
Research Article
Austin J Nutri Food Sci. 2016; 4(1): 1074.
Simplified Adequate Energy Requirements for Patients on Maintaince Hemodialysis in Taiwan: A Cross- Sectional Observation Study
Lu YJ¹, Chen TW², Chen TH³, Yang SH¹, Lin WC¹, Wu PY¹ and Yang SH¹*
¹School of Nutrition and Health Sciences, Taipei Medical University, Taiwan
²Nephrology, Taipei Medical University Hospital, Taiwan
³Nephrology, Taipei Wan Fang Hospital, Taiwan
*Corresponding author: Yang SH, School of Nutrition and Health Sciences, Taipei Medical University, No. 250, Wu-Xin Street, Taipei City, Taiwan
Received: September 28, 2015; Accepted: January 07, 2016; Published: January 11, 2016
Abstract
Background: Taiwanese studies have typically shown that the average Dietary Energy Intake (DEI) of Hemodialysis (HD) patients was lower than that recommended by the National Kidney Foundation (NKF) Kidney Disease Outcomes Quality Initiative (K/DOQI) guidelines; however, these patients did not display a poor nutritional status. This study aimed to use the energy prediction equation to estimate simplified Appropriate Energy Requirements (AER) for Taiwanese HD patients.
Design: This was a cross-sectional study with 108 HD patients. The demographic, anthropometric, and laboratory measurement data of the patients were obtained from a chart review. The dietary data were obtained from 3-day dietary records at the baseline and follow-up periods of the current study.
Result: To maintain an ideal body weight and a healthy nutritional status, the AER for males is approximately 24.6 (kcal/IBW kg/day) and the AER for females is approximately 21.0 (kcal/IBW kg/day). Based on the DEI reached AER, a comparison of the nutritional parameters between the baseline and follow-up was non-significant. Male patients older than 60 years with adequate dietary energy showed higher serum albumin values and Geriatric Nutritional Risk Index (GNRI) scores in the follow-up compared with those at the baseline.
Conclusion: The AER of Taiwanese HD patients may be lower than the recommendation of the K/DOQI guideline. We recommend an AER of approximately 21 to 25 (kcal/kg/day) to maintain an ideal body weight and a healthy nutritional status.
Keywords: Hemodialysis; Energy requirement; Equation of energy requirements; Nutritional status; Energy intake
Abbreviations
AER: Appropriate Energy Requirements; APR: Appropriate Protein Requirement; BUN: Blood Urea Nitrogen; BMI: Body Mass Index; Cr: Creatinine; DEI: Dietary Energy Intake; DRIs: Dietary Reference Intakes; ESRD: End-Stage Renal Disease; ER: Energy Requirement; GNRI: Geriatric Nutritional Risk Index; Hct: Hematocrit; HD: Hemodialysis; Hb: Hemoglobin; HE: High Energy; HP: High Protein; HOMA-IR: Homeostasis Model Assessment of Insulin Resistance; K/DOQI: Kidney Disease Outcomes Quality Initiative; LE: Low Energy; LP: Low Protein; NKF: National Kidney Foundation; nPNA: Normalization of Protein Equivalent of total Nitrogen Appearance (nPNA); PD: Peritoneal Dialysis; P: Phosphate; K: Potassium; PEW: Protein-Energy Wasting; RRT: Renal Replacement Therapy; Alb: Serum Albumin; HEMO: The National Institutes of Health-Sponsored Hemodialysis; TC: Total Cholesterol; TG: Triglyceride; USRDS: United States Renal Data System; WBC: White Blood Cell Count
Introduction
In the 2014 United States Renal Data System (USRDS) annual data report, it was showed that the size of the prevalent dialysis population (HD and Peritoneal Dialysis (PD)) increased 3.8 percent in 2012, and is now 57.4 percent larger than in 2000. In 2012, nearly 90 percent of all dialysis patients received HD. Among incident end-stage renal disease (ESRD) patients starting Renal Replacement Therapy (RRT) by HD in 2012, 84.0% had Medicare coverage [1]. In Taiwan, the total number of HD patients in 2011 was 57615 – a 3.1 percent increase from 2010 [2]. In the National Health Insurance Statistics, 2011, it was also showed broken down by the global budget payment system, the outpatient benefits claimed by dialysis was being the highest of all [3]. Because of HD population growing year by year, it would be a danger to national health and increase the burden of medical cost.
Adequate nutrition support in HD patients is one of the most important factors of increased longevity, decreased hospitalization and burden of medical cost. The NKF K/DOQI Clinical Practice Guidelines for Nutrition in Chronic Renal Failure has referenced several studies [4-8] and developed age-specific guidelines for Dietary Energy Intake (DEI) in HD patients. The recommended DEI is 30 to 35 kcal/kg/day for HD patients = 60 years old and 35 kcal/kg/day for HD patients < 60 years old [5,9,10]. However, data of the National Institutes of Health-sponsored Hemodialysis (HEMO) study (n = 1397) which compared the DEI in patients of various ages determined that the mean DEI was lower than that recommended by the K/DOQI [11], which is similar with studies of Bossola et al. and As’habi et al. [12,13]. Hung and Tarng observed that the mean DEI of Taiwanese HD patients are lower than K/DOQI energy recommendation, but not in malnutrition status [14]. Moreover, the same groups asserted that if DEI exceeds the current recommendations, it could increase adiposity and inflammation [14]. Several studies in Taiwan and Japan have determined that the range of DEI in HD patients is approximately 25–29 kcal/kg/day [14-16]. Considering the DEI less than recommendation, Milano et al. concluded that energy supplementation alone in HD patients resulted in an increase in body weight because of an increase in body fat; however, the nutritional status of the patients did not improve [17]. In summary, the DEI of HD patients commonly ranges from 21 to 29 kcal/kg/day.
The causes of the increased death rates in HD patients are multifaceted. Protein-energy wasting (PEW) is a common phenomenon in patients undergoing dialysis and a risk factor for poor clinical outcomes [18-20]. Such is the case of the “obesity paradox,” whereby a high body mass index (BMI) or body weight gain has been associated with longer survival in many studies [21-23] but not all studies [24-27] of dialysis patients. Chazot et al. concluded that despite overweigh and obese patients on maintenance HD carry a significant lower mortality risk than patients in the normal and lower BMI ranges, but also increased comorbidities [22]. Amongst these, to provide optimal nutrition care to HD patients, a clear understanding of their energy requirements is paramount [28].
