
Special Article - Food Supplements: Clinical Cases & Short Reports
Austin J Nutri Food Sci. 2015; 3(4): 1072.
Total Selenium Content of Commercial Food Supplements: Label Accuracy Evaluation
Almeida IMC¹, Oliva-Teles MT², Santos J¹, Delerue-Matos C² and Oliveira MBPP¹*
¹Department of Chemistry, University of Porto, Portugal
²Department of Chemical Engineering, Polytechnic Institute of Porto, Portugal
*Corresponding author: Oliveira MBPP, Department of Chemistry, Faculty of Pharmacy, University of Porto, Portugal
Received: September 24, 2015; Accepted: December 04, 2015; Published: December 11, 2015
Abstract
The determination of selenium in food supplements is of major interest due to the low range between beneficial and toxic effects of this element. The total selenium content of eight commercially available food supplements were determined and compared with the labeled values. Microwave-assisted acid digestion was used for sample mineralization and the selenium was determined by high-resolution continuum source atomic absorption spectrometry with electrothermal atomization (HR-CS ETAAS) after optimization of the electro thermal behavior of selenium in the presence of different chemical modifiers, and of pyrolysis and atomization temperatures. Palladium nitrate-magnesium nitrate was selected as matrix modifier and 1050 °C and 2000 °C were the optimum pyrolysis and atomization temperatures, respectively. The LOD and LOQ were of 0.10 and 0.34 μg g-1, respectively. A 3.2 % of intra-day precision was obtained, and inter-day precision RSD did not exceeded 6.7 %. The accuracy of the method was checked with a certified reference material and showed a good agreement between the obtained results and certified selenium content (p > 0.05). The determined total selenium content of the food supplements varied between 15.4 ± 0.9 and 205.3 ± 9.9 μg/ unit, with a difference from the stated amount ranging from -12% to +14%. Also, all supplements were in compliance with the recent recommendations made by the European Community regarding the acceptable difference between labeled and measured values for minerals and vitamins in food supplements, fixed in -20% to +45% of the declared on label.
Keywords: Food supplements; Selenium; High-resolution continuum source electrothermal atomic absorption spectrometry; Label accuracy
Introduction
In the European Union (EU), the Food Supplements Directive [1] defines ‘food supplements’ as concentrated sources of nutrients or other substances with a nutritional and/or physiological effect whose purpose is to supplement the normal diet. They are marketed in a dose form (capsules, tablets, pills, powders, liquids, etc.), alone or in combination, and are designed to be taken in measured small unit amounts [1].
Food supplements are generally used to overcome nutritional deficiencies, prevent or reduce the risk of disease, and/or to promote general well-being. Generally, consumers assume these products as natural and safe, using them in addition to, or as a replacement or alternative to pharmaceuticals. However, food supplements, unlike pharmaceutical drugs, do not require approval for safety and efficacy prior to their marketing. Manufacturers and/or distributors are only obliged to notify their national competent authorities before marketing their product, and are responsible to ensure its compliance with the requirements of applicable legislation both in terms of safety and of consumer information [1]. With the widespread use of food supplements, it is essential to ensure the safety of these products for human consumption. There have been reports of the presence of impurities and the adulteration of several food supplements, lack of batch-to-batch consistency, and misformulated products [2- 4].
Selenium is an essential trace element required for the normal growth, development and metabolism of both humans and animals [5]. Selenium is an integral part of important selenoproteins, including Glutathione Peroxidases (GPx), an antioxidant enzyme that protects cell membranes from free radicals damage, iodothyronine deiodinases, involved in the thyroid hormone metabolism, and thioredoxin reductase that, in conjunction with the compound thioredoxin, participates in the regeneration of antioxidants from their oxidized forms, regulating cell growth and viability [6,7]. Prospective studies provide some evidence that selenium intakes of 200-300 μg/ day may prevent certain cancers [8,9] and cardiovascular disease [10], and improve immune response and male fertility [11,12].
The selenium content of foods and fodders depends on their geographical origin and the respective selenium content and availability of the soil. Consequently, the selenium intake by humans varies considerably between countries and regions [13]. Some European countries, including Portugal, register selenium dietary levels below RDA guidelines [13-15]. Although evident selenium deficiencies are rare, suboptimal selenium status can lead to cancer, heart disease, and an impaired immune system [16].
