
Special Article - Antioxidants in Foods
Austin J Nutri Food Sci. 2015;3(1): 1057.
Comparison of Extraction Methods for the Determination of Antioxidant Activity in Extracts of Hippophae Rhamnoides L. and Lippia Citriodora. The Effect of Seasonal Collection
Roidaki A1, Zoumpoulakis PG2 and Proestos C1*
1Department of Chemistry, National and Kapodistrian University of Athens, Greece
2Institute of Biology, Medicinal Chemistry & Biotechnology, National Hellenic Research Foundation, Greece
*Corresponding author: Charalampos Proestos, Department of Chemistry, National and Kapodistrian University of Athens, Laboratory of Food Chemistry, Zografou, 157 84 Athens, Greece
Received: January 09, 2015; Accepted: February 26, 2015; Published: February 27, 2015
Abstract
In this work, the presence of natural antioxidants has been studied by measuring the antioxidant activity, of two selected samples sea buckthorn (Hippophae rhamnoides L.) and verbena (Lippia Citriodora). Leaves and fruits of two varieties (Leikora and Hergo) of sea buckthorn which are grown in different regions of Greece and verbena leaves grown in the same environment with the variety of sea buckthorn (Leikora) commercially sold as mixtures of the two plants to produce a specific beverage were selected and studied. easonal collection (Leikora variety) of two different crops was studied, in order to investigate the change in the content of antioxidant compounds in the same area at every year of cultivation. Specifically, the aim of this study was to determine the concentration of Total Phenolic Compounds (TPC) in the samples and the assessment of their antioxidant action by different spectrophotometric methods. Extracts were studied using different extraction solvents (methanol/ water 80/20 v/v and water to create beverages). Different extraction methods were compared (Ultrasound Assisted Extraction versus a conventional method) in order to determine the best method of extraction along with the extraction solvent suited for the quantitative release of phenolic components. The total phenolic content was determined by the Folin-Ciocalteu method using gallic acid as the standard and the antioxidant capacity of the plant extracts was measured by their ability to scavenge commercially available free radicals such as (a) DPPH (2,2-diphenyl-1-picrylhydrazyl) and, (b) ABTS (2,2'-azinobis-(3- ethylbenzothiaziline-6- sulfonate) using L- ascorbic acid and Trolox as standards respectively.
Keywords: Antioxidants, Hippophae rhamnoides L; Extraction comparison; Lippia citriodora; Seasonal collection
Abbreviations
UAE: Ultrasound-Assisted Extraction; DPPH: 2,2-Diphenyl- 1-Picrylhydrazyl; ABTS: 2,2’-Azino-Bis(3-ethylbenzothiazoline-6- Sulphonic acid); FC: Folin-Ciocalteu; GAE: Gallic Acid Equivalents; AAE: Ascorbic Acid Equivalents; TE: Trolox Equivalents; TPC: Total Phenolic Content
Introduction
In an effort to minimize the undesirable effects of synthetic food preservatives in human health, food industries and scientists have recently turned their interest to new preservatives. Sea buckthorn and verbena contain antioxidants which are known to prevent rancidity of fats in foods and dietary antioxidants that reduce the adverse effects of reactive species such as free radicals of oxygen and nitrogen, in the normal functioning of the human body. Sea Buckthorn (Hippophae L.) is a deciduous shrub that belongs to the family of Elaiagnoeidon and considered according to several studies, an important ally of our health [1]. The common species of sea Buckthorn (Hippophae rhamnoides L.) is by far the most widespread and contains a number of compounds including carotenoids, tocopherols, sterols, flavonoids, lipids, ascorbic acid, tannins. Such compounds are of interest not only from chemical point of view, but also because many of them have biological and therapeutic effects including antioxidant properties [2,3].
The official name of verbena is lippia citriodora and is a deciduous shrub belonging to the family of vervenakia and comprises about 200 species of herbs, shrubs and small trees [4]. Verbena contains active substances such as vervenalin, berberine, essential oils and tannin strange that unlike conventional tannins have not yet been investigated. These substances confer to the diuretic-decongestant action, antimicrobial, astringent, soothing and relaxing, anticonvulsive properties of the plant [5,6]. The chemical composition of the essential oils of many types of Lippia has been investigated by using several chromatographic techniques. Elements found in higher frequency in these essential oils are: limonene, caryophyllene, p-cymene, camphor, linalool, pinene and thymol.
Phytochemicals are a heterogeneous group of substances found in all plant products and are an important part of human diet. In the category of phytochemicals widespread redox secondary metabolites of phenolic nature can be found. Polyphenols give color to stem, leaves, flowers and fruits, and anthocyanins are the color component of most red and blue parts of the plant [7,8]. They occur very frequently in young leaves, which probably exert repulsive action in herbivorous insects. It is well known that the antioxidant and pharmacological properties of the plants are associated with the presence of phenolic compounds. These phytochemical compounds are derived from phenylalanine and tyrosine and do not contain any nitrogenous functional group. The structure varies from low molecular weight compounds with a single aromatic ring to large and complex tannins, polyphenols and phenolic molecules havingat least one aromatic ring with one or more hydroxyl groups attached. Some phenolic compounds can be classified according to the number of phenolic compounds and their components [7-9].