Humans require adequate energy to maintain their body temperature and metabolic conditions and to expend energy during physical activities. Energy is regulated by a complex set of feedback mechanisms. Changes in energy intake or expenditure trigger metabolic and behavioral responses that restore the balance of energy in adults [29]. Stability of body weight and body composition requires that energy intake matches energy expenditure and that nutrient balance is achieved [30,31].
Because of the energy recommendations of the 2012 edition of the Dietary Reference Intakes (DRIs) in Taiwan [32,33] are lower than those in the United States [34,35], may be the energy recommendations of the K/DOQI are higher than necessary for Taiwan HD patients. In this study, we established the energy calculate equation and estimate Appropriate Energy Requirements (AER) for Taiwanese HD patients.
Materials and Methods
Study design
This was a cross-sectional observation study and it comprising patients recruited from August 2010 to March 2011. The demographic, anthropometric, and laboratory measurement data of the patients were obtained from a chart review, and the dietary data were obtained from the 3-day dietary records at baseline and followup (Month 2) periods of the current study. We derived an equation of energy prediction to calculate the AER (Figure 1). And we compared the nutritional parameters and dietary data at the baseline and followup period to determine whether the proposed AER was appropriate for maintaining a positive nutritional status. The study was approved by the Institutional Review Board of the Taipei Medical University (NO: 201005004, NO: 99053). All participants provided their written consent.
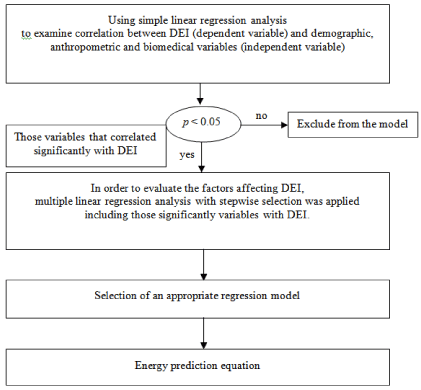
Figure 1: Model-building procedure for developing energy prediction equation
in Taiwan maintenance hemodialysis patients.
Study population
We recruited 116 HD patients from the Taipei Medical University Hospital Hemodialysis Center (n = 45) and Taipei Wan Fang Hospital Hemodialysis Center (n = 71). The patients included in the study must have been = 20 years old and on maintenance dialysis for at least 3 months, thrice a week. Patients with malignance, liver failure, liver cirrhosis, and planned surgeries, or those who were on tube-feeding regimens, were excluded.
Data collection
We obtained demographic data, such as age, sex, medical history, dialysis history, complications [diabetes, hypertension, cardiovascular disease history (coronary artery disease, ischemia heart disease, congestive heart failure, stroke, and cerebrovascular accident)], by reviewing the charts of patients. The following anthropometric parameters were measured: body height from the chart review, postdialysis body weight (UWE, TAIWAN or JWI-586, TAIWAN), and calculated BMI [weight (kg) /height (m2)]. We obtained blood samples before the HD session, after the patients fasted overnight. The serum fasting blood glucose, serum albumin (Alb) (Bromocresol green), blood urea nitrogen (BUN), creatinine (Cr), Kt/V, Normalization Oof Protein Equivalent of total Nitrogen Appearance (nPNA), Total Cholesterol (TC), Triglyceride (TG), White Blood Cell Count (WBC), Potassium (K), Phosphate (P), Hemoglobin (Hb), and Hematocrit (Hct) were measured by the laboratories of the Taipei Medical University Hospital and Taipei Wan Fang Hospital. The nPNA value was calculated using the following equation: nPNA = [pre BUN / (25.8 + 1.15/Kt/V + 56.4/Kt/V) + 0.168].
Nutritional assessment
The nutritional statuses of HD patients were evaluated based on their serum Alb, Cr, TC, nPNA, and GNRI scores. Serum Alb levels < 3.5 g/dL [7], serum Cr levels < 10 mg/dL [7], serum TC levels < 150 or > 199 mg/dL [7], nPNA levels < 1.2 g/kg [7], or GNRI scores < 91.2 [36-38] indicated a poor nutritional status. The GNRI was the simplest and most accurate index for identifying HD patients who were at nutritional risk [36-38], and the score was calculated based on the serum Alb and body weight by using the following equation: GNRI = [14.89 * Alb (g/dL)] + [41.7 * (body weight/ideal body weight]. The body weight / ideal body weight was set to 1 when the weight of the patient exceeded the ideal body weight. The ideal body weight was defined as height2 (m2) * 22. NKF K/DOQI energy recommendation was calculated by 30 kcal/bw kg/day for HD patients = 60 years old and 35 kcal/kg/day for HD patients < 60 years old.
Dietary data
We assessed the dietary data of HD patients by using a 3-day dietary record (HD day, non-HD day, and Sunday). A dietitian instructed the participants to record their food intake, including intake of condiments and beverages. They used typical tools to estimate their portion sizes [i.e., bowl (250 c.c.) / tablespoon (15 c.c.) / cup (240 c.c.)]. The dietitians used a 24-hour dietary recall to review the records with the patients. The Taiwanese food composition table was used to evaluate the nutritional intake of the patients, and we analyzed the nutrients by using the Nutritionist Edition, Enhancement plus 3, version 2009.
Statistical analysis
We performed a statistical analysis by using SAS 9.3 for windows.
We compared the variables for males and females by using a Student t-test and compared the variables at baseline and follow-up by using a Wilcoxon rank sum test. Using a simple linear regression analysis and multiple regression analysis, we derived an equation of energy and protein prediction (Figure 1). As the DEI reached the AER, we used ROC association statistics to predict the correct percentage of nutritional status (p < 0.05 was considered statistically significant). The higher percentage is represented the higher prediction disease sensitivity and lower false positive rate, which is better discrimination of this tool.
Results
Demographic, anthropometric and biomedical data of the participants
A total of 108 patients completed the current study (Figure 2). Table 1 lists the demographic, anthropometric and dietary data, and nutritional characteristics of the patients. The ages of the patients ranged from 28.1 to 94.0 years, averaging 63.5 ± 14.6 years. The average dialysis vintage was 51.1 ± 50.9 months. The complications in patients included diabetes (n = 48; 44.4%), hypertension (n = 59; 54.6%), and a history of cardiovascular disease (n = 51; 47.2%). The BMI was within the normal range (18.5 to 24 kg/m2) in 54.6% (n = 59) of the patients, whereas it indicated that only 8.3% (n = 9) of patients were underweight (< 18.5 kg/m2). The average nutritional parameters were a serum Alb level of 4.0 ± 0.4 g/dL, a serum Cr level of 10.4 ± 3.0 mg/dL, a serum TC level of 174.9 ± 45.8 mg/dL, a nPNA level of 1.1 ± 0.3 g/kg, and a GNRI score of 99.2 ± 7.7.