In the United States (US), the Recommended Dietary Allowance (RDA) for selenium determined by the Food and Nutrition Board of the Institute of Medicine is 55 μg/day for both men and women [17], while in the European Union (EU), the European Food Safety Authority (EFSA), has set an Adequate Intake of 70 μg/day [18]. The Tolerable Upper Intake Level (UL) is of 400 μg / day in the US [17] and of 300 μg/ day in Europe [19].
Selenium supplementation is becoming a common practice among consumers of developed countries to compensate for dietary deficiencies and/or to prevent certain cancers and aging effects. However, in view of the narrow range between deficiency, essentiality and toxicity of selenium in human nutrition, and the documented cases of intoxication caused by selenium supplements, makes particularly important the control of these products.
Selenium has been measured in food supplements using different analytical techniques including Inductively Coupled Plasma Mass Spectrometry (ICP-MS) [20], Cathodic Stripping Voltammetry (CSV) [21], Hydride Generation Atomic Fluorescence Spectrometry (HGAFS) [22,23], and electrothermal atomic absorption spectrometry (ETAAS) [24,25]. Line source- ETAAS has been extensively employed for the elemental analysis of several matrices [26] due to its versatility, low limits of detection, and selectivity. Recently, High-Resolution Continuum Source Atomic Absorption Spectrometry (HR-CS AAS) has extended the capabilities of conventional AAS methods. Novel features like the use of a high-intensity xenon short-arc lamp as a continuum radiation source and a linear Charge-Coupled Device (CCD) array detector with 588 pixels, 200 of which are dedicated to the analytical signal, allows for the simultaneous visualization, with high resolution, of the spectral environment around the analytical line, and for an automated background correction to reduce the spectral interferences, reducing noise levels and improve the detection limits [27]. HR-CS AAS has been employed for elemental analyses of diverse matrices [28-30]. Recently, Krawczyk [31] determined macro and trace elements in multivitamin dietary supplements by HR-CS AAS, with slurry sampling.
The aim of this work was to optimize and validate a method using microwave-assisted acid digestion and HR-CS ETAAS to quantify total selenium contents in commercially available food supplements and to compare the results against the amounts referred on the supplement label.
Materials and Methods
Reagents and solutions
Ultrapure water from a Simplicity 185 system 148 (resistivity 18.2 MΩ.cm; Millipore, Belford, USA) was used for the preparation of samples and standards. Chemicals were of analytical reagent grade unless otherwise stated. Suprapur® grade nitric acid (65%) and hydrogen peroxide (30%) were obtained from Merck (Darmstadt, Germany). Selenium working standards were prepared by dilution of a 1000 mg L–1 selenium stock solution (Panreac, Barcelona, Spain). The Pd and Mg modifier solutions (10.0±0.2 g L-1 in 15% (v/v) HNO3 (Merck, Darmstadt, Germany) were made by dilution of commercially available stock solutions. A 1% (m/v) nickel nitrate solution, used as chemical modifier, was prepared by dissolving an appropriated amount of Ni(NO3)2 6H2O (Merck, Darmstadt, Germany) in water.
Sampling and sample preparation
Eight different food supplements containing selenium, for adult consumption, were purchased from local retail and herbal stores. The samples were selected to encompass different selenium species (organic and inorganic) and formulations (tablet or capsule dosage). The supplements were designated as A, B, C, D, E, F, G, and H, respectively. The specifications of the selected supplements, according to the manufacturer, are summarized in (Table 1).
Ten tablets or capsules were taken from each product, and then crushed and homogenized manually in a mortar, after carefully removing tablets film coats, if present, and the hard-gelatin of the capsules. Powdered samples were stored in screw capped vials and kept at 4°C until analysis.
Microwave-assisted digestion
Samples digestion was performed with a MARS X 1500W Microwave Accelerated Reaction System (CEM Corp., Mathews, NC, and USA) and 100 mL Teflon HP-500 Plus closed-system vessels (CEM Corporation, Matthews, NC). All glassware and plastic materials were washed with an appropriate detergent, immersed in 10% HNO3 for 24 h and rinsed with ultrapure water, prior to use.
