
Research Article
Austin J Nutri Food Sci. 2015;3(2): 1062.
Preharvest Treatment of 1-methylcyclopropene Reduces the Occurrence of the Water-soaking and Enhances the Quality of Watermelons (Citrullus Vulgaris)
Chao Zhang, Jie Huo, Wu Li, Yue Ma, Guoyi Gong, Xiaoyan Zhao and Yong Xu*
Beijing Vegetable Research Center, Beijing Academy of Agriculture and Forestry Sciences, China
*Corresponding author: Yong Xu, Beijing Vegetable Research Center, Beijing Academy of Agriculture and Forestry Sciences, Banjing, Haidian District, Beijing 100097, China
Received: February 27, 2015; Accepted: May 11, 2015; Published: May 14, 2015
Abstract
Exposure to exogenous ethylene is closely related to occurrence of water-soaking in watermelons. 1-Methylcyclopropene (1-MCP) inhibits the physiological action of ethylene in fruits and vegetables. Consequently, a preharvest treatment of 1-MCP was a possible option to reduce the occurrence of the water-soaking in watermelons. Two cultivars were used to validate the effect of the preharvest 1-MCP treatment at 10 days prior to their harvest. The 1-MCP treatment of 1μL/L enhanced the soluble solid content and fruit weight of the 2 cultivars, and lowered the water-soaking percentage and respiration rate. The 1-MCP blocked the physiological action of exogenous ethylene and slowed down the maturation of the watermelons. In summary, the preharvest 1-MCP treatment of 1μL/L reduced the occurrence of the water-soaking and enhanced the quality of watermelons by slowing down the maturation.
Keywords: Watermelon; 1-MCP; Preharvest treatment; Water-soaking; Exogenous ethylene
Introduction
Water-soaking of watermelons (Citrullus vulgaris) is characterized by tissue translucency, softening and maceration of the endocarp and placental tissues [1]. A number of factors are suspected to contribute to this syndrome [1-5]. Among the factors, the exposure to exogenous ethylene has been demonstrated to induce the watersoaking in both the immature and fully ripe fruits [1,5-7]. Exposure to exogenous ethylene is difficult to avoid during the growth of watermelons, as ethylene may be produced from other plants or fruits [8]. Therefore, inhibiting the effect of exogenous ethylene could be an effective strategy to reduce the occurrence of the watersoaking in watermelons. 1-Methylcyclopropene (1-MCP) inhibits the physiological action of ethylene in fruits and vegetables [9,10]. Postharvest treatment of 1-MCP inhibits water-soaking and extends the shelf life of fresh-cut watermelons [8,11]. Moreover, the 1-MCP treatment to watermelons benefits the commercial storage conditions (up to 3 weeks at 13°C ) even with exposure to exogenously ethylene [1]. However, the effect of preharvest treatment of 1-MCP on watersoaking of watermelon fruits has not been fully explored.
The aim of the current study was to evaluate the effect of preharvest treatments of 1-MCP on water-soaking and quality of watermelon fruits. Preharvest 1-MCP treatments either 1μL/L or 5μL/L were applied 10 days prior to their harvest. Water-soaking and quality parameters of the fruits were then evaluated. Moreover, 2 cultivars, one of whom is more susceptible to the water-soaking, were used to corroborate and reinforce our results.
Materials and Methods
Plant material and cultivation
Two cultivars of watermelon (Citrullus vulgaris, var Jingying series), F and M that have not been used commercially, were used in this study. The Jingying series that are round with serrated stripes are harvested 26 d after the pollination. The average weight of the fruits is 2000 g. Both cultivars were planted in August and harvested in October (2010 and 2011) at the Sijiqing Farm of the Beijing Vegetable Research Center, Beijing, China, following standard commercial practices. Two separated greenhouses (4 m × 6 m) were used as the controls to raise the fruit number. The other 2 separated greenhouses (4 m × 6 m) were subjected to preharvest 1-MCP treatments either 1μL/L or 5μL/L. In each green houses, the cultivar F (about 60 seeds) and M (about 60 seeds) were sown on in rows that were 3 m apart. At the six-true-leaf stage, all vines except 2 per plant were pinched to promote branching. The female flowers on the 11th node were self-pollinated by using more than 2 staminate flowers per pistil at anthesis. Only one pollinated flower was left on each of the 2 branches, and all the other female flowers were removed. The fruits were harvested after the pollination for 26 d. For each cultivar, more than 40 fruits were harvested and weighed for each treatment.