Ultrasound-Assisted Extraction (UAE) is a hight energy extraction technique. The ultrasonic radiation, which has frequencies higher than 20 kHz, facilitates the extraction of organic and inorganic compounds from solid substrates using liquid solvents. Sonication is the production of sound waves that generate bubbles close to the tissue sample, which are cleaved and disrupt the cell walls, thereby releasing the contents of the cells [10,11]. A suitable solvent is mixed with a sample and sonication is performed under controlled temperature for a specified period. The quality of the extract is influenced not only by the sonication time, temperature and choice of solvent, and the ultrasonic wave frequency and wavelength distribution [12]. The two most common ways of applying ultrasonic wave’s extraction samples are that of a probe and bath [13-15].
The antiradical activity characterizes the ability of compounds to react with a radical, while the in vitro antioxidant action is the ability of compounds to inhibit the oxidation process in a system in vivo. Through spectrophotometry it was possible to determine the concentration of total phenols in the extracts of the sea buckthorn and verbena samples for both crops (2012-2013), as well as extracts ability to bind two different free radicals (DPPH, ABTS) [16].
A method for the assessment of antioxidant activity is referred to as a discoloration test, as for lipophilic and hydrophilic antioxidants, including the carotenoids, and antioxidants in plasma is the method with the use of ABTS free radical. First, the process comprises the direct production of the ABTS free radical without involving an intermediate radical. Secondly, is a discoloration assay thus radical cation pre–formed prior to addition of the antioxidant system, instead of the radical produced continuously in the presence of antioxidant [17]. By the presence of molecules which are hydrogen donors, the radical ABTS reduced quantitatively depending on the activity of the hydrogen donor. The scanning of the radical ABTS either by transferring a hydrogen or by transfer of an electron from a compound antiradical [18].
ABTS•+ (blue-green) + ?? —> ABTS (colorless) + [??]•+
ABTS• (blue-green ) + ?? —> ABTS-? (colorless) + ?•
The (DPPH •) test is often employed as the ABTS method as well, to characterize the scavenging ability of free radicals of the phenolic compounds (AH). Detailed published protocols differ in more than one experimental conditions and results for the relative order or magnitude of activity is often contradictory. The DPPH • do not dimerize, remains in its monomeric form in solution, exhibits a stable absorption over a wide pH range, and it resists oxidation. Moreover, the reaction conditions are mild and the results provide basic information about the activity of the compounds in relation to their structure. The free radical has a maximum absorbance at 516 nm is purple. Presence of a scanner free radical root receives an electron or a hydrogen molecule and so is diamagnetic and purple color gradually becomes yellow [19,20].
DPPH• (purple)+ AH DPPH-H (yellow)+ A• →
The total phenolic content of the extracts from samples of sea buckthorn and verbena were measured by using the Folin-Ciocalteu (FC) reagent. The FC reagent consists of salts of molybdenum (Mo) and tungsten (W). In an alkaline environment, the phenolic compound is oxidized and the reagent is reduced to oxides having the characteristic blue color of pentavalent molybdenum. The color intensity is proportional to the phenolic content, the concentration of which is expressed in equivalents of a selected model in this case was chosen gallic acid [21].
Materials and Methods
Plant materials and reagents
To conduct the experiments two sea buckthorn varieties (Hippophaë rhamnoides L.) and verbena leaves were selected. Buckthorn mixture of ramnoeides and Leikora (Hippophae rhamnoides-Leikora) namely: sea buckthorn leaves, sea buckthorn dried fruit, sea buckthorn fresh fruit from Konitsa region Ioannina (Greece) and verbena leaves from the same domain. (Samples September 2012 and September 2013). Buckthorn ramnoeides ?ergo (Hippophae rhamnoides -Hergo) namely: sea buckthorn leaves, sea buckthorn dried fruit, sea buckthorn fresh fruit from Nea Gonia Chalkidiki in Northenn Greece (Samples September 2013). All samples were analyzed within three months of collection.
Sodium Carbonate anhydrous (?a2C?3) is purchased from Carlo Erba agents, Italy. Gallic acid (3,4,5-Trihydroxybenzoic acid anhydrous) and DPPH radical (2,2-D?phenyl-1-picrylhydrazyl) were purchased from Alfa Aesar GmbH &Co KG, Germany and the Folin-Ciocalteu reagent was purchased from Merck (KGaA, Germany). ABTS: ABTS 2,2’-Azino-b?s(3-ethylbenzoth?azol?ne- d-sulfon?c acid ammonium salt ?) was purchased from Chemical Industry Co. L?D, Japan. L-Ascorbic acid, analytical reagent grade, was purchased from Fischer Chem?cal, UK and Trolox: 6-Hydroxy- 2,5,7,8-tetramethylchroman-2-carboxyl?c acid 97%, was from Sigma- Aldr?ch, Germany.