Approximately 0.2 g of each powdered sample was weighed into 100 mL microwave Teflon vessels and 9 mL of concentrated nitric acid and 1 mL of hydroxide peroxide were added to each vessel. The vessels were left open for 15 minutes before sealing to allow samples to predigest, and were then positioned inside the microwave digestion system for a three-step microwave temperature program. First, samples were digested at 50 °C for 3 minutes, with 3 min ramp to reach the temperature. Subsequently, samples were irradiated to a temperature of 90 °C, with 10 min ramp and 10 min hold. Finally, the microwave program used included a step where samples were digested at 190°C for 20 minutes, using a ramp time of 10 minutes. Once the vessels were cooled, the digested samples were transferred to volumetric flasks and diluted to 15 mL with Milli-Q water. One reagent blank vessel was added to each batch of samples. All the experiments were performed in triplicate.
A certified reference material SELM-1 (selenium-enriched yeast) obtained from the National Research Council of Canada (NRCC) (Ottawa, Québec, Canada) and was submitted to the same procedure applied to samples.
HR-CS ETAAS measurement conditions
Selenium was determined in an Analytik 123 Jena contr AA 700 (Analytik Jena, Jena, Germany) High-Resolution Continuum Source Atomic Absorption Spectrometer (HR-CS-AAS) equipped with a transversely heated graphite furnace, a high-intensity xenon shortarc lamp (XBO 301, GLE, Berlin, Germany), a high-resolution double monochromator, and a charge-coupled device (CCD) array detector and an MPE 60 auto sampler. Pyrolytically coated graphite tubes with integrated platform (Analytik Jena, Jena, Germany) were used. Argon (99.95% purity, Linde Sogás, Portugal) was used as purge gas. The optimized electrothermal program used for selenium determination is shown in (Table 2). Quantification was performed using 5 μL of the matrix modifier solution selected and 10 μL of sample volume, sequentially pipette by the auto sampler into the graphite tube. The measurements were performed in a spectral interval of 0.2209 nm (200 pixels) around the primary selenium line 196.0267 nm (pixel 101). The integrated absorbance (Aint) was optimized and the values obtained for seven pixels (the central pixel ± 3), corresponding to the wavelength range of 7.7 pm was used. A dynamic automated background correction was used. Analytical blanks and standards were tested routinely to check instrument performance. Four replicate measurements were carried out for all solutions.
Step
Temperature
(oC)
Ramp
(oC/s)
Hold time
(s)
Ar flow rate L/min-1
Drying
90
3
20
Max
Drying
110
5
10
Max
Pyrolysis
1050
300
10
Max
Atomization
2000
1500
4
Stop
Cleaning
2450
500
4
Max
Table 2: Optimized graphite furnace program used for the determination of selenium by HR-CS ETAAS.
Statistical analysis
Data are reported as mean ± standard deviation. One-sample t-test was used to compare means. Statistical analysis was carried out using IBM SPSS Statistics for Windows, version 21.0 (IBM Corp., Armonk, New York). Significant differences were considered when p < 0.05.
Results and Discussion
Selenium determination by HR-CS ETAAS: Optimization and method validation
The electrothermal behavior of selenium in the presence of the different chemical modifiers, and pyrolysis and atomization temperatures, was optimized to maximize the absorbance signal and minimize the background and matrix interferences. The determination of selenium by ETAAS requires the use of an adequate chemical modifier to avoid elemental volatilization during the different stages of the electrothermal process, and the consequent loss of analytes. Solutions of nickel (1%), palladium (0.1%), magnesium (0.1%), and palladium-magnesium (0.1% Pd+0.05% Mg) nitrates, prepared in water from the respective commercial stock solutions, were tested during the development of this analytical method. A 50 μg L-1 selenium standard solution and four digested food supplement samples were analyzed with the referred chemical modifiers, using the default values suggested in the equipment manual for selenium analysis (provided by Analytik Jena). The best sensitivity was achieved using 5 μL of the palladium nitrate-magnesium nitrate (0.1% Pd+0.05% Mg) solution, and was further used for the optimization of the electrothermal program.