Total
Male
Female
p-value1
n = 108
n = 54 (50.0%)
n =54 (50.0%)
Age
63.5
±
14.6
64.4
±
13.8
62.5
±
15.4
0.504
Dialysis vintage, month
51.1
±
50.9
50.4
±
55.1
51.9
±
46.9
0.879
Diabetes3, n (%)
48 (44.4%)
27 (50.0%)
21 (38.9%)
0.245
Hypertension3, n (%)
59 (54.6%)
26 (48.1%)
33 (61.1%)
0.176
Cardiovascular disease history2,3, n (%)
51 (47.2%)
25 (46.3%)
26 (48.1%)
0.847
Anthropometric data
Dry body weight, kg
61.1
±
12.4
65.5
±
12.4
56.7
±
10.7
0.000*
Height, cm
162.1
±
8.7
167.8
±
6.5
156.5
±
6.5
< 0.000*
BMI, kg/m2
23.2
±
3.9
23.1
±
3.5
23.2
±
4.3
0.973
Dietary intake
Energy intake, kcal/day
1362.9
±
433.5
1569.1
±
427.8
1156.7
±
331.2
<0.000*
Energy intake, kcal/kg/day
22.9
±
8.0
24.5
±
7.6
21.4
±
8.2
0.040*
Protein intake, g/day
55.2
±
22.4
63.9
±
25.4
46.5
±
14.7
<0.000*
Protein intake, g/kg/day
0.9
±
0.4
1.0
±
0.5
0.9
±
0.3
0.062
Protein, % energy
16.2
±
4.0
16.4
±
4.8
16.1
±
3.0
0.682
Nutritional status
Alb, g/dL
4.0
±
0.4
4.0
±
0.4
3.9
±
0.5
0.433
Cr, mg/dL
10.4
±
3.0
11.0
±
3.1
9.9
±
2.7
0.061
TC, mg/dL
174.9
±
45.8
165.9
±
44.5
183.9
±
45.7
0.041*
nPNA, g/kg
1.1
±
0.3
1.1
±
0.3
1.2
±
0.3
0.408
GNRI score
99.2
±
7.7
99.9
±
6.8
98.6
±
8.5
0.388
Values are expressed as number, percentage, mean ± SD.
1Comparisons of demographic, anthropometric, dietary data and nutritional characteristics between male and female subjects
2Cardiovascular disease included coronary artery disease, ischemia heart disease, congestive heart failure, stroke, cerebrovascular accident.
BMI: Body Mass Index; Alb: Albumin; Cr: Creatinine; TC: Total Cholesterol; nPNA: Normalization of Protein Equivalent of total Nitrogen Appearance; GNRI: Geriatric Nutritional Risk Index
*Means significantly different at p < 0.05, Chi-square test3 (categorical variable) or Student’s t-test (continuous variable).
Table 1: Demographic, anthropometric, dietary data and nutritional characteristics on subjects.
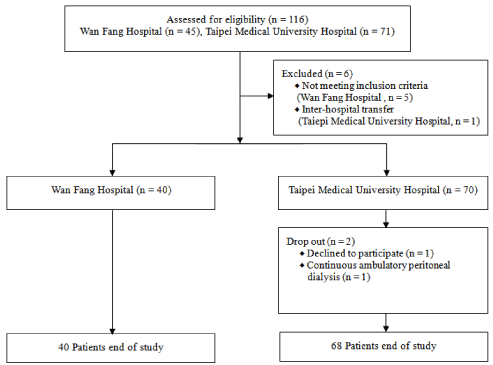
Figure 2: Study Protocol.
Dietary protein and energy intake
The average dietary protein intake (DPI) and DEI were 0.9 ± 0.4 g/kg/day and 22.9 ± 8.0 kcal/kg/day, respectively. The DEI showed a significant linear regression with the DPI (p < 0 .000) (Fig. 2). The mean DEI values in the current study (1362.9 ± 433.5 kcal/day) were significantly lower than the means of the NKF K/DOQI energy recommendations (1971.6 ± 456.3 kcal/day; p < 0.000) and only 16 patients displayed a DEI higher than those recommended by the K/ DOQI guidelines (data not shown). No significant differences were observed among participants whether or not their DEI was lower than that recommended by the K/DOQI.
Established predictive energy and protein equation
Because (1) the DEI (kcal/kg/day) shows a significant difference for weight and sex (Table 1) and (2) the DEI has a significant linear regression with DPI (p < 0.000; Fig. 3), the DEI was adjusted for sex, weight (kg), and DPI (g/kg/day) in the simple and multiple linear regression models.
In the simple linear regression analysis, the DEI was positively correlated with Cr (β = 0.3920, r2 = 0.6570, p = 0.024) and negatively correlated with age (β = -0.0833, r2 = 0.6598, p = 0.015). In the multiple linear regression analysis in which DEI was used as a dependent variable, the final model showed that sex, age, weight (kg) and DPI (g/ kg/day) were the independent determinants of DEI (Table 2). Table 2 lists the DEI prediction equation.
Dependent Variable
Independent Variable
Partial r2
Model r2
p-Value
Regression equation
DEI, kcal/kg/day
DPI, g/kg/day
0.6009
0.6009
< 0.000
DEI (kcal/kg/day) = 2.95618*Sex (Male=1, Female=0) - 0.18025*Weight (kg) + 12.27989*DPI (g/kg/day) - 0.08326*Age + 26.31441
Dry body weight, kg
0.0197
0.6206
0.022
Sex
0.0189
0.6395
0.021
Age
0.0203
0.6598
0.015
DPI, g/kg/day
Dry body weight, kg
0.1179
0.1179
0.000
DPI (g/kg/day) = 0.32479*Sex (Male=1, Female=0) - 0.01726*Weight (kg) + 0.11589*K (mEq/dL) + 1.30721
Sex
0.1051
0.2230
0.000
K, mEq/dL
0.0422
0.2652
0.016
1Adjusted for sex, weight (kg) and DPI (g/kg/day)
DEI: Dietary Energy Intake; DPI: Dietary Protein Intake; K: Potassium
p < 0.05, Multiple regression analysis with stepwise selection
Table 2: Multiple regression results for prediction of DEI (kcal/kg/day) and DPI (g/kg/day) for subjects1.
In the simple linear regression analysis, the DPI was positively correlated with the serum potassium (K) level (mEq/dL) (β = 0.1159, r2 = 0.2652, p = 0.016). In the multiple linear regression analysis in which DPI was used as a dependent variable, the final model showed that sex, weight (kg), and serum K level (mEq/dL) were the independent determinants of DPI (Table 2). Table 2 lists the DPI prediction equation.