Quantification was done via calibration curve with standards (external standard method). All standards were prepared as stock solutions in methanol (L-ascorbic acid), in water (Gallic acid) and in ethanol (Trolox). Stock/working solutions of the standards were stored in darkness. All solvents and reagents from various suppliers were of the highest purity needed for each application.
Plant preparation
All samples were processed as quickly as possible and in a shady and cool atmosphere, where fresh fruit frozen, dried leaves and fruits were stored in a cool, shady place. Samples from 2012 crop variety Leikora used for comparing extraction methods (classical extraction, UAE) in different parts of sea buckthorn plant. Both extracts were prepared from the dried fruit and of dried leaves, as well as extracts prepared from dried verbena leaves. Samples of the crop variety Leikora 2013 and samples of the variety Hergo were used for comparing the two varieties. Also all sample extracts were prepared by using the conventional extraction procedure. It should be mentioned that decoctions were also prepared from the above samples. Also, fresh and dried fruits from the two varieties were used to estimate the difference in concentration of antioxidants between fresh and dried fruit.
Samples of fresh fruit were lyophilized (freeze drying) for better maintenance as the absence of water suspends microbial and enzyme activity. Also, the dry sample is easier to be pulverized compared to the fresh sample, thus increasing the surface of the material and thereby increasing the extraction process [22,23].
Extraction Procedure
Ultrasound-Assisted Extraction was the first extraction method applied. In a special oval bottle each time, 1 g of powdered sample was placed and 50 mL of methanol:water, 80:20 (v/v) was added, followed by adjusting the ultrasound generating device. Also, the flask was immersed in ice, so that the temperature during extraction did not exceed 35°C. Specifically, the power of ultrasound ranged in percentage 80 % and the function of pulses (Pulse mode) adjusted to 10 sec On then 5 sec Off (power intensity device probe 750Watt). Overall, the time for each extraction was 15 minutes. Upon completion of the extraction, filtration was followed by using Buchner funnel, to remove solids and to have clear extracts, while the final volume was 50 mL.
For conventional extraction method, 1 g of each powdered material was placed in separate beakers, then 50 mL of methanol:water, 80:20 (v/v) was added for the extraction of the phenolic content by diffusion having samples shaken at regular intervals. After 48 h in the dark and at ambient temperature extraction is completed, followed by filtration using Buchner funnel, to remove solids and the clarified extract diluted to a final volume of 50 mL. The extracts were kept frozen at -200C in sealed containers.
For the preparation of decoctions, 1 g of each powdered material was used. Then 50 mL of deionized water at 80 °C was added. The samples were left to extract components for 3min, as it is normally performed for infusion preparation, and then filtered and diluted to a final volume of 50 mL. The spectrophotometric analyses were performed within 1h after preparation of infusions which were freshly prepared daily.
Determination of Total Phenolics
The same procedure was applied for all extracts to determine the total phenolic content using the same quantities of reagents. Initially, a solution of saturated sodium hydrogen carbonate Na2C03 was prepared as follows: 20 g anhydrous sodium carbonate (Na2C03) were dissolved in 80 mL of boiling distilled H20. After the solution of sodium carbonate reached ambient temperature, 8 g of excess Na2C03, was added and the solution was left for 24 h in the dark and well sealed. Finally, the solution was filtered through a fluted filter and diluted to 100 mL with distilled H20 to a volumetric flask. This solution is stable and suitable to use over a long period. Gallic acid (GA) was used as the standard phenolic substance to compare with extracts. Originally a stock solution of GA (stock) concentration 5g GA / L was prepared from which aqueous solutions were prepared with concentration of 25 to 600 mg GA / L. In plastic cuvettes of 2,5 mL, 20.0 ML standard or diluted sample were placed using electronic pipettes, plus 1500 mL distilled H20 and 100 mL of Folin-Ciocalteu reagent (commercially available). Following vigorous stirring and after waiting for 8 min, 300 mL of saturated Na2C03 solution were added and the mixture was sealed with parafilm followed by vigorous shake again. Then, the cuvettes were placed for 30 min in a water bath at 40 °C. Then the absorbance was measured at 750 nm (A750) for each sample or standard. The error correction in the value of the absorption due to the solvent of samples and standards is the “blank” sample. The experimental procedure and calculations were made in triplicate for each sample or standard solution, while different series of experiments were performed in the same day, but on different days as well. Final results are expressed in mg GAE g-1.
Determination of Antioxidant Activity Using the 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Radical Scavenging Method
Initially the DPPH radical was dissolved in methanol to prepare a concentrated (stock) solution of 0.001M from which a diluted solution of 100 μM was prepared, which is later used for measuring the antiradical activity of samples. 20.0 μL of diluted samples were placed in plastic cuvettes together with 1500.0 μL of DPPH 100 μM solution, left in the dark for 1 min and then the absorbance was measured at 516 nm (A516) every 10 min until the absorbance was stabilized in a minimum point at a plateau time.24 The time for the reaction to reach this point, denoted plateau depends on the type of sample, the concentration, the ambient conditions (temperature and light), and obviously the concentration of DPPH. At the same time, the absorbance of DPPH solution used was measured so as to calculate the percentage of the halting at the plateau time. Also measurements of the blank were made to correct the error caused by the solvent.
