanta A. Development and evaluation of intestinal targeted mucoadhesive microspheres of Bacillus coagulans. Drug Dev Ind Pharm. 2011; 37: 1329-1338.
- Haverson K, Rehakova Z, Sinkora J, Sver L, Bailey M. Immune development in jejunal mucosa after colonization with selected commensal gut bacteria: a study in germ-free pigs. Vet Immunol Immunopathol. 2007; 119: 243-253.
- Hapfelmeier S, Lawson MA, Slack E, Kirundi JK, Stoel M, Heikenwalder M, et al. Reversible microbial colonization of germ-free mice reveals the dynamics of IgA immune responses. Science. 2010; 328: 1705-1709.
- Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011; 331: 337-341.
- Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005; 122: 107-118.
- Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009; 139: 485-498.
- Lührs H, Gerke T, Müller JG, Melcher R, Schauber J, Boxberge F, et al. Butyrate inhibits NF-kappaB activation in lamina propria macrophages of patients with ulcerative colitis. Scand J Gastroenterol. 2002; 37: 458-466.
- Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009; 461: 1282-1286.
- Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009; 9: 313-323.
- Gibson GR, Wang X. Regulatory effects of bifidobacteria on the growth of other colonic bacteria. J Appl Bacteriol. 1994; 77: 412-420.
- Yurdaydin C, Walsh TJ, Engler HD, Ha JH, Li Y, Jones EA, et al. Gut bacteria provide precursors of benzodiazepine receptor ligands in a rat model of hepatic encephalopathy. Brain Res. 1995; 679: 42-48.
- Cani PD, Delzenne NM. The role of the gut microbiota in energy metabolism and metabolic disease. Curr Pharm Des. 2009; 15: 1546-1558.
- Cani PD, Delzenne NM. Interplay between obesity and associated metabolic disorders: new insights into the gut microbiota. Curr Opin Pharmacol. 2009; 9: 737-743.
- Larsen N, Vogensen FK, van den Berg FW, Nielsen DS, Andreasen AS, Pedersen BK, et al. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS One. 2010; 5: e9085.
- Furet JP, Kong LC, Tap J, Poitou C, Basdevant A, Bouillot JL, et al. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade inflammation markers. Diabetes. 2010; 59: 3049-3057.
- Ley RE. Obesity and the human microbiome. Curr Opin Gastroenterol. 2010; 26: 5-11.
- Aronsson L, Huang Y, Parini P, Korach-André M, Håkansson J, Gustafsson JÅ, et al. Decreased fat storage by Lactobacillus paracasei is associated with increased levels of angiopoietin-like 4 protein (ANGPTL4). PLoS One. 2010; 5.
- Iyer LM, Aravind L, Coon SL, Klein DC, Koonin EV. Evolution of cell-cell signaling in animals: did late horizontal gene transfer from bacteria have a role? Trends Genet. 2004; 20: 292-299.
- Bercik P, Verdú EF, Foster JA, Lu J, Scharringa A, Kean I, et al. Role of gut-brain axis in persistent abnormal feeding behavior in mice following eradication of Helicobacter pylori infection. Am J Physiol Regul Integr Comp Physiol. 2009; 296: R587-594.
- Torii H, Hosoi J, Asahina A, Granstein RD. Calcitonin gene-related peptide and Langerhans cell function. J Investig Dermatol Symp Proc. 1997; 2: 82-86.
- Goehler LE, Gaykema RP, Nguyen KT, Lee JE, Tilders FJ, Maier SF, et al. Interleukin-1beta in immune cells of the abdominal vagus nerve: a link between the immune and nervous systems? J Neurosci. 1999; 19: 2799-2806.
- Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000; 405: 458-462.
- Ghia JE, Blennerhassett P, Kumar-Ondiveeran H, Verdu EF, Collins SM. The vagus nerve: a tonic inhibitory influence associated with inflammatory bowel disease in a murine model. Gastroenterology. 2006; 131: 1122-1130.