Preharvest 1-MCP treatment and experiment design
Each fruit was sealed separately in a polyethylene bag for 24 h at 10 d prior to the harvest. An appropriate weight of the 1-MCP powder (Lanzhou Jiacheng Biotechnology Ltd., Lanzhou, China) and water were sealed in a bag to obtain an initial 1-MCP concentration of either 1μL/L or 5μL/L that was validated by the GC analysis. The control-F and control-M stood the control of the cultivars F and M, respectively. The 1-MCP treatments of 1μL/L were named as F1 and M1 for cultivars F and M, respectively. In a similar manner, the treatments of 5μL/L were named as F5 and M5 for cultivars F and M, respectively.
For each treatment, more than 40 fruits were harvested. Among the fruits, 10 fruits were used to measure the respiration rate after harvest immediately, and the remaining fruits (more than 30 fruits) were used for the other analyses. The remaining fruits were cut in half through the placenta tissues and the cross surface was evaluated for the water-soaking. The half of the fruit was peeled and juiced in a HR1861food mixer (Philips, Dongguan, China) to acquire the watermelon juice. The juice was used to measure the Soluble Solid Content (SSC), titratable acid, and vitamin C content. The juice for the vitamin C analysis was frozen at -80°C for the future analyses. The other half of the fruits was peeled and cut into a cube with a dimension of 5 cm × 5 cm × 3 cm for the texture analysis.
Determination of vitamin C content
The vitamin C content of the fruits was determined by an HPLC method that was recently reported [12]. An aliquot of 25 mL of the juice was mixed with 25 mL of a solution containing 45 g/L of metaphosphoric acid and 7.2 g/L of dithiothreitol. The mixture was centrifuged at 20,000 × g for 15 min at 4°C and the supernatant was vacuum-filtered through the Whatman No. 1 paper. The sample was then passed through a millipore 0.45 μm membranes into an opaque vial and kept at -80°C until being used. An aliquot of 20 μL was injected into Agilent 1200 series HPLC (Agilent Technologies, Palo Alto, California) fitted with a reverse-phase C18 Spherisorb® ODS2 (5 μm) stainless-steel column (4.6 mm × 250 mm). The mobile phase was a 0.01% sulphuric acid solution adjusted to a pH of 2.6. The flow rate was fixed at 1 mL/min and monitored at 245 nm at 25°C. Vitamin C was quantified using a calibration curve based on ascorbic acid pure standards and results were expressed as relative vitamin C concentration.
Determination of SSC and titratable acid
An aliquot of 1 ml juice was dropped on a pocket digital refractometer (Pal-a, ATAGO Co., Ltd., Japan) to measure the SSC. An aliquot of 10 mL juice was titrated automatically with a standardized 10 mmol/L NaOH to the end point (pH = 8.1) by a pH meter (TitroLine- SI Analytics GmbH, Mainz, Germany). The volume of NaOH (by multiplying with 0.67) was converted to mg malic acid per ml juice.
Texture analysis
The fruit cube was placed on the test platform of a TA.XT2i plus texture analyzer (Stable Micro Systems Ltd., Godalming, and Surrey, UK). The trigger (10 mm stainless cylinder) was compressed to 50 % of the total height with a pre-test speed of 5 mm/s, test speed of 1 mm/s, and post-test speed of 10 mm/s. During the compression, the force of the trigger increased quickly, and then vibrated in a small range at a certain level. The time of the vibration was defined as the statistical interval. The average force of the statistical interval indicated the firmness of fruits. The ratio of the linear distance to the time difference in the statistical interval indicated the fragility of fruits. The higher the ratio, the more fragile is the fruit.
Determination of respiration rate
Respiration rate was determined at 22°C by an infrared CO2 analyzer (LI-6400XT, LI-COR, Inc., Lincoln, NE, USA) with a 5 L sample chamber. The fruit was sealed in the chamber. The carrier gas (O2 of 21%, and N2 of 79%) was used to transport the CO2 produced by the fruit to the CO2 analyzer. The respiration rate was recorded when the CO2 reached equilibrium. The results were the average of about 10 replicates per treatment.
Water-soaking of fruits
For each treatment, about 30 fruits were cut in half through the placenta tissues for the water-soaking percentage evaluation. The water-soaking percentage, which was the average percentage of the water-soaking area in the total fruit area through the placenta tissues, was determined through the visual estimation by 3 experienced panelists.
Statistical analysis
The results were the average of 2 years (2010 and 2011) that were expressed as the average ± standard deviation (n = 3). Analysis of variance was used to compare mean differences of the results. If the differences among means were detected, multiple comparisons were performed using Duncan’s Multiple Range Test. All analyses were conducted by SPSS (Window Version 19).