L-ascorbic acid (AA), was used as standard compound to prepare the standard curve for quantification because ascorbic acid reacts rapidly and completely with DPPH radical. A stock solution of 1 mg / mL was prepared by dissolving 0,1 g of ascorbic acid in 100 mL of methanol. Then the preparation of dilute solutions of ascorbic acid was followed with concentrations ranged between 800 to 1800 μg AA / mL in order for the preparation of standard curve. The solutions of ascorbic acid were prepared on each day of conducting the experiment. The experimental procedure and calculations were made in triplicate for each sample or standard solution. Final results are expressed in mg AAE g-1.
Determination of Antioxidant Activity Using the ABTS Free Radical Scavenging Method
The first step was to prepare the ABTS • +radical. To do so, an aqueous solution containing the dissolved substance in concentration of 7 mM ABTS was prepared, which is the concentrated solution of the radical (stock solution). Also sodium persulfate (Na2S208) 2,45 mM was also prepared. The mixture of the aforementioned solution was left in darkness for 16h at room temperature. Oxidation of ABTS by the persulfate ions starts directly, but the stoichiometry of the reaction is 1: 0.5, whereby the oxidation is incomplete. The radical in this form is stable for more than 2 up to 4 days in the dark, hence solution was stored in the refrigerator. Before using the ABTS+ for estimating antiradical capacity of the sample solution, the radical solution was appropriately diluted with ethanol to give absorbance between 0,70 ± 0,02. As standard compound Trolox was chosen, which is prepared as ethanolic stock solution of 0.006 M, from which solutions with concentration between 0.20 to 1,50 mM were prepared for conducting the standard curve for quantification. In plastic cuvettes of 2,5 mL, 15.0 μL standard or diluted sample using electronic pipettes were placed, plus 1500 μl of the diluted radical solution of ABTS+. The cells are sealed with parafilm and stirred vigorously prior to storage in dark. Measurements were performed at 734nm. The error correction in the value of the absorption due to the solvent for samples and standard was deducted. The experimental procedure and calculations were made in triplicate for each sample or standard solution. Final results are expressed in mg Trolox Equivalents (TE) g-1.
Results and Discussion
In Table 1 below, the abbreviations for each of the samples studied are given for the two different crop seasons of collection.
SAMPLES
CROP 2012
CROP 2013
Ultrasound-Assisted Extraction
Sea buckthorn leaves (Leikora)
US-SL-L1
-
Sea buckthorn dried fruit (Leikora)
US -SDF-L1
-
Verbena leaves
US -VL-L1
-
Conventional Extraction Method
Sea buckthorn leaves (Leikora)
CM - SL -L1
CM - SL -L2
Sea buckthorn dried fruit (Leikora)
CM - SDF -L1
CM - SDF -L2
Sea buckthorn fresh fruit (Leikora)
-
CM -SFF-L2
Verbena leaves
CM - VL -1
CM - VL -2
Sea buckthorn leaves (Hergo)
-
CM - SL -H
Sea buckthorn dried fruit (Hergo)
-
CM - SDF -H
Sea buckthorn fresh fruit (Hergo)
-
CM-SFF-H
Decoctions (infusions)
Sea buckthorn leaves (Leikora)
D- SL -L1
D - SL -L2
Sea buckthorn dried fruit (Leikora)
D - SDF -L1
D - SDF -L2
Sea buckthorn fresh fruit (Leikora)
-
D - SFF -L2
Verbena leaves
D - VL -1
D - VL -2
Sea buckthorn leaves (Hergo)
-
D - SL -H
Sea buckthorn dried fruit (Hergo)
-
D - SDF -H
Sea buckthorn fresh fruit (Hergo)
-
D - SFF –H
* Result ± standard deviation (SD)
Table 1: Abbreviations for samples studied.
Determination of Total Phenolic Content (TPC)
The results of the determination of total phenolic content (TPC) are presented below in Table 2. Results are expressed in mg GAE g-1.
SAMPLES
mg GAE*g-1
US-SL-L1
107.4±1.0
US -SDF-L1
9.5±0.5
US -VL-L1
61.2±0.8
CM - SL -L1
82.1±0.4
CM - SDF -L1
18.0±0.1
CM - VL -1
41.0±0.5
D- SL -L1
23.9±0.3
D - SDF -L1
11.2±0.2
D - VL -1
20.6±0.9
CM - SL -L2
45.0±0.4
CM - SDF -L2
13.5±0.4
CM -SFF-L2
20.2±0.3
CM - VL -2
19.9±0.6
D - SL -L2
24.2±0.3
D - SDF -L2
3.4±0.2
D - SFF -L2
6.4±0.3
D - VL -2
10.5±1.5
CM - SL -H
97.0±0.4
CM - SDF -H
20.6±0.1
CM-SFF-H
6.9±0.5
D - SL -H
52.6±0.4
D - SDF -H
8.7±0.4
D - SFF -H
6.4±0.1
*Result ± standard deviation (SD)
Table 2: Total phenolic content of all samples mg GAE g-1
As it can be seen from the above table, regardless of extraction, the leaves of sea buckthorn contain in all cases the higher amount of TPC. For samples of 2012 crop a series of measurements for determination of the total phenolic content was conducted by using the Folin- Ciocalteu method for extracts obtained from different extractions and different parts (fruit, leaves) of the plant sea buckthorn as well as leaves of verbena, a plant developed in the same environment with sea buckthorn.