- Chae CS, Kwon HK, Hwang JS, Kim JE, Im SH. Prophylactic effect of probiotics on the development of experimental autoimmune myasthenia gravis. PLoS One. 2012; 7: e52119.
- Strauch UG, Obermeier F, Grunwald N, Gürster S, Dunger N, Schultz M, et al. Influence of intestinal bacteria on induction of regulatory T cells: lessons from a transfer model of colitis. Gut. 2005; 54: 1546-1552.
- Honda K, Takeda K. Regulatory mechanisms of immune responses to intestinal bacteria. Mucosal Immunol. 2009; 2: 187-196.
- Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology. 2008; 134: 577-594.
- Quigley EM. Therapies aimed at the gut microbiota and inflammation: antibiotics, prebiotics, probiotics, synbiotics, anti-inflammatory therapies. Gastroenterol Clin North Am. 2011; 40: 207-222.
- Bonfrate L, Tack J, Grattagliano I, Cuomo R, Portincasa P. Microbiota in health and irritable bowel syndrome: current knowledge, perspectives and therapeutic options. Scand J Gastroenterol. 2013; 48: 995-1009.
- Barbara G, Stanghellini V, Cremon C, De Giorgio R, Gargano L, Cogliandro R, et al. Probiotics and irritable bowel syndrome: rationale and clinical evidence for their use. J Clin Gastroenterol. 2008; 42 Suppl 3 Pt 2: S214-217.
- Pimentel M, Chang C. Inflammation and microflora. Gastroenterol Clin North Am. 2011; 40: 69-85.
- Lee BJ, Bak YT. Irritable bowel syndrome, gut microbiota and probiotics. J Neurogastroenterol Motil. 2011; 17: 252-266.
- Hofer U, Speck RF. Disturbance of the gut-associated lymphoid tissue is associated with disease progression in chronic HIV infection. Semin Immunopathol. 2009; 31: 257-266.
- Liu CH, Yang XQ, Liu CH, He Y, Wang LJ. Allergic airway response associated with the intestinal microflora disruption induced by antibiotic therapy. Zhonghua Er Ke Za Zhi. 2007; 45: 450-454.
- Penders J, Stobberingh EE, van den Brandt PA, Thijs C. The role of the intestinal microbiota in the development of atopic disorders. Allergy. 2007; 62: 1223-1236.
- Verhulst SL, Vael C, Beunckens C, Nelen V, Goossens H, Desager K. A longitudinal analysis on the association between antibiotic use, intestinal microflora, and wheezing during the first year of life. J Asthma. 2008; 45: 828-832.
- Kolida S, Gibson GR. Synbiotics in health and disease. Annu Rev Food Sci Technol. 2011; 2: 373-393.
- Klein M, Sanders ME, Duong T, Young HA. Probiotics: from bench to market. Ann N Y Acad Sci. 2010; 1212 Suppl 1: E1-14.
- de Vrese M, Schrezenmeir J. Probiotics, prebiotics, and synbiotics. Adv Biochem Eng Biotechnol. 2008; 111: 1-66.
- Guarner F, Sanders ME, Gibson G, Klaenhammer T, Cabana M, Scott K, et al. Probiotic and prebiotic claims in Europe: seeking a clear roadmap. Br J Nutr. 2011; 106: 1765-1767.
- Brooks SP, Kalmokoff ML. Prebiotics and probiotics: some thoughts on demonstration of efficacy within the regulatory sphere. J AOAC Int. 2012; 95: 2-4.
- Suvarna VC, Boby VU. Probiotics in human health: a current assessment. Curr Sci. 2005; 88: 1744-1748.
- Ouwehand AC, Salminen S, Isolauri E. Probiotics: an overview of beneficial effects. Antonie Van Leeuwenhoek. 2002; 82: 279-289.