Appropriate protein and energy requirements
The AER and appropriate protein requirement (APR) are estimated using the DEI and DPI regression equations to indicate an ideal body weight and a healthy nutritional status.
The normal range for serum K levels is 3.5–5.5 mEq/dL, “3.5” is the basic goal of the normal range. Therefore, we substituted 3.5 into the serum K level variables and the ideal body weight into weight variables, using the DPI regression prediction to determine the APR. After substituting the ideal body weight into the weight variables and APR into the DPI variables, we used a DEI regression prediction to calculate the AER. Table 3 lists the APR and AER. For maintaining an ideal body weight and a healthy nutritional status, the AER for males is approximately 24.6 kcal/kg/day and the AER for females is approximately 21.0 kcal/kg/day. For male patients < 60 years old, the AER is 24.9 kcal/kg/day; for males = 60 years old, it is 24.4 kcal/kg/ day; for females < 60 years old, it is 21.6 kcal/kg/day; and for females = 60 years old, it is 20.5 kcal/kg/day.
Dependent Variable
Total
Male
Female
n = 108 (100.0%)
n = 54 (50.0%)
n = 54 (50.0%)
AER, kcal/kg/day2
22.8
±
2.5
24.6
±
1.7
21
±
1.7
APR, g/kg/day3
0.9
±
0.1
1
±
0.1
0.8
±
0.1
(< 60 years old)
n = 47 (43.5%)
n = 23 (42.6%)
n = 24 (44.4%)
AER, kcal/kg/day2
23.2
±
2.3
24.9
±
1.4
21.6
±
1.6
APR, g/kg/day3
0.8
±
0.1
0.9
±
0.1
0.8
±
0.1
(= 60 years old)
n = 61 (56.5%)
n = 31 (57.4%)
n = 30 (55.6%)
AER, kcal/kg/day2
22.5
±
2.6
24.4
±
1.9
20.5
±
1.7
APR, g/kg/day3
0.9
±
0.1
1
±
0.1
0.8
±
0.1
1The AER is estimated from regression equations to maintain in ideal body weight and healthy nutrition status.
2AER (kcal/kg/day) = 2.95618*Sex (Male=1, Female=0) - 0.18025*Ideal body weight (kg) + 12.27989*APR (g/kg/day) - 0.08326*Age + 26.31441
3APR (g/kg/day) = 0.32479*Sex (Male=1, Female=0) - 0.01726* Ideal body weight (kg) + 0.11589*K (mEq/dL) + 1.30721
K (mEq/dL) normal values: 3.5-5.5
AER: Appropriate Energy Requirement; APR: Appropriate Protein Requirement; K: Potassium
Table 3: Appropriate energy requirement (kcal/kg/day) and appropriate protein requirement (g/kg/day) for subjects1.
< 60 years old
Male (n = 20, 87.0%)
= 24.9 kcal/kg/day
(n = 10, 50.0%)
p-value
<24.9 kcal/kg/day
(n = 10, 50.0%)
p-value
Baseline
Follow-up
Baseline
Follow-up
Dietary intake
Energy intake, kcal/kg/day
31.6
±
5.1
32.3
±
4.0
.3915
20.2
±
2.6
18.6
±
2.9
0.116
Protein intake, g/day
71.7
±
18.6
76.6
±
16.9
.3464
52.8
±
12.3
53.7
±
11.9
0.816
Protein intake, g/kg/day
1.1
±
0.2
1.2
±
0.2
.2555
0.7
±
0.2
0.8
±
0.2
0.864
Protein, % energy
14.4
±
2.3
15.0
±
1.7
.5815
14.2
±
2.2
20.7
±
14.3
0.187
Nutritional status
Alb, g/dL
4.1
±
0.4
4.1
±
0.3
.8049
4.2
±
0.3
4.2
±
0.3
0.726
Cr, mg/dL
12.1
±
4.2
12.2
±
3.8
.9437
13.5
±
2.7
13.6
±
2.5
0.826
TC, mg/dL
197.4
±
63.9
196.0
±
73.1
.7851
170.3
±
28.5
173.3
±
21.8
0.688
nPNA, g/kg
1.0
±
0.3
1.2
±
0.2
.0796
1.2
±
0.2
1.3
±
0.3
0.270
GNRI score
100.1
±
6.4
100.3
±
4.4
.8826
104.4
±
4.2
104.1
±
4.6
0.716
= 60 years old
Male (n = 26, 83.9%)
= 24.4 kcal/kg/day
(n = 8, 30.8%)
p-value
<24.4 kcal/kg/day
(n = 18, 69.2%)
p-value
Baseline
Follow-up
Baseline
Follow-up
Dietary intake
Energy intake, kcal/kg/day
33.7
±
6.5
32.6
±
7.8
.5548
19.3
±
3.2
19.2
±
3.1
0.951
Protein intake, g/day
90.3
±
32.6
83.4
±
20.0
.5460
56.5
±
20.6
54.3
±
19.0
0.344
Protein intake, g/kg/day
1.6
±
0.8
1.5
±
0.5
.5336
0.8
±
0.3
0.8
±
0.3
0.570
Protein, % energy
18.9
±
6.4
18.0
±
2.9
.5839
17.6
±
5.4
16.7
±
4.8
0.214
Nutritional status
Alb, g/dL
3.6
±
0.3
3.7
±
0.3
.0835
4.0
±
0.4
3.9
±
0.4
0.202
Cr, mg/dL
9.9
±
2.1
10.3
±
2.2
.1945
9.8
±
1.8
9.9
±
2.0
0.787
TC, mg/dL
130.6
±
32.7
137.0
±
25.7
.5239
153.0
±
42.0
167.7
±
37.8
0.092
nPNA, g/kg
1.3
±
0.3
1.1
±
0.2
.1304
1.2
±
0.2
1.1
±
0.3
0.399
GNRI score
92.3
±
4.5
94.6
±
4.0
.0665
101.4
±
6.5
100.5
±
6.4
0.189
Values are expressed as number, percentage, mean ± SD.
1The data was shown that the subjects whose DEI was higher or lower than AER was still higher or lower in follow-up compared with in baseline.
The AER was estimated form model 2 regression equation.
(< 60 years old) AER: 24.9 kcal/kg/day.
(= 60 years old) AER: 24.4 kcal/kg/day.
DEI: Dietary Energy Intake; AER: Appropriate Energy Requirement; Alb: Albumin; Cr: Creatinine; TC: Total Cholesterol; nPNA: Normalization of Protein Equivalent of total Nitrogen Appearance; GNRI: Geriatric Nutritional Risk Index
*Means significantly different at p < 0.05, Wilcoxon sign rank test.