The optimization of pyrolysis and atomization temperatures was carried out automatically, using a 50 μg L-1 selenium standard solution and four different food supplements samples, in order to assure equal responses from selenium originated from selenite, selenomethionine and selenized yeast, present in the supplements. The optimum pyrolysis and atomization temperatures were 1050 °C and 2000 °C, respectively. The complete and optimized electrothermal program used for selenium determination by HR-CS ETAAS is shown in (Table 2).
Due to the features of the equipment used, the optimization of the selenium signal included also the analysis of the spectral environment, where two selenium peaks (196.027 nm and 196.026 nm) were observed. The use of these two spectral lines was carefully tested, as the sensitivity and the precision of the methods depends on the wavelength selected. Selenium signal showed two overlapping peaks, being necessary to evaluate the integrated absorbance of a standard solution for different number of pixels (Figure 1). The best precision values were achieved when 7 pixels were used, i.e. from central pixel (196.027 nm) ± 3.
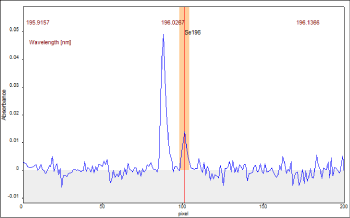
Figure 1: Wavelength-resolved absorbance spectrum obtained for sample A.
Under the optimized conditions, the linear range, the Limits of Detection (LOD) and quantification (LOQ), the precision and the accuracy for the determination of selenium by HR-CS ETAAS were assessed. Calibration curves were obtained using five selenium standard solutions (0, 12.5, 25, 50, 75, and 150 μg L-1). The correlation coefficient obtained was of 0.998. The LOD and LOQ were calculated as 3 and 10 times the standard deviation estimated from the regression line divided by the slope of the calibration curve, respectively, and taking into account the dilutions done, and are expressed in μg g-1. The LOD and LOQ for selenium were 0.10 μg g-1and 0.34 μg g-1, respectively. The intra- and inter-day precisions were evaluated by analyzing one sample (supplement E) six times, under the same experimental conditions on the same day, and on three different days. The intra-day was precision was of 3.2 % and the inter-day RSD did not exceeded 6.7 %.
The accuracy of the method was verified by the analysis of a certified reference material, SELM-1, Selenium Enriched Yeast, which had a total certified amount of selenium of 2059 ± 64 μg g-1. The material was analyzed in triplicate by the proposed method and an average concentration of 1999 ± 41 μg g-1of total selenium was obtained (98.6 ± 2.8 % recovery). No significant differences were found between the certified and the experimental values (p > 0.05), confirming the accuracy of the method for the determination of total selenium food supplements with added selenium.
Quantification of total selenium in food supplements
The market offers a plethora of food supplements with added selenium. In this work, the samples analyzed were selected to encompass supplements with different selenium chemical species and different formulations. In the samples A, B, C, and D the selenium chemical species present was the L-selenomethionine, while selenized yeast was present in samples E and F. Accordingly to their label, samples G and H contained sodium selenite. The selected samples comprised single-component selenium supplements and multi-ingredient formulations that, besides selenium, could contain minerals, vitamins, amino acids, and other antioxidants, in capsules or tablets (Table 1). The concentrations of total selenium were determined by HR-CS ETAAS, after microwave-assisted digestion of samples, being the results summarized in (Table 3). Determined selenium levels in samples varied between 15.4 ± 0.9 and 205.3 ± 9.9 μg/ unit, with a difference from the stated amount ranging from -12% to +14%. In 2012, the EU Standing Committee on the Food Chain and Animal Health published guidance establishing a tolerance threshold for nutrient values declared on nutrition labeling. This “tolerance threshold” states that the mineral content determined by official controls of food supplements should be in the range of -20 % to +45 % of the value declared on the label [32]. According to this, the differences between claimed and the selenium content determined in all supplements were acceptable.
Sample
Description
Claims
RDD
A
Selenium (L-selenomethionine) (yeast free).
No claim
1-2 tablets
B
Selenium (L-selenomethionine). Aged garlic extract, Sylibum marianum extract, green tea (powder), vitamins A, C and E, grape seed extract, pine bark extract.
Antioxidant and anti-aging
4 capsules
C
Selenomethionine. Vitamins A, C, and E, L-cysteine chloridrate, powdered extracts of green tea, red wine and pycnogenol, zinc glycinate, taurine, L-glutathione, manganese glycinate, powdered active plant base (Spirulina, Ginkgo biloba, Sylibum marianum and Gotu kola extracts), copper lysinate, riboflavin-5-phosphate.