- Perdigon G, Alvarez S, Rachid M, Agüero G, Gobbato N. Immune system stimulation by probiotics. J Dairy Sci. 1995; 78: 1597-1606.
- Górska S, Jarzab A, Gamian A. Probiotic bacteria in the human gastrointestinal tract as a factor stimulating the immune system. Postepy Hig Med Dosw (Online). 2009; 63: 653-667.
- Isolauri E, Sütas Y, Kankaanpää P, Arvilommi H, Salminen S. Probiotics: effects on immunity. Am J Clin Nutr. 2001; 73: 444S-450S.
- Khachatryan ZA, Ktsoyan ZA, Manukyan GP, Kelly D, Ghazaryan KA, Aminov RI. Predominant role of host genetics in controlling the composition of gut microbiota. PLoS One. 2008; 3: e3064.
- Parks BW, Nam E, Org E, Kostem E, Norheim F, Hui ST, et al. Genetic control of obesity and gut microbiota composition in response to high-fat, high-sucrose diet in mice. Cell Metab. 2013; 17: 141-152.
- Zareie M, Johnson-Henry K, Jury J, Yang PC, Ngan BY, McKay DM, et al. Probiotics prevent bacterial translocation and improve intestinal barrier function in rats following chronic psychological stress. Gut. 2006; 55: 1553-1560.
- Messaoudi M, Lalonde R, Violle N, Javelot H, Desor D, Nejdi A, et al. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br J Nutr. 2011; 105: 755-764.
- Gruenwald J, Graubaum HJ, Harde A. Effect of a probiotic multivitamin compound on stress and exhaustion. Adv Ther. 2002; 19: 141-150.
- Rao AV, Bested AC, Beaulne TM, Katzman MA, Iorio C, Berardi JM, et al. A randomized, double-blind, placebo-controlled pilot study of a probiotic in emotional symptoms of chronic fatigue syndrome. Gut Pathog. 2009; 1: 6.
- Bochner BS, Hamid Q. Advances in mechanisms of allergy. J Allergy Clin Immunol. 2003; 111: S819-823.
- van der Waaij D. The ecology of the human intestine and its consequences for overgrowth by pathogens such as Clostridium difficile. Annu Rev Microbiol. 1989; 43: 69-87.
- Macfarlane GT, Cummings JH. Probiotics, infection and immunity. Curr Opin Infect Dis. 2002; 15: 501-506.
- Miraglia del Giudice M, De Luca MG. The role of probiotics in the clinical management of food allergy and atopic dermatitis. J Clin Gastroenterol. 2004; 38: S84-85.
- Pessi T, Sütas Y, Hurme M, Isolauri E. Interleukin-10 generation in atopic children following oral Lactobacillus rhamnosus GG. Clin Exp Allergy. 2000; 30: 1804-1808.
- Marteau PR, de Vrese M, Cellier CJ, Schrezenmeir J. Protection from gastrointestinal diseases with the use of probiotics. Am J Clin Nutr. 2001; 73: 430S-436S.
- Vanderhoof JA. Probiotics and intestinal inflammatory disorders in infants and children. J Pediatr Gastroenterol Nutr. 2000; 30 Suppl 2: S34-38.
- Pessi T, Sütas Y, Marttinen A, Isolauri E. Probiotics reinforce mucosal degradation of antigens in rats: implications for therapeutic use of probiotics. J Nutr. 1998; 128: 2313-2318.
- Cremonini F, Di Caro S, Nista EC, Bartolozzi F, Capelli G, Gasbarrini G, et al. Meta-analysis: the effect of probiotic administration on antibiotic-associated diarrhoea. Aliment Pharmacol Ther. 2002; 16: 1461-1467.
- Kajander K, Hatakka K, Poussa T, Färkkilä M, Korpela R. A probiotic mixture alleviates symptoms in irritable bowel syndrome patients: a controlled 6-month intervention. Aliment Pharmacol Ther. 2005; 22: 387-394.