As it can be seen from the above Figure 1 for sea buckthorn leaves and verbena leaves of 2012 crop, the ultrasonic assisted extraction process produced higher amounts of total phenolic compounds, while lower amounts were produced by the conventional extraction method and finally the smallest amount of TPC extracted were from decoctions. This is probablybecause the sonication is the production of sound waves that generate bubbles (pit) close to the tissue of the plant cell, which are cleaved and disrupt the cell walls, which facilitates penetration of the solvent, thereby releasing the contents of cells [12]. Also sonication accelerates the movement of the molecules thus bringing together the molecules of solvent with those of the sample, unlike the conventional method that simply infused material with the solvent and the quantity of the mass of extractable compounds is significantly reduced. Regarding the sea buckthorn fruit, higher concentration of TPC had the samples derived by conventional extraction method, followed by the beverage and then samples extracted with the use of ultrasound. This may be due to different substrate between the leaves and the fruit of sea buckthorn. For infusions, due to the increased temperature of the water the cells are heated and thereby lose moisture through evaporation. The steam produced swells and eventually breaks the cells, releasing some of the active ingredients.
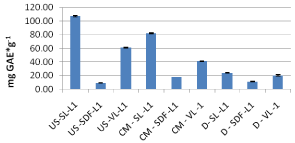
Figure 1: Comparative diagram of the TPC for 2012 crop samples.
For samples of 2013 crop two different varieties of sea buckthorn and extracts were derived by the conventional method and infusions as well. Also extracts from both fresh and dried sea buckthorn fruits and leaves of verbena were prepared (Figure 2).
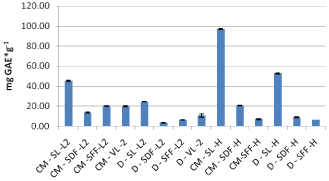
Figure 2: Comparative Chart for TPC samples of 2013 crop.
As mentioned above for samples of the 2012 crop, similar results found for the Leikora variety of 2013 crop, where most total phenolic compounds were contained in the leaves followed by the fresh fruit and finally the dried fruit (Figure 3). The extracts of verbena contained similar amounts of TPC to that of the fresh fruit. Extracts of Leikora variety for the 2013 crop had less concentration in TPC compared to those of the 2012 crop which might be due to different growth conditions, harvesting time and drying procedures (e.g. temperature, weather conditions). As far as it concerns samples of the variety Hergo more TPC found to be present in the extracts of leaves, followed by dried fruits and finally the fresh fruit which all have been extracted by the use of conventional extraction method. Decoctions of the Hergo variety had similar results with methanol:water extracts (conventional extraction). The variety Hergo based on Figure 2 had higher content in TPC in relation to the Leikora variety for the 2013 crop.
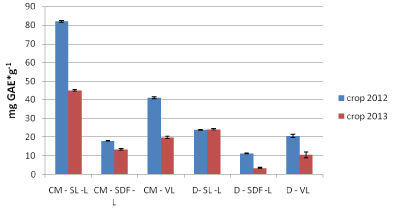
Figure 3: Total phenolic content (TPC) for 2012 and 2013crop (Leikora).
Determination of Antioxidant Activity Using the 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Radical Scavenging Method
The DPPH method was introduced by Blois [24] and it is widely used to test the ability of compounds to act as free radical scavengers or hydrogen donors, and to evaluate antioxidant capacity. The estimation of the binding capacity of the DPPH radical, in this case by sea buckthorn and verbena samples, is a common and widely used method to estimate the antioxidant activity of herbal extracts and herbal infusions. To quantify the antioxidant activity of the samples a standard curve for ascorbic acid was conducted and results were expressed as mg Ascorbic Acid Equivalents (AAE) per g of sample. The parameter EC50 (Efficient Concentration value), is used for the interpretation of the results from the DPPH method and is defined as the concentration of substrate that causes 50% loss of the DPPH activity (color). The following Table 3 lists the AAE g-1 for each sample.
SAMPLES
mg AAE g-1*
US-SL-L1
1890.2±2.9
US -SDF-L1
33.1±1.1
US -VL-L1
169.1±0.1
CM - SL -L1
812.8±6.0
CM - SDF -L1
104.3±1.2
CM - VL -1
256.5±3.4
D- SL -L1
595.5±5,0
D - SDF -L1
59.2±3.8
D - VL -1
60.7±1.2
CM - SL -L2
762.6±5.7
CM - SDF -L2
52.2±0.3
CM -SFF-L2
65.8±2.6
CM - VL -2
147.5±7.3
D - SL -L2
102.9±1.3
D - SDF -L2
27.2±1.1
D - SFF -L2
46.3±2.0
D - VL -2
60.7±1.2
CM - SL -H
825.1±5
CM - SDF -H
133.7±0.8
CM-SFF-H
57.6±0.5
D - SL -H
559.6±2.1
D - SDF -H
43.4±2
D - SFF -H
49.9±0.5
* Result ± standard deviation (SD)
Table 3: Aggregate results for extracts and decoctions by the use of DPPH assay.