Antioxidant
2 tablets
D
Selenium (L-selenomethionine). Vitamins A, B1, B2, B3, B, B6, B7, B11, B12, C, D and E, magnesium, zinc, chromium, manganese, copper.
Antioxidant
1 tablet
E
Selenium:brewer´s yeast.
Helps support the immune system
1 tablet
F
Selenium (yeast).Vitamins A, C and E, broccoli sprouts powder, red fruit (grape, blueberry, cranberry, cherry, strawberry and raspberry).
Antioxidant
1 tablet
G
Disodium selenium. Vitamins A, C and E.
No claim
1 tablet
H
Sodium selenite•Zinc sulphate, vitamins A, C and E.
Antioxidant
1 capsule
Table 1: Description of the analyzed food supplements. (RDD, recommended daily dose).
Sample
Declared Se content/unit (μg)
Measured Se content/ unit (μg)
% difference from labelled Se/unit level*
A
100
87.9±8.0
-12
B
17.5
15.4±0.9
-10
C
25
21.6±1.7
-14
D
62.5
70.6±3.3
+13
E
200
205.3±9.9
+3
F
100
102.0±7.3
+2
G
100
100.6±6.3
+1
H
50
49.4±4.8
-1
*Percent difference of label claim calculated as (measured Se content/ unit - Declared Se content/unit)/ Declared Se content/unit*1000.
Table 3: Total selenium contents in 8 food supplements. Experimental results as mean value ± standard deviation (n=3).
The results obtained in our study are similar to the difference range (–19% to+23%) reported by Feifer et al. [33] after analyzing five selenium supplement brands used for prostate disease. In a previous study, eight different commercial supplements were analyzed by GFAAS and results found were also in good agreement with the labeled content [24]. B’Hymer et al. [20] have also obtained total selenium levels reasonably close to the labeled content of six different brands of yeast-based supplements (91 to 111% of the label claim), analyzed using microwave digestion and ICP-MS. A few studies have identified discrepancies between stated and actual contents in selenium supplements. Valiente et al. [34] analyzed three different selenium supplements brands for total selenium by ETAAS and found significant differences between tablets and between batches of the same brand, with differences over +300% in one brand. More recently, a work by Stibilj et al. [35] revealed that 2 of the 9 food supplements containing selenium analyzed by HG-AFS did not comply with US Pharmacopoeia and that there was a high variability between different batches of the same brand. In the same year, Veatch et al. [36] analyzed 15 food supplements based on selenized yeast and selenate by neutron activation analysis technique and verified that there were significant differences between the labeled and determined selenium levels.
The Recommended Daily Dose (RDD) suggested by the food supplements producers was also in accordance with the RDA and the Tolerable Upper Intake Level guidelines for selenium. When consumed according to the RDD, all supplements exceed the RDA (55μg/ day), with exception of supplement C. Nevertheless, none of the studied food supplements represented a realistic hazard of exceeding the UL (300-400 μg/day) [17, 19].
In recent decades the chemical species and levels of selenium present in food supplements has been the target of several studies. The control of selenium levels in these products is important not only to control label accuracy to avoid frauds, but also to avoid harmful that can be caused by selenium toxicity when ingest above the UL. In the last years, a few cases of intoxication caused by selenium based food supplements have been reported. Although improperly formulated supplements formulations is uncommon, the consequences of that can be serious. In 1996, Clark et al. [37] reported a case of intoxication in a man taking a nutritional supplement for fatigue. Although the product label specified 5 μg of selenium per six tablets, a subsequent analysis revealed a level 500-1000 times higher to the declared amount per tablet. In May 2008, 201 cases of selenium poisoning were reported by the FDA in the USA. Poisoning was caused by the ingestion of a misformulated liquid food supplement containing almost 200 times the intended concentration of selenium [3].