Table 4: Comparisons of dietary intake and nutrition status by age and appropriate energy requirement between baseline and follow-up on male subjects1.
< 60 years old
Female (n = 19, 79.2%)
= 21.6 kcal/kg/day
(n = 8, 42.1%)
p-value
<21.6 kcal/kg/day
(n = 11, 57.9%)
p-value
Baseline
Follow-up
Baseline
Follow-up
Dietary intake
Energy intake, kcal/kg/day
29.9
±
5.9
28.2
±
4.9
.4652
14.1
±
3.0
16.1
±
3.2
0.036*
Protein intake, g/day
58.6
±
13.7
57.4
±
16.0
.5904
37.1
±
14.0
41.2
±
14.5
0.319
Protein intake, g/kg/day
1.1
±
0.2
1.1
±
0.3
.6183
0.6
±
0.2
0.6
±
0.2
0.319
Protein, % energy
15.5
±
3.3
20.5
±
13.0
.4405
16.0
±
3.3
15.6
±
3.6
0.598
Nutritional status
Alb, g/dL
4.1
±
0.5
4.1
±
0.4
.7318
4.2
±
0.3
4.2
±
0.3
0.916
Cr, mg/dL
12.0
±
3.7
11.9
±
3.3
.8474
11.3
±
1.0
11.0
±
1.2
0.323
TC, mg/dL
194.1
±
34.4
193.4
±
32.9
.8226
192.3
±
48.3
206.0
±
53.6
0.272
nPNA, g/kg
1.3
±
0.4
1.3
±
0.4
.6263
1.2
±
0.2
1.4
±
0.3*
0.039*
GNRI score
99.8
±
6.8
99.6
±
5.2
.8367
104.3
±
4.4
104.4
±
4.2
0.915
= 60 years old
Female (n = 22, 71.0%)
= 20.5 kcal/kg/day
(n = 11, 50.0%)
p-value
<20.5 kcal/kg/day
(n = 11, 50.0%)
p-value
Baseline
Follow-up
Baseline
Follow-up
Dietary intake
Energy intake, kcal/kg/day
30.1
±
6.7
30.7
±
6.0
.5159
15.0
±
3.7
16.1
±
4.0
0.337
Protein intake, g/day
54.5
±
13.5
60.2
±
17.8
.0818
41.1
±
9.9
46.2
±
16.3
0.361
Protein intake, g/kg/day
1.2
±
0.4
1.3
±
0.5
.0998
0.7
±
0.2
0.7
±
0.2
0.439
Protein, % energy
15.8
±
2.9
17.2
±
4.3
.1548
17.3
±
3.1
18.5
±
3.6
0.396
Nutritional status
Alb, g/dL
3.7
±
0.3
3.7
±
0.4
.5780
3.8
±
0.4
3.8
±
0.3
0.441
Cr, mg/dL
8.6
±
2.3
9.2
±
2.7
.3579
9.5
±
2.9
9.9
±
2.4
0.453
TC, mg/dL
186.7
±
54.3
180.6
±
43.7
.5467
177.0
±
55.6
189.8
±
54.4
0.035*
nPNA, g/kg
1.1
±
0.3
1.2
±
0.4
.4981
1.1
±
0.2
1.3
±
0.2
0.074
GNRI score
92.1
±
9.0
91.4
±
8.9
.5920
98.4
±
5.6
97.6
±
5.8
0.435
Values are expressed as number, percentage, mean ± SD.
1The data was shown that the subjects whose DEI was higher or lower than AER was still higher or lower after 2 months compared with baseline.
The AER was calculate form model 2 regression equation.
(< 60 years old) AER: 21.6 kcal/kg/day.
(= 60 years old) AER: 20.5 kcal/kg/day.
DEI, dietary energy intake; AER, appropriate energy requirement; Alb, albumin; Cr, creatinine; TC, total cholesterol; nPNA, normalization of protein equivalent of total nitrogen appearance; GNRI, geriatric nutritional risk index.
*Means significantly different at p < 0.05, Wilcoxon sign rank test.
Table 5: Comparisons of dietary intake and nutrition status by age and appropriate energy requirement between baseline and follow-up on female subjects1.
A comparison of nutritional parameters at the baseline and follow-up based on the DEI reached AER
As DEI reached AER, we compared the nutritional parameters at baseline and follow-up periods to determine whether the AER is appropriate for maintaining a healthy nutritional status. The result showed no significant difference between the baseline and follow-up values (Tables 4 and 5). In male HD patients = 60 years old, the Alb values and GNRI scores at the follow-up were higher compared with those at baseline; however, the difference was not significant (p = 0.084, p = 0.067). As DEI reached AER, we used the ROC association statistics to predict the correct percentage indicating a healthy nutritional status. The correct percentage for the five nutritional parameters were higher than 50% (Table 6).
Correct percentage (%)
Male
Female
Alb = 3.5 g/dL
60.9
54.8
Cr = 10 mg/dL
60.9
54.8
TC: 150-199 mg/dL
60.9
61.9
nPNA = 1.2 g/kg
60.9
59.5
GNRI = 91.2 score
60.9
59.5
Alb: Albumin; Cr: Creatinine; TC: Total Cholesterol; nPNA: Normalization of Protein Equivalent of total Nitrogen Appearance; GNRI: Geriatric Nutritional Risk Index
Table 6: The correct percentage on five nutritional parameters.
Discussion
The patients in this study had similar mean ages and serum Alb levels compared with the population of HD patients in Taiwan. According to the 2013 Medical Service Quality of Dialysis [39], Taiwanese HD patients were 62.89 ± 13.31 years old, and the mean serum Alb level was 3.9 g/dL. 10.2 percent of Taiwanese HD patients have serum Alb levels lower than 3.5 g/dL. Taipei has the highest number of HD patients in Taiwan. Therefore, the characteristics of patients in this study could represent the population of Taiwanese HD patients.