- Bibiloni R, Fedorak RN, Tannock GW, Madsen KL, Gionchetti P, Campieri M, et al. VSL#3 probiotic-mixture induces remission in patients with active ulcerative colitis. Am J Gastroenterol. 2005; 100: 1539-1546.
- Sanders ME, Klaenhammer TR. Invited review: the scientific basis of Lactobacillus acidophilus NCFM functionality as a probiotic. J Dairy Sci. 2001; 84: 319-331.
- Campieri M, Gionchetti P. Probiotics in inflammatory bowel disease: new insight to pathogenesis or a possible therapeutic alternative? Gastroenterology. 1999; 116: 1246-1249.
- Shanahan F. Inflammatory bowel disease: immunodiagnostics, immunotherapeutics, and ecotherapeutics. Gastroenterology. 2001; 120: 622-635.
- Camilleri M. Probiotics and irritable bowel syndrome: rationale, putative mechanisms, and evidence of clinical efficacy. J Clin Gastroenterol. 2006; 40: 264-269.
- Verdu EF, Collins SM. Microbial-gut interactions in health and disease. Irritable bowel syndrome. Best Pract Res Clin Gastroenterol. 2004; 18: 315-321.
- Salminen S, Nybom S, Meriluoto J, Collado MC, Vesterlund S, El-Nezami H. Interaction of probiotics and pathogens--benefits to human health? Curr Opin Biotechnol. 2010; 21: 157-167.
- Alander M, Satokari R, Korpela R, Saxelin M, Vilpponen-Salmela T, Mattila-Sandholm T, et al Persistence of colonization of human colonic mucosa by a probiotic strain, Lactobacillus rhamnosus GG, after oral consumption. Appl Environ Microbiol. 1999; 65: 351-354.
- Collado MC, Isolauri E, Salminen S. Specific probiotic strains and their combinations counteract adhesion of Enterobacter sakazakii to intestinal mucus. FEMS Microbiol Lett. 2008; 285: 58-64.
- Collado MC, Meriluoto J, Salminen S. Measurement of aggregation properties between probiotics and pathogens: in vitro evaluation of different methods. J Microbiol Methods. 2007; 71: 71-74.
- Salminen SJ, Gueimonde M, Isolauri E. Probiotics that modify disease risk. J Nutr. 2005; 135: 1294-1298.
- De Simone C, Baldinelli L, Di Fabio S, Tzantzoglou S, Jirillo E, Bianchi-Salvadori B, et al. The adjuvant effect of yogurt on production of gamma-interferon by con A-stimulated human peripheral blood lymphocytes. Nutr Rep Int. 1986; 33: 419-433.
- Bonifait L, Chandad F, Grenier D. Probiotics for oral health: myth or reality? J Can Dent Assoc. 2009; 75: 585-590.
- Luman W, Williams AJ, Merrick MV, Eastwood MA. Idiopathic bile acid malabsorption: long-term outcome. Eur J Gastroenterol Hepatol. 1995; 7: 641-645.
- El-Nezami HS, Chrevatidis A, Auriola S, Salminen S, Mykkänen H. Removal of common Fusarium toxins in vitro by strains of Lactobacillus and Propionibacterium. Food Addit Contam. 2002; 19: 680-686.
- Haskard CA, El-Nezami HS, Kankaanpää PE, Salminen S, Ahokas JT. Surface binding of aflatoxin B(1) by lactic acid bacteria. Appl Environ Microbiol. 2001; 67: 3086-3091.
- Hussein HS, Brasel JM. Toxicity, metabolism, and impact of mycotoxins on humans and animals. Toxicology. 2001; 167: 101-134.
- Turbic A, Ahokas JT, Haskard CA. Selective in vitro binding of dietary mutagens, individually or in combination, by lactic acid bacteria. Food Addit Contam. 2002; 19: 144-152.