Conclusion
Considering the widespread use of food supplements containing selenium, and the fact that these products are not required to be compliant with the same standards enforced for pharmaceuticals, it is essential their monitoring to ensure the safety of these products for human consumption. Microwave-assisted digestion followed by analysis by HR-CS ETAAS was found to be a suitable procedure for the determination of total selenium in food supplements. The analytical method was proven to be accurate and precise, and the determined detection and quantification limits are adequate for the routine analysis of selenium in food supplements, since selenium is usually present in food supplements at concentrations >10 μg g-1. Generally, the total contents of selenium were in good agreement with the labeled average levels, with an error lower than ±15%.
Acknowledgement
This work has been supported by FCT through grant no. PEst-C/ EQB/LA0006/2013 and NORTE-07-0124-FEDER-000069- Food Science. Ivone M.C. Almeida is thankful to Fundação para a Ciência e Tecnologia for the PhD grant (SFRH/BD/66032/2009) financed by POPH-QREN and subsidised by European Science Foundation and Ministério da Ciência, Tecnologia e Ensino Superior.
References
- EU, 2010. Directive 2002/46/EC of the European Parliament and of the Council of 10 June 2002 on the approximation of the laws of the Member States relating to food supplements. Official Journal of the European Union: L136/85. 2002.
- Gilroy CM, Steiner JF, Byers T, Shapiro H, Georgian W. Echinacea and truth in labeling. Arch Intern Med. 2003; 163: 699-704.
- MacFarquhar JK, Broussard DL, Melstrom P, Hutchinson R, Wolkin A, Martin C, et al. Acute selenium toxicity associated with a dietary supplement. Arch Intern Med. 2010; 170: 256-261.
- Aldosary BM, Sutter ME, Schwartz M, Morgan BW. Case series of selenium toxicity from a nutritional supplement. Clin Toxicol (Phila). 2012; 50: 57-64.
- Bellinger FP, Raman AV, Reeves MA, Berry MJ. Regulation and function of selenoproteins in human disease. Biochem J. 2009; 422: 11-22.
- Burk RF. Selenium, an antioxidant nutrient. Nutr Clin Care. 2002; 5: 75-79.
- Gladyshev VN. Selenoproteins and selenoproteomes. Selenium: Its molecular biology and role in human health. Hatfield DL, Berry MJ, Gladyshev VN, editors. 2nd edition. In: Springer, New York. 2006; 99-114.
- Combs GF, Lu J. Selenium as a cancer preventative agent. Selenium: Its molecular biology and role in human health. Hatfield DL editors. In: Kluwer Academic 347 Publishers, Boston. 2001; 205-219.
- Brozmanová J, Mániková D, Vlčková V, Chovanec M. Selenium: a double-edged sword for defense and offence in cancer. Arch Toxicol. 2010; 84: 919-938.
- Alehagen U, Johansson P, Björnstedt M, Rosén A, Dahlström U. Cardiovascular mortality and N-terminal-proBNP reduced after combined selenium and coenzyme Q10 supplementation: A 5-year prospective randomized double-blind placebo-controlled trial among elderly Swedish citizens. Int J Cardiol. 2013; 167: 1860-1866.
- Arthur JR, McKenzie RC, Beckett GJ. Selenium in the immune system. J Nutr. 2003; 133: 1457S-1459S.
- Maiorino M, Ursini F. Oxidative stress, spermatogenesis and fertility. Biol Chem. 2002; 383: 591-597.
- Rayman MP. Food-chain selenium and human health: emphasis on intake. Br J Nutr. 2008; 100: 254-268.
- Pappa EC, Pappas AC, Surai PF. Selenium content in selected foods from the Greek market and estimation of the daily intake. Sci Total Environ. 2006; 372: 100-108.
- Ventura MG, Freitas MC, Pacheco AMG. Determination of selenium daily intakes in two small groups of the Portuguese population by replicate sample neutron activation analysis. Radioanal Nucl Chem. 2009; 281: 193-196.
- Rayman MP. The importance of selenium to human health. Lancet. 2000; 356: 233-241.
- Institute of Medicine, Selenium, in: Reference dietary intakes for vitamin C, vitamin E, selenium, and carotenoids. National Academy Press Washington, DC. 2000; 284-324.
- Scientific Opinion on Dietary Reference Values for Selenium. EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). 2014; 12: 3846.
- SCF, 2000. Opinion of the Scientific Committee on Food on the tolerable upper intake level of selenium expressed on October 19, 2000. Scientific Committee on Food (SCF), Unit C3 - Management of scientific committee II, Directorate C - Scientific Health Opinions, Health and Consumer Protection Directorate-General, European Commission, Brussels, Belgium. SCF/CS/NUT/UPPLEV/25 Final. 2000.