Results and Discussion
Preharvest treatments of 1-MCP enhanced the quality of watermelons
The vitamin C, SSC, titratable acid, and average weight of the fruits are shown in Figure 1. The data described herein were the summary of the fruits from two harvest seasons. The 1-MCP treatment showed no influence on the vitamin C content of the cultivar M, while the treatment of the 5μL/L enhanced the vitamin C content of the cultivar F significantly. This difference maybe resulted from the nature of the cultivars.
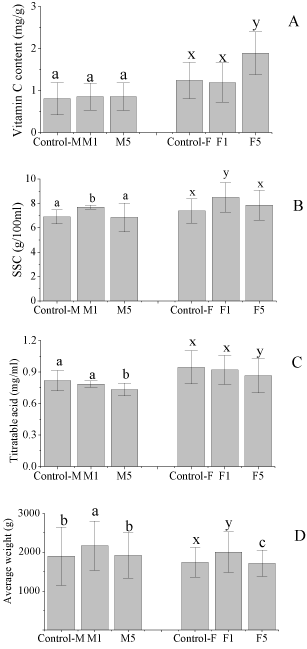
Figure 1: Preharvest1-MCP treatments on the vitamin C (A), SSC (B),
titratable acid (C), and average weight (D) of watermelons. Data are means ±
standard deviation (n = 60). Means with different letter represent a significant
difference (p<0.05). The control-M and control-F were the controls for the
cultivars F and M, respectively. The M1 and M5 were the 1-MCP treatments
of 1μL/L and 5μL/L, respectively for cultivars M. The similar rule was applied
for the cultivars F.
SSC increases with the ripening of watermelons and reaches a plateau at maturation [13], while titratable acid decreases during the maturation of watermelons [13,14]. Remarkably, the 1-MCP treatments influence the SSC in different manners that depend on the fruit type or cultivar [15]. For the 2 cultivars, the SSC of the 1μL/L treatment was significantly higher than that of the control, while the SSC of the 5μL/L treatment was similar to that of the control. Meanwhile, the 1-MCP treatment of 5μL/L reduced the titratable acid content of the fruits when compared to the control. The average weight of the 1μL/L treatment was higher than that of the control, while the average weight of the 5μL/L treatment was similar to that of the control. Hence, the preharvest 1-MCP treatments of 1μL/L raised the SSC and average weight of the fruits.
The preharvest 1-MCP treatment showed no influence on the firmness of the fruits (Figure 2). The 1-MCP treatment of 1μL/L showed on influence on both the fragility and hardness of the fruits. The 1-MCP treatment of 5μL/L reduced the fragility of the fruits compared with the control, while showed no influence on their hardness. Being similar to our results, the postharvest treatment of 1-MCP (1μL/L) shows no significant influence on the firmness of the fresh-cut watermelons [8].
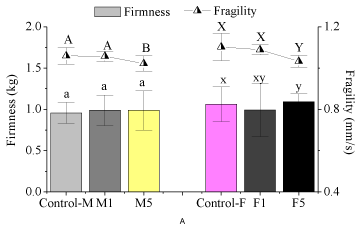
Figure 2: Preharvest 1-MCP treatments on the texture of watermelons.
Data are means ± standard deviation (n = 180). Means with different letter
represent a significant difference (p<0.05). The control-M and control-F were
the controls for the cultivars F and M, respectively. The M1 and M5 were
the 1-MCP treatments of 1μL/L and 5μL/L, respectively for cultivars M. The
similar rule was applied for the cultivars F.
The 1-MCP treatment of 1μL/L showed the similar influence on the quality parameters of the 2 cultivars, except the vitamin C content. These results validated that the preharvest 1-MCP treatment of 1μL/L enhanced the quality of the watermelons.
Preharvest treatments of 1-MCP reduced the watersoaking and respiration rate of watermelons
The effect of the preharvest treatments of 1-MCP on the watersoaking and respiration rate of watermelons is shown in Figure 3. The preharvest 1-MCP treatment lowered the water-soaking percentage of the fruits, which was speculated as the slowing down the maturation of watermelons by 1-MCP regulation. The similar phenomenon also presents on tomato [16]. The results of the respiration rate of the fruits further confirmed our speculation. The respiration rate of the treated fruits was significantly lower than that of the control. Consequently, the preharvest 1-MCP treatment was supposed to reduce the watersoaking by slowing down the maturation of fruits, which would also be good for their postharvest storage. Furthermore, the water-soaking percentage of the 1μL/L treatment was lower than that of the 5μL/L treatment. The reason for this phenomenon is not clear. Based on previous studies, the effective concentrations of 1-MCP vary widely for different commercial purposes that differ with respect to time, temperature and the method of application [17,18].