For the 2012 crop samples, leaves of sea buckthorn extracted by ultrasonic assisted extraction shows greater antioxidant activity against fruit. On the contrary by conventional extraction fruits had greater antioxidant capacity compare to leaves (Leikora) (Figure 4). Decoctions of Leikora had lower antioxidant activity compared to leaves and fruit aqueous methanolic extracts for both extraction methods (UAE and conventional) possibly to the same reasons already mentioned in paragraph 3.1 and can be seen in Figure 4.
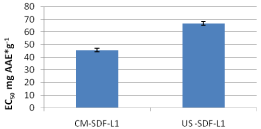
Figure 4: EC50 of dried Leikora variety fruits (2012 crop).
As it can be seen in Table 3 leaf samples of sea buckthorn, whatever variety and extraction performed, resulted in greater antioxidant activity, which is consistent with the results of the Folin- Ciocalteu method. As for verbena plant, the antiradical activity was in descending order as follows: leaves extracts by ultrasound extraction > leaves extracts by conventional extraction > leaves decoctions. For all the extracts of the 2012 crop, the higher antiradical activity was found to be for the ultrasonic assisted extracts of the sea buckthorn leaves (Figure 5).
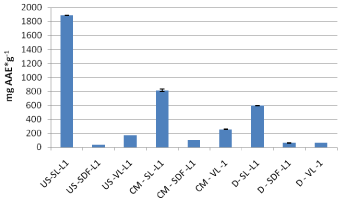
Figure 5: DPPH assay for extracts and decoctions of 2012 crop.
Figure 6 shows that samples of the Leikora variety (2012 crop) had under conventional extraction higher antioxidant activity in comparison with specimens of the same variety of 2013.
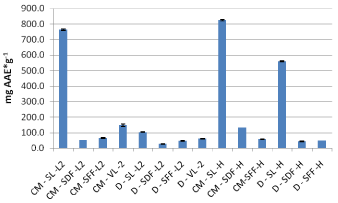
Figure 6: Assessment of binding capacity of DPPH radical of Leikora samples
(2012-2013 crop).
Between the two varieties of sea buckthorn (Hergo and Leikora) as shown below in Figure 6, under conventional extraction, the leaves of Hergo variety appear to have higher antioxidant activity both for extracts and decoctions.
Comparing extracts from both varieties which were extracted by conventional extraction, both leaves and dried fruits of Hergo variety had higher content in antiradical compounds than the corresponding variety Leikora, as presented by the EC50 value in Figure 8 below.
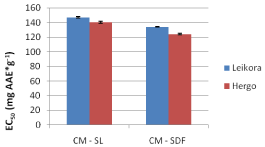
Figure 8: EC50 values for Leikora and Hergo varieties of sea buckthorn leaves
and dried fruit (conventional extraction method).
Decoctions made out of leaves, dried fruits and fresh fruit of sea buckthorn (Leikora variety) had all lower antiradical activity compared to those of the Hergo variety (Figure 9). As far as the verbena extracts are concerned, aqueous methanolic extracts (by conventional extraction) had higher activity as presented in Figure 7.
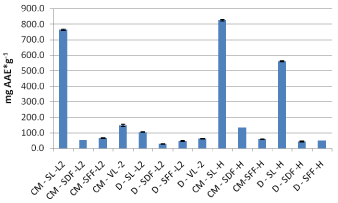
Figure 7: DPPH assay for sea buckthorn (Hergo and Leikora) under
conventional extraction (2013 crop).
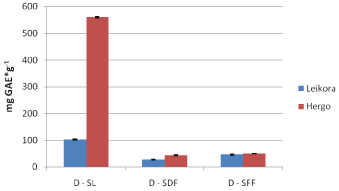
Figure 9: DPPH assay for decoctions of the two varieties of sea buckthorn
studied (2013 crop).
Determination of Antioxidant Activity Using the ABTS Free Radical Scavenging Assay
Another evaluation of the antioxidant activity of the samples studied is given by their ability to scavenge the cation radical ABTS•+. Results of this assay are expressed as mg Trolox Equivalents (TE) per g sample. Table 4 shows the summary of results in mg TE g-1 of all extractions for both 2012 and 2013 crops.
SAMPLES
mg TE*g-1
US-SL-L1
240.5±0.2
US -SDF-L1
10.0±0.5
US -VL-L1
55.9±0.5
CM - SL -L1
227.9±0.3
CM - SDF -L1
12.9±0.6
CM - VL -1
39.5±0.5
D- SL -L1
71.4±0.5
D - SDF -L1
17.6±0.1
D - VL -1
6.5±0.4
CM - SL -L2
105.7±0.6
CM - SDF -L2
14.3±0.5
CM -SFF-L2
11.5±0.1
CM - VL -2
17.7±0.1
D - SL -L2
60.2±1.1
D - SDF -L2
15.3±0.3
D - SFF -L2
18.9±0.2
D - VL -2
6.6±0.1
CM - SL -H
145.3±1.0
CM - SDF -H
25.3±1.2
CM-SFF-H
8.3±0.2
D - SL -H
77.0±0.3
D - SDF -H
29.6±0.2
D - SFF -H
8.7±0.1
* Result ± standard deviation (SD)
Table 4: Aggregate results of all samples of ABTS method.