In our study, nutritional status showed no significant difference between participants whether or not their DEI was lower than the recommended K/DOQI values. Several studies showed that energy expenditure of patients undergoing maintenance HD is similar to other healthy individuals [4-6]. Some studies have demonstrated a 20% increase in energy expenditure during HD [7]. Slomowitz et al. established the requirements for DEI in a study that examined 6 HD patients [8]. These 6 HD patients ingested 25, 35, and 45 kcal/kg/day and had a DPI of 1.13 g/kg/day for 21 days each. The experts used a regression equation to indicate that the mean DEI necessary to maintain both neutral nitrogen balance and unchanging body composition was approximate to 35 kcal/kg/day [8]. Based on this study, the NKF KDOQI guideline suggests that the energy requirements (ERs) of HD patients are approximately similar to normal adults of the same age who are engaged in mild daily physical activity, as indicated in the Recommended Dietary Allowances. This energy recommendation is 35 kcal/kg/day for HD patients < 60 years old and 30 to 35 kcal/kg/day for HD patients = 60 years old [5,9,10]. However, no Asian patients were recruited in previous studies, and the body compositions and ERs differ among various ethnicities. Because of various differences in ethnicity, dietary habits, and physical activity between Taiwanese and Americans, the energy recommendations of the 2012 edition of the Dietary Reference Intakes (DRIs) in Taiwan [32,33] are lower than those in the United States [34,35]. The energy recommendation of the K/DOQI is might potentially higher than necessary for Taiwanese HD patients.
The average DEI was about 23 kcal/kg/day in current study. The majority of studies in Asia and elsewhere have demonstrated the similar result with our study, the DEI was lower than the recommended K/DOQI values [12-16,40-43]. The mean DEI of HD patients in the Europe and America area were 22.8–29.0 kcal/kg/day [44]. In Japan, in Ichikawa et al., the nutritional status and the body composition were compared among 4 groups of patients in each gender that were divided by the combination of DEI and DPI; high energy (HE)/high protein (HP), HE/low protein (LP), low energy (LE)/HP and LE/ LP groups. HE means DEI?30 kcal/kg IBW/day and HP means nPNA?1.0 g/kg IBW/day. In the two low energy groups (LE/HP and LE/LP) were approximately 24.2–25.0 kcal/kg BW/d, the mean serum albumin value is 3.7–3.8 g/dL. When they compared the serum albumin and other biochemical indices among the 4 groups, except for minor exceptions they were not significantly different [16]. In Taiwan, in Hung and Tarng, the mean DEI and albumin are 29 kcal/ kg BW/d and 3.8 g/kg BW/day at baseline, when increasing energy intakes above K/DOQI guidelines recommendations, it appears to increase body fat mass and subsequently increase homeostasis model assessment of insulin resistance (HOMA-IR) and might worsen insulin resistance and other associated metabolic disorders [14]. There are several studies proposed that strategies to attenuate adverse metabolic responses without negating the beneficial effects of increases in energy intake need to be evaluated in future studies [45- 46]. In Thus, we suggest that increasing energy intakes above the K/ DOQI guidelines are might not suitable for Taiwanese HD patients.
The DEI (kcal/kg/day) differed significantly with weight and sex (Table 1), and the DEI had a significant linear regression with DPI (P< .0001; Figure 2). This is consistent with previous studies [11,12,16,36]. The majority of previous studies have determined a significant negative correlation between age and DEI [11,12,41,47]. Morley founded that reducing food intake with aging is considered to the decrease in physical activity and early satiation that occurs in response to large meals and a decrease in snacking between meals [48], and it was similar with the study by Wakimoto and Block [49]. Some studies of the general population have shown that multiple factors, such as decreased gastric fundal compliance, lead to rapid antral filling [50], decreased ghrelin levels [51], or high basal levels of cholecystokinin [52], which are involved in the pathogenesis of anorexia of aging [12].
Our results were similar to those of the Nutritional and Inflammatory Evaluation in Dialysis (NIED) Study (n = 893), showing that DPI positively correlated with dietary potassium intake and dietary potassium intake positively correlated with serum potassium levels [53]. Kovesdy et al. used nPNA to represent DPI, determining that the nPNA was positively correlated with serum potassium level [54]. However, some previous studies have suggested that malnourished HD patients have muscle protein degradation, which leads to potassium release from the muscle protein and explains why HD patients show increased serum K concentrations [55].
To maintain an ideal body weight and a healthy nutritional status, the optimal AER is approximate to 24.6 kcal/kg/day for males and 21.0 kcal/kg/day for females. Those particpants had DEI reached AER, but they showed not significant improvement in nutritional status in the follow-up compared with the baseline. However, in male patients = 60 years old, the serum Alb values and GNRI scores in the follow-up increased from the baseline, although non-significantly. We suggest that the proposed AER is appropriate for maintaining a healthy nutritional status.
Measuring energy expenditure is the most accurate method for assessing ERs. The common methods for measuring energy expenditure are indirect calorimetry or prediction equations. Compared with indirect calorimetry, predictive equations are easily accessible, inexpensive, and require no specialized equipment [56,57]. Although numerous nutritional authorities have encouraged using the Harris and Benedict equation or the Schofield equation [58,59], these methods could reduce the level of accuracy among patients with different diseases. In Kamimura et al., both the Harris and Benedict and Schofield equations overestimated the resting energy expenditures of HD patients, but the errors were minimized by the presence of comorbidities [57]. To date, few studies have addressed the reliability of energy prediction equations for HD patients. When more recent studies were reviewed, there is a significant gap of knowledge regarding the accurate estimation of energy needs for patients undergoing HD.
Our study is advantageous because we develop population and disease-specific equations to adequately estimate the AER of HD patients Taiwan. The study populations are Taiwanese HD patients, compared with other common energy prediction equation, it will reduce the body compositions and AER differ among various ethnicities and enhance the level of accuracy among HD patients. We used the simple linear regression analysis to examine correlation between DEI (dependent variable) and demographic, anthropometric and biomedical variables (independent variable). And using multiple linear regression analysis with stepwise selection to evaluate the factors affecting DEI, then consider and adjust the factor which will reduce the level of accuracy for the result of prediction equation, selection of an appropriate regression model to derive the adequate energy prediction equation. This is the first paper to derive the energy prediction equation for HD patients in Taiwan.
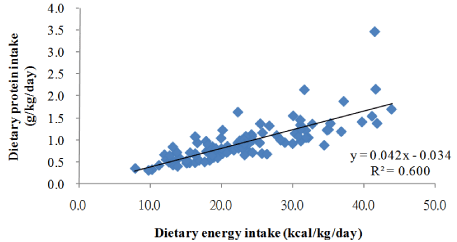
Figure 3: Correlation between dietary protein and energy intake.
This study was limited by the small number of patients, which increased the difficulty of determining significant differences and confounding factors. Future studies should use a large sample size to more accurately determine additional significant consequences. Referring to the K/DOQI guidelines, we used the DEI to develop a population- and disease-specific ER equation. However, an underreported DEI of participants could affect the accuracy of the results. Therefore, to confirm the result for Taiwanese HD patients, we suggest that future studies use indirect calorimetry to measure energy expenditure and estimate the AER.