- Haskard C, Binnion C, Ahokas J. Factors affecting the sequestration of aflatoxin by Lactobacillus rhamnosus strain GG. Chem Biol Interact. 2000; 128: 39-49.
- El-Nezami H, Mykkänen H, Kankaanpää P, Salminen S, Ahokas J. Ability of Lactobacillus and Propionibacterium strains to remove aflatoxin B, from the chicken duodenum. J Food Prot. 2000; 63: 549-552.
- el-Nezami H, Kankaanpää P, Salminen S, Ahokas J. Physicochemical alterations enhance the ability of dairy strains of lactic acid bacteria to remove aflatoxin from contaminated media. J Food Prot. 1998; 61: 466-468.
- Peltonen K, el-Nezami H, Haskard C, Ahokas J, Salminen S. Aflatoxin B1 binding by dairy strains of lactic acid bacteria and bifidobacteria. J Dairy Sci. 2001; 84: 2152-2156.
- Honkanen RE, Zwiller J, Moore RE, Daily SL, Khatra BS, Dukelow M, et al. Characterization of microcystin-LR, a potent inhibitor of type 1 and type 2A protein phosphatases. J Biol Chem. 1990; 265: 19401-19404.
- MacKintosh C, Beattie KA, Klumpp S, Cohen P, Codd GA. Cyanobacterial microcystin-LR is a potent and specific inhibitor of protein phosphatases 1 and 2A from both mammals and higher plants. FEBS Lett. 1990; 264: 187-192.
- Jones GJ, Orr PT. Release and degradation of microcystin following algicide treatment of a Microcystis aeruginosa bloom in arecreational lake, as determined by HPLC and protein phosphatase inhibition assay. Water Res. 1994; 28: 871-876.
- Nybom SM, Collado MC, Surono IS, Salminen SJ, Meriluoto JA. Effect of glucose in removal of microcystin-LR by viable commercial probiotic strains and strains isolated from dadih fermented milk. J Agric Food Chem. 2008; 56: 3714-3720.
- Bolte ER. Autism and Clostridium tetani. Med Hypotheses. 1998; 51: 133-144.
- Finegold SM, Molitoris D, Song Y, Liu C, Vaisanen ML, Bolte E, et al. Gastrointestinal microflora studies in late-onset autism. Clin Infect Dis. 2002; 35: S6-6S16.
- Madsen K, Cornish A, Soper P, McKaigney C, Jijon H, Yachimec C, et al. Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology. 2001; 121: 580-591.
- Oxman T, Shapira M, Klein R, Avazov N, Rabinowitz B. Oral administration of Lactobacillus induces cardioprotection. J Altern Complement Med. 2001; 7: 345-354.
- de Roos NM, Katan MB. Effects of probiotic bacteria on diarrhea, lipid metabolism, and carcinogenesis: a review of papers published between 1988 and 1998. Am J Clin Nutr. 2000; 71: 405-411.
- Naruszewicz M, Johansson ML, Zapolska-Downar D, Bukowska H. Effect of Lactobacillus plantarum 299v on cardiovascular disease risk factors in smokers. Am J Clin Nutr. 2002; 76: 1249-1255.
- Lam V, Su J, Koprowski S, Hsu A, Tweddell JS, Rafiee P, et al. Intestinal microbiota determine severity of myocardial infarction in rats. FASEB J. 2012; 26: 1727-1735.
- Leclerc PL, Gauthier SF, Bachelard H, Santure M, Roy D. Antihypertensive activity of casein-enriched milk fermented by Lactobacillu shelveticus. Int Dairy J. 2002; 12: 995-1004.
- Hata Y, Yamamoto M, Ohni M, Nakajima K, Nakamura Y, Takano T. A placebo-controlled study of the effect of sour milk on blood pressure in hypertensive subjects. Am J Clin Nutr. 1996; 64: 767-771.