As shown in Table 4 leaf extracts had the best antioxidant capacity regardless of crop and variety. UAE samples of the 2012 crop, more specifically the leaves of sea buckthorn have shown greater antioxidant activity as shown by the higher amount of mg TE g-1 sample, while decoctions had the lower antiradical capacity. As Figure 9 shows the extracts of the fruit extracted by UAE had better antioxidant activity than samples extracted by conventional extraction, which is consistent with the results of Folin-Ciocalteu method, since extracts by UAE had higher TPC than that of conventional method for fruit extract of the Leikora variety.
Decoctions of the sea buckthorn dried fruit unlike leaf extracts had shown the best antiradical activity and this may be due to the different substrate (fruit-leaves) and the differential effect of ABTS in aqueous solutions. Verbena aqueous methanol extracts extracted by UAE had the best antioxidant activity followed by conventional extraction extracts while decoctions had the lower activity (Figure 10). Comparing verbena extracts with those of sea buckthorn (leaves and fruit) it was proven that antioxidant capacity did not show large differences. For samples of the 2013 crop higher concentration in antioxidant components found for the sea buckthorn leaves for both varieties (Figure 11)
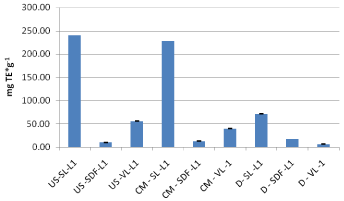
Figure 10: ABTS results for 2012 crop of the Leikora variety (mg TE*g-1 ).
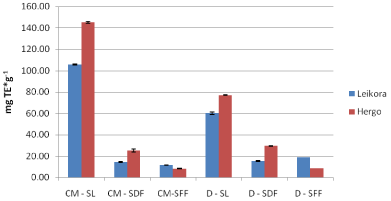
Figure 11: ABTS results of 2013 crop (mg TE*g-1).
Concerning the conventional extraction of leaves and dried fruits of sea buckthorn, Hergo variety, had better antioxidant activity than Leikora variety. On the contrary fresh fruit of the variety Leikora had better antioxidant activity than the Hergo variety. For the variety Leikora, in descending order for antioxidant activity was as follows: leaves > fresh fruit > dried fruits. Similarly is the order for the samples of the variety Hergo. As far as decoctions of leaves of sea buckthorn are regarded the antioxidant activity was higher for both varieties. Decoctions from leaves of both varieties (Leikora and Hergo) had comparable antioxidant activity with the conventional extraction samples (Figure 11). Also decoctions of leikora and hergo fruits (dried and fresh), had higher antioxidant activity than the corresponding aqueous methanolic extracts by conventional extraction (Figure 11). Finally, verbena aqueous methanolic extracts extracted by conventional extraction had higher antioxidant activity than decoctions prepared by the same extraction method (Figure12).
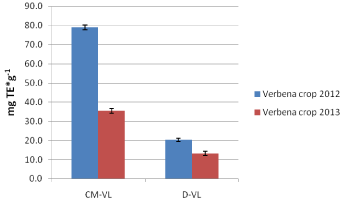
Figure 12: Verbena leaves samples, conventional extraction vs decoctions
(2012-2013 crops, results expressed in mg TE*g-1).
Conclusion
The presence of natural antioxidants has been proven by measuring the antioxidant activity, of two selected samples sea buckthorn (Hippophae rhamnoides L.) and verbena (Lippia Citriodora) cultivated in Greece. This is the first time to our knowledge that these plants were analyzed and compared. Hergo variety has shown better antioxidant activity than that of leikora variety for both UAE and conventional method used. UAE was proven to be more effective for leaves extraction for Leikora variety, concerning the antioxidant activity measured by DPPH and ABTS, also for TPC. However, conventional extraction was more effective for dried fruits of the same variety. Seasonal collection showed that 2013 crop for the Leikora variety was better than that of 2012, concerning the antioxidant capacity of extracts and decoctions. Finally decoctions of sea buckthorn and verbena had comparable but less antioxidant activity compared with extracts. Decoction prepared from leaves had higher antioxidant capacity that those prepared by dried or fresh fruits. Hence, for consumers it is better to prepare decoction made out of leaves than fruits to gain more antioxidant compounds as proven by this study. Verbena can be used together with sea buckthorn leaves to prepare infusions because it can increase amount of natural antioxidants and improve taste of final infusion.
References
- Sindhu Mathew, Carl Grey, Kimmo Rumpunen, Patrick Adlercreutz. Analysis of carbonyl compounds in sea buckthorn for the evaluation of triglyceride oxidation, by enzymatic hydrolysis and derivatisation methodology, F Chem. 2011; 126: 1399–1405.