Conclusion
For Taiwanese HD patients, we recommend AER of 21 to 25 kcal/ kg/day to maintain an ideal body weight and a healthy nutritional status, especially in HD patients = 60 years old.
Acknowledgment
The authors wish to thank all nurses of the hemodialysis center in Taipei Medical University Hospital and Wan Fang Hospital.
References
- United States Renal Data System (2014) 2014 Atlas of CKD & ESRD.
- Bureau of National Health Insurance, Department of Health, Executive Yuan, ROC 2011 Medical Service Quality of Dialysis.
- Bureau of National Health Insurance, Department of Health, Executive Yuan, ROC, The National Health Insurance Statistics, 2011.
- Monteon FJ, Laidlaw SA, Shaib JK, Kopple JD. Energy expenditure in patients with chronic renal failure. Kidney Int. 1986; 30: 741-747.
- Schneeweiss B, Graninger W, Stockenhuber F, Druml W, Ferenci P, Eichinger S, et al. Energy metabolism in acute and chronic renal failure. Am J Clin Nutr. 1990; 52: 596-601.
- Tabakian A, Juillard L, Laville M, Joly MO, Laville M, Fouque D. Effects of recombinant growth factors on energy expenditure in maintenance hemodialysis patients. Miner Electrolyte Metab. 1998; 24: 273-278.
- Ikizler TA, Wingard RL, Sun M, Harvell J, Parker RA, Hakim RM. Increased energy expenditure in hemodialysis patients. J Am Soc Nephrol. 1996; 7: 2646-2653.
- Slomowitz LA, Monteon FJ, Grosvenor M, Laidlaw SA, Kopple JD. Effect of energy intake on nutritional status in maintenance hemodialysis patients. Kidney Int. 1989; 35: 704-711.
- Cuppari L, de Carvalho AB, Avesani CM, Kamimura MA, Dos Santos Lobo RR, Draibe SA. Increased resting energy expenditure in hemodialysis patients with severe hyperparathyroidism. J Am Soc Nephrol. 2004; 15: 2933-2939.
- Clinical practice guidelines for nutrition in chronic renal failure. K/DOQI, National Kidney Foundation. Am J Kidney Dis. 2000; 35: S1-140.
- Burrowes JD, Cockram DB, Dwyer JT, Larive B, Paranandi L, Bergen C, et al. Cross-sectional relationship between dietary protein and energy intake, nutritional status, functional status, and comorbidity in older versus younger hemodialysis patients. J Ren Nutr. 2002; 12: 87-95.
- Bossola M, Muscaritoli M, Tazza L, Panocchia N, Liberatori M, Giungi S, et al. Variables associated with reduced dietary intake in hemodialysis patients. J Ren Nutr. 2005; 15: 244-252.
- As’habi A, Tabibi H, Houshiar Rad A, Nozary Heshmati B, Mahdavi-Mazdeh M, Hedayati M. Dietary assessment of hemodialysis patients in Tehran, Iran. Hemodial Int. 2011; 15: 530-537.
- Hung SC, Tarng DC. Adiposity and insulin resistance in nondiabetic hemodialysis patients: effects of high energy supplementation. Am J Clin Nutr. 2009; 90: 64-69.
- Hung SC, Tung TY, Yang CS, Tarng DC. High-calorie supplementation increases serum leptin levels and improves response to rHuEPO in long-term hemodialysis patients. Am J Kidney Dis. 2005; 45: 1073-1083.
- Ichikawa Y, Hiramatsu F, Hamada H, Sakai A, Hara K, Kogirima M, et al. Effect of protein and energy intakes on body composition in non-diabetic maintenance-hemodialysis patients. J Nutr Sci Vitaminol (Tokyo). 2007; 53: 410-418.
- Milano MC, Cusumano AM, Navarro ET, Turín M. Energy supplementation in chronic hemodialysis patients with moderate and severe malnutrition. J Ren Nutr. 1998; 8: 212-217.
- Carrero JJ, Stenvinkel P, Cuppari L, Ikizler TA, Kalantar-Zadeh K, Kaysen G, et al. Etiology of the Protein-Energy Wasting Syndrome in Chronic Kidney Disease: A Consensus Statement From the International Society of Renal Nutrition and Metabolism (ISRNM). J Ren Nutr. 2013; 23: 77–90.
- Kovesdy CP, Kalantar-Zadeh K. Why is protein-energy wasting associated with mortality in chronic kidney disease? Semin Nephrol. 2009; 29: 3-14.
- Fouque D, Kalantar-Zadeh K, Kopple J, Cano N, Chauveau P, Cuppari L, et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. 2008; 73: 391-398.
- Kalantar-Zadeh K, Abbott KC, Salahudeen AK, Kilpatrick RD, Horwich TB. Survival advantages of obesity in dialysis patients. Am J Clin Nutr. 2005; 81: 543-554.
- Chazot C, Gassia JP, Di Benedetto A, Cesare S, Ponce P, Marcelli D. Is there any survival advantage of obesity in Southern European haemodialysis patients? Nephrol Dial Transplant. 2009; 24: 2871–2876.
- Kalantar-Zadeh K, Streja E, Kovesdy CP, Oreopoulos A, Noori N, Jing J, et al. The obesity paradox and mortality associated with surrogates of body size and muscle mass in patients receiving hemodialysis. Mayo Clin Proc. 2010; 85: 991–1001.
- Kaizu Y, Tsunega Y, Yoneyama T, Sakao T, Hibi I, Miyaji K, et al. Overweight as another nutritional risk factor for the long-term survival of non-diabetic hemodialysis patients. Clin Nephrol. 1998; 50: 44-50.
- Wong JS, Port FK, Hulbert-Shearon TE, Carroll CE, Wolfe RA, Agodoa LY, et al. Survival advantage in Asian American end-stage renal disease patients. Kidney Int. 1999; 55: 2515-2523.
- de Mutsert R, Snijder MB, van der Sman-de Beer F, Seidell JC, Boeschoten EW, Krediet RT, et al. Association between body mass index and mortality is similar in the hemodialysis population and the general population at high age and equal duration of follow-up. J Am Soc Nephrol. 2007; 18: 967-974.
- Cabezas-Rodriguez I, Carrero JJ, Zoccali C, Qureshi AR, Ketteler M, Floege J, et al. Influence of body mass index on the association of weight changes with mortality in hemodialysis patients. Clin J Am Soc Nephrol. 2013; 8: 1725- 1733.
- Byham-Gray L, Parrott JS, Ho WY, Sundell MB, Ikizler TA. Development of a predictive energy equation for maintenance hemodialysis patients: a pilot study. J Ren Nutr. 2014; 24: 32-41.