- B'Hymer C, Caruso JA. Evaluation of yeast-based selenium food supplements using high-performance liquid chromatography and inductively coupled plasma mass spectrometry. J Anal At Spectrom. 2000; 15: 1531-1539.
- Holak W, Specchio JJ. Determination of selenium in food supplements by differential-pulse cathodic stripping voltammetry in the presence of added copper. Analyst. 1994; 119: 2179-2182.
- Gámiz-Gracia L, Luque de Castro MD. Determination of selenium in nutritional supplements and shampoos by flow injection-hydride generation-atomic fluorescence spectrometry. Talanta. 1999; 50: 875-880.
- Smrkolj P, Stibilj V. Determination of selenium in vegetables by hydride generation atomic fluorescence spectrometr. Anal Chim Acta. 2004; 512: 11-17.
- Larsen EH, Ekelund J. Determination of Total Selenium in Nutritional Supplements and Selenised Yeast by Zeeman-effect Graphite Furnace Atomic Absorption Spectrometry. Analyst. 1989; 114: 915-917.
- Vale G, Rodrigues A, Rocha A, Rial RA, Mota AM, Gonçalves ML, et al. Ultrasonic assisted enzymatic digestion (USAED) coupled with high performance liquid chromatography and electrothermal atomic absorption spectrometry as a powerful tool for total selenium and selenium species control in Se-enriched food supplements. Food Chem. 2010; 121: 268-274.
- Taylor A, Branch S, Day MP, Patriarca M, White M. Atomic spectrometry update. Clinical and biological materials, foods and beverages. J Anal At Spectrom. 2010; 25: 453-492.
- Welz B, Becker-Ross H, Florek S, Heitmann U. High-Resolution Continuum Source AAS: The Better Way to Do Atomic Absorption Spectrometry, Wiley-VCH: Weinheim. 2005.
- Huang MD, Becker-Ross H, Florek S, Heitmann U, Okruss M, Patz CD. Determination of sulfur forms in wine including free and total sulfur dioxide based on molecular absorption of carbon monosulfide in the air-acetylene flame. Anal Bioanal Chem. 2008; 390: 361-367.
- Oliveira SR, Gomes-Neto JA, Nóbrega JA, Jones BT. Determination of macro- and micronutrients in plant leaves by high-resolution continuum source flame atomic absorption spectrometry combining instrumental and sample preparation strategies, Spectrochim. Acta Part B. 2010; 65: 316-320.
- Brandao GC, Matos GD, Ferreira SLC. Slurry sampling and high-resolution continuum source flame atomic absorption spectrometry using secondary lines for the determination of Ca and Mg in dairy products. Microchem J. 2011; 98: 231-233.
- Krawczyk M. Determination of macro and trace elements in multivitamin dietary supplements by high-resolution continuum source graphite furnace atomic absorption spectrometry with slurry sampling, J Pharm Biomed Anal. 2014; 88: 377-384.
- EU. Guidance document for competent authorities, tolerances for the control of compliance of nutrient values declared on a label with EU legislation. EU, Luxembourg. 2012.
- Feifer AH, Fleshner NE, Klotz L. Analytical accuracy and reliability of commonly used nutritional supplements in prostate disease. J Urol. 2002; 168: 150-154.
- Valiente L, Piccinna M, Ale ER, Grillo A, Smichowski P. Determination of selenium in dietary supplements by ETAAS and HG-AAS: a comparative study, At Spectrosc. 2002; 23: 129-134.
- Stibilj V, Smrkolj P, Krbavcic A. Investigation of the Declared Value of Selenium in Food Supplements by HG-AFS. Microchim Acta. 2005; 150: 323-327.
- Veatch AE, Brockman JD, Spate VL, Robertson JD, Morris JS. Selenium and nutrition: The accuracy and variability of the selenium content in commercial supplements. J Radioanal Nucl Chem. 2005; 264: 33-38.
- Clark RF, Strukle E, Williams SR, Manoguerra AS. Selenium poisoning from a nutritional supplement. JAMA. 1996; 275: 1087-1088.