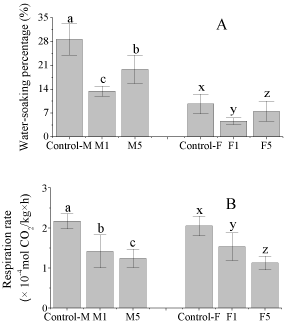
Figure 3: Preharvest 1-MCP treatments on the water-soaking occurrence
(n = 60) (A) and the respiration rate (n = 20) (B) of watermelons. Data are
means ± standard deviation. Means with different letter represent a significant
difference (p<0.05). The control-M and control-F were the controls for the
cultivars F and M, respectively. The M1 and M5 were the 1-MCP treatments
of 1μL/L and 5μL/L, respectively for cultivars M. The similar rule was applied
for the cultivars F.
The water-soaking percentage of the cultivar M was much higher than that of the cultivar F, which proved that the cultivar M was more susceptible to the water-soaking than the cultivar F under the same conditions.
Acknowledgement
The authors are grateful to the financial support of the earmarked fund for Modern Agro-industry Technology Research System (CARS-26-22) and Beijing Academy of Agricultural and Forestry Sciences Special Funding (KJCX20140204).
References
- DeLuca HF. History of the discovery of vitamin D and its active metabolites. BoneKEy Reports. 2014; 3: Article number 479.
- Editorial: A dose of vitamin D history. Nat Struct Biol. 2002; 9: 77.
- Neal S, Sykes J, Rigby M, Hess B. A review and clinical summary of vitamin D in regard to bone health and athletic performance. PhysSportsmed. 2015; 43: 161-168.
- Hyppönen E, Läärä E, Reunanen A, Järvelin MR, Virtanen SM. Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. Lancet. 2001; 358: 1500-1503.
- Embry AF, Snowdon LR, Vieth R. Vitamin D and seasonal fluctuations of gadolinium-enhancing magnetic resonance imaging lesions in multiple sclerosis. Ann Neurol. 2000; 48: 271-272.
- Gelfand JM, Cree BA, McElroy J, Oksenberg J, Green R, Mowry EM, et al. Vitamin D in African Americans with multiple sclerosis. Neurology. 2011; 6: 1824-1830.
- Munger KL, Zhang SM, O'Reilly E, Hernán MA, Olek MJ, Willett WC. et al. Vitamin D intake and incidence of multiple sclerosis. Neurology. 2004; 62: 60-65.
- Merlino LA, Curtis J, Mikuls TR, Cerhan JR, Criswell LA, Saag KG. Vitamin D intake is inversely associated with rheumatoid arthritis: results from the Iowa Women's Health Study. Arthritis Rheum. 2004; 50: 72-77.
- Anglin RE, Samaan Z, Walter SD, McDonald SD. Vitamin D deficiency and depression in adults: systematic review and meta-analysis. Br J Psychiatry. 2013; 202: 2100-107.
- Ginde AA, Liu MC, Camargo CA. Demographic differences and trends of vitamin D insufficiency in the U.S. population, 1988–2004. Arch Intern Med. 2009; 169: 626–632.
- Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006; 84: 18-28.
- Ebeling PR. Vitamin D and bone health: Epidemiologic studies. Bonekey Rep. 2014; 3: 511.
- Ceglia L, Harris SS. Vitamin D and its role in skeletal muscle. Calcif Tissue Int. 2013; 92: 151-162.
- Ward KA, Das G, Berry JL, Roberts SA, Rawer R, Adams JE, et al. Vitamin D status and muscle function in post-menarchal adolescent girls. J ClinEndocrinolMetab. 2009; 94: 559-563.
- Close GL, Russell J, Cobley JN, Owens DJ, Wilson G, Gregson W, et al. Assessment of vitamin D concentration in non-supplemented professional athletes and healthy adults during the winter months in the UK: implications for skeletal muscle function. J Sports Sci. 2013; 31: 344-353.
- Close GL, Leckey J, Patterson M, Bradley W, Owens DJ, Fraser WD, et al. The effects of vitamin D(3) supplementation on serum total 25[OH]D concentration and physical performance: a randomised dose-response study. Br J Sports Med. 2013; 47: 692-696.
- Shindle MK, Voos JE, Gulotta L, Weiss L, Rodeo SA, Kelly B, et al. Vitamin D Status in a Professional American Football Team. Presented at AOSSM 2011 Annual Meeting. San Diego, CA. 2011.
- Maroon JC, Mathyssek CM, Bost JW, Amos A, Winkelman R, Yates AP, et al. Vitamin D Profile in National Football League Players. Am J Sports Med. 2015.