- Vahid Bilaloglu Guliyev, Mustafa Gul, Ali Yildirim. Review Hippophae rhamnoides L.: Chromatographic methods to determine chemical composition, use in traditional medicine and pharmacological effect. J of Chrom. B. 2004; 812: 291-307.
- Lalit M Bal, Venkatesh Meda, Naik SN, Santosh Satya. Sea buckthorn berries: A potential source of valuable nutrients for nutraceuticals and cosmoceuticals. F Res Intern. 2011; 44: 1718-1727.
- Catherine Argyropoulou, Dimitra Daferera, Petros A Tarantilis, Costas Fasseas, Moschos Polissiou. Chemical composition of the essential oil from leaves of Lippia citriodora (Verbenaceae) at two developmental stages, Biochem System and Eco. 2007; 35: 831-837.
- Valentão P, Fernandes E, Carvalho F, Andrade PB, Seabra RM, de Lourdes Basto M. Studies on the Antioxidant Activity of Lippia citriodora Infusion: Scavenging Effect on Superoxide Radical, Hydroxyl Radical and Hypochlorous Acid, Bio and Pharm Bul. 2002; 25: 1324-1327.
- Yogendra Kumar MS, Tirpude RJ, Maheshwari DT, Bansal A, Misra K. Antioxidant and antimicrobial properties of phenolic rich fraction of Seabuckthorn (Hippophae Rhamnoides L.) leaves in vitro. Food Chem, 2013, 141: 3443-3450
- Crozier A, Clifford MN, Ashihara H. Plant Secondary Metabolites: Occurrence, Structure and Role in the Human Diet. Blackwell Publishing Ltd. 2007; 2-19.
- Özlem Tokusoglu, Clifford Hall III. Fruit and Cereal Bioactives Sources. Chem and App, CRC Press. 2011: 3-7.
- Hasna El Gharras. Polyphenols: food sources, properties and applications – a review. Inter J of F Scien & Tech. 2009; 44: 2512-2518.
- Kamaljit Vilkhu, Raymond Mawson, Lloyd Simons, Darren Bates. Applications and opportunities for ultrasound assisted extraction in the food industry — A review. Innov F Scien & Emer Tech. 2009; 9: 161-169.
- Chemat F, Zill-e-Huma, Khan MK. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason Sonochem. 2011; 18: 813-835.
- Vinatoru M1. An overview of the ultrasonically assisted extraction of bioactive principles from herbs. Ultrason Sonochem. 2001; 8: 303-313.
- Muhammad Kamran Khan, Maryline Abert-Vian, Anne-Sylvie Fabiano-Tixier, Olivier Dangles, Farid Chemat. Ultrasound-assisted extraction of polyphenols (flavanone glycosides) from orange (Citrus sinensis L.) peel. Food Chem. 2010; 119: 851-858.
- Ying Z, Han X, Li J. Ultrasound-assisted extraction of polysaccharides from mulberry leaves. Food Chem. 2011; 127: 1273-1279.
- Dey S, Rathod VK. Ultrasound assisted extraction of β-carotene from Spirulina platensis. Ultrason Sonochem. 2013; 20: 271-276.
- Ruth Martínez, Paulina Torres, Miguel A Meneses, Jorge G. Figueroa, Jose A Perez-Alvarez, Manuel Viuda-Martos. Chemical, technological and in vitro antioxidant properties of mango, guava, pineapple and passion fruit dietary fibre concentrate. Food Chem. 2012; 135: 1520–1526.
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999; 26: 1231-1237.
- Vikas Jaitak, Kapil Sharma, Kalpana Kalia, Neeraj Kumar, Singh HP, Kaul VK, et al. Antioxidant activity of Potentilla fulgens: An alpine plant of western Himalaya. Journal of Food Composition and Analysis. 2010; 23: 142–147.
- Carmona-Jiménez Y, García-Moreno MV, Igartuburu JM, Garcia Barroso C. Simplification of the DPPH assay for estimating the antioxidant activity of wine and wine by-products. Food Chem. 2014; 165: 198-204.
- Om P Sharma, Tej K Bhat. DPPH antioxidant assay revisited. Food Chem. 2009; 113: 1202-1205.
- Andressa Blainski, Gisely Cristiny Lopes, João Carlos Palazzo de Mello. Application and Analysis of the Folin Ciocalteu Method for the Determination of the Total Phenolic Content from Limonium Brasiliense. Mol (MDPI). 2013; 18: 6852-6865.
- Haminiuk CWI, Maciel GM, Plata-Oviedo MSV, Peralta RM. Phenolic compounds in fruits - an overview. Inter J of Food Scien & Tech. 2012; 47: 2023-2044.
- Aline Meda, Charles Euloge Lamien, Marco Romito, Jeanne Millogo, Odile Germaine Nacoulma. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005; 91: 571–577.
- Brand-Williams W, Cuvelier ME, Berset C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT - F Scien and Tech. 1995; 28: 25-30.