
Review Article
Austin J Nutri Food Sci. 2016; 4(2): 1078.
An Overview on Edible Vaccines and Immunization
Naeema Jan¹, Fouzia Shafi¹, Omar bin Hameed¹, Khalid Muzaffar², Shuaib Mohammad Dar², Ishrat Majid² and Nayik GA²*
¹Division of Post Harvest Technology, SKUAST