- Yamamoto N, Akino A, Takano T. Antihypertensive effect of the peptides derived from casein by an extracellular proteinase from Lactobacillus helveticus CP790. J Dairy Sci. 1994; 77: 917-922.
- A vervik E, Rafter J, Nord CE, Gustafsson JA. Effect of Lactobacillus acidophilus supplements on mutagen excretion in faeces and urine in humans. Microb Ecol Health Dis. 1992; 5: 59-67.
- Isolauri E. Dietary modification of atopic disease: Use of probiotics in the prevention of atopic dermatitis. Curr Allergy Asthma Rep. 2004; 4: 270-275.
- Motta L, Blancato G, Scornavacca G, De Luca M, Vasquez E, Gismondo MR, et al. Study on the activity of a therapeutic bacterial combination in intestinal motility disorders in the aged. Clin Ter. 1991; 138: 27-35.
- Horie H, Zeisig M, Hirayama K, Midtvedt T, Moller L, Rafter J. Probiotic mixture decreases DNA adduct formation in colonic epithelium induced by the food mutagen 2-amino-9H-pyrido [2,3-b] indole in a human-flora associated mouse model. Eur J Cancer Prev. 2003; 12: 101-107.
- Parvez S, Malik KA, Ah Kang S, Kim HY. Probiotics and their fermented food products are beneficial for health. J Appl Microbiol. 2006; 100: 1171-1185.
- Reddy BS. Possible mechanisms by which pro- and prebiotics influence colon carcinogenesis and tumor growth. J Nutr. 1999; 129: 1478S-82S.
- Pericleous M, Mandair D, Caplin ME. Diet and supplements and their impact on colorectal cancer. J Gastrointest Oncol. 2013; 4: 409-423.
- Simenhoff ML, Dunn SR, Zollner GP, Fitzpatrick ME, Emery SM, Sandine WE, et al. Biomodulation of the toxic and nutritional effects of small bowel bacterial overgrowth in end-stage kidney disease using freeze-dried Lactobacillus acidophilus. Miner Electrolyte Metab. 1996; 22: 92-96.
- Dunn SR, Simenhoff ML, Ahmed KE, Gaughan WJ, Eltayeb BO, Fitzpatrick Mary-Ellen D, et al. Effect of oral administration of freeze-dried Lactobacillus acidophilus on small bowel bacterial overgrowth in patients with end stage kidney disease: reducing uremic toxins and improving nutrition. Int Dairy J. 1998; 8: 545-553.
- Gilliland SE, Kim HS. Effect of viable starter culture bacteria in yogurt on lactose utilization in humans. J Dairy Sci. 1984; 67: 1-6.
- Marteau P, Flourie B, Pochart P, Chastang C, Desjeux JF, Rambaud JC. Effect of the microbial lactase (EC 3.2.1.23) activity in yoghurt on the intestinal absorption of lactose: an in vivo study in lactase-deficient humans. Br J Nutr. 1990; 64: 71-79.
- Fuller R. Probiotics in man and animals. J Appl Bacteriol. 1989; 66: 365-378.
- Salminen S, Ouwehand A, Benno Y, Lee YK. Probiotics: how should they be defined? Trends Food Sci Technol. 1999; 10: 107-110.
- Food and Agriculture Organization of the United Nations/World Health Organization [FAO/WHO]. Joint FAO/WHO working group report on guidelines for the evaluation of probiotics in food. FAO/WHO, London, Ontario, Canada, April 30 and May 1 2002, 2002: 1-11.
- Campbell A. Probiotics and prebiotics: parts of a healthy diet. Diabetes Self Manag. 2011; 28: 9-10, 12, 14-6.
- Nazzaro F, Fratianni F, Nicolaus B, Poli A, Orlando P. The prebiotic source influences the growth, biochemical features and survival under simulated gastrointestinal conditions of the probiotic Lactobacillus acidophilus. Anaerobe. 2012; 18: 280-285.