- Cuppari L, Ikizler TA. Energy balance in advanced chronic kidney disease and end-stage renal disease. Semin Dial. 2010; 23: 373-377.
- Jéquier E, Tappy L. Regulation of body weight in humans. Physiol Rev. 1999; 79: 451-480.
- Mehrotra R, Kopple JD. Nutritional management of maintenance dialysis patients: why aren’t we doing better? Annu Rev Nutr. 2001; 21: 343-379.
- Bureau of National Health Insurance, Department of Health, Executive Yuan, ROC. Daily Food Guide, 2012.
- Food and Drug Administration, Department of Health, Executive Yuan, ROC. 2012 edition of the Dietary Reference Intakes (DRIs). Taipei, ed. Wei Pai Wen. In: Energy recommendation. 2012: 10-26.
- The National Academies Press, Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients), Energy. 2005; 107-264.
- U.S. Department of Agriculture 2010 Dietary Guidelines for Americans, 2012; 14.
- Yamada K, Furuya R, Takita T, Maruyama Y, Yamaguchi Y, Ohkawa S, et al. Simplified nutritional screening tools for patients on maintenance hemodialysis. Am J Clin Nutr. 2008; 87: 106-113.
- Kobayashi I, Ishimura E, Kato Y, Okuno S, Yamamoto T, Yamakawa T, et al. Geriatric Nutritional Risk Index, a simplified nutritional screening index, is a significant predictor of mortality in chronic dialysis patients. Nephrol Dial Transplant. 2010; 25: 3361-3365.
- Ferng SH, Chen MY, Fan WC. Use of the Geriatric Nutritional Risk Index for Nutritional Screening in Patients on Maintenance Hemodialysis. Acta Nephrologica. 2013; 27: 41-47.
- Bureau of National Health Insurance, Department of Health, Executive Yuan, ROC 2013 Medical Service Quality of Dialysis.
- Loerenzo V, De Bonis E, Rufino M, Hernandez D, Rebollo S, Rodriguez A, et al. Caloric rather than protein deficiency predominates in stable chronic haemodialysis patients. Nephrol Dial Transplant. 1995; 10: 1885-1889.
- Kalantar-Zadeh K, Kopple JD, Deepak S, Block D, Block G. Food intake characteristics of hemodialysis patients as obtained by food frequency questionnaire. J Ren Nutr. 2002; 12: 17-31.
- Burrowes JD, Larive B, Cockram DB, Dwyer J, Kusek JW, McLeroy S, etal. Effects of dietary intake, appetite, and eating habits on dialysis and nondialysis treatment days in hemodialysis patients: Cross-sectional results from the HEMO study. J Renal Nutr. 2003; 13: 191-198.
- Bovio G, Montagna G, Brazzo S, Piazza V, Segagni S, Cena H. Energy balance in haemodialysis and peritoneal dialysis patients assessed by a 7-day weighed food diary and a portable armband device. J Hum Nutr Diet. 2013; 26: 276-285.
- Rocco MV, Paranandi L, Burrowes JD, Cockram DB, Dwyer JT, Kusek JW, et al. Nutritional status in the HEMO Study cohort at baseline. Hemodialysis. Am J Kidney Dis. 2002; 39: 245-256.
- Kwan BC, Murtaugh MA, Beddhu S. Associations of body size with metabolic syndrome and mortality in moderate chronic kidney disease. Clin J Am Soc Nephrol. 2007; 2: 992-998.
- O’Keefe JH, Gheewala NM, O’Keefe JO. Dietary strategies for improving post-prandial glucose, lipids, inflammation, and cardiovascular health. J Am Coll Cardiol. 2008; 51: 249-255.
- Thunberg BJ, Swamy AP, Cestero RV. Cross-sectional and longitudinal nutritional measurements in maintenance hemodialysis patients. Am J Clin Nutr. 1981; 34: 2005-2012.
- Morley JE. Decreased food intake with aging. J Gerontol A Biol Sci Med Sci. 2001; 56 Spec No 2: 81-88.
- Wakimoto P, Block G. Dietary intake, dietary patterns, and changes with age: an epidemiological perspective. J Gerontol A Biol Sci Med Sci. 2001; 56 Spec No 2: 65-80.
- Rayner CK, MacIntosh CG, Chapman IM, Morley JE, Horowitz M. Effects of age on proximal gastric motor and sensory function. Scand J Gastroenterol. 2000; 35: 1041-1047.
- Rigamonti AE, Pincelli AI, Corrà B, Viarengo R, Bonomo SM, Galimberti D, et al. Plasma ghrelin concentrations in elderly subjects: comparison with anorexic and obese patients. J Endocrinol. 2002; 175: R1-5.
- MacIntosh CG, Andrews JM, Jones KL, Wishart JM, Morris HA, Jansen JB, et al. Effects of age on concentrations of plasma cholecystokinin, glucagon-like peptide , and peptide YY and their relation to appetite and pyloric motility. Am J Clin Nutr. 1999; 69: 999-1006.
- Noori N, Kalantar-Zadeh K, Kovesdy CP, Murali SB, Bross R, Nissenson AR, et al. Dietary potassium intake and mortality in long-term hemodialysis patients. Am J Kidney Dis. 2010; 56: 338-347.
- Kovesdy CP, Regidor DL, Mehrotra R, Jing J, McAllister CJ, Greenland S, et al. Serum and dialysate potassium concentrations and survival in hemodialysis patients. Clin J Am Soc Nephrol. 2007; 2: 999-1007.
- Garibotto G, Barreca A, Russo R, Sofia A, Araghi P, Cesarone A, et al. Effects of recombinant human growth hormone on muscle protein turnover in malnourished hemodialysis patients. J Clin Invest. 1997; 99: 97-105.
- Haugen HA, Chan LN, Li F. Indirect calorimetry: a practical guide for clinicians. Nutr Clin Pract. 2007; 22: 377-388.
- Kamimura MA, Avesani CM, Bazanelli AP, Baria F, Draibe SA, Cuppari L. Are prediction equations reliable for estimating resting energy expenditure in chronic kidney disease patients? Nephrol Dial Transplant. 2011; 26: 544-550.
- De Lorenzo A, Tagliabue A, Andreoli A, Testolin G, Comelli M, Deurenberg P. Measured and predicted resting metabolic rate in Italian males and females, aged 18-59 y. Eur J Clin Nutr. 2001; 55: 208-214.
- Fouque D, Vennegoor M, ter Wee P, Wanner C, Basci A, Canaud B, et al. EBPG guideline on nutrition. Nephrol Dial Transplant. 2007; 22 Suppl 2: ii45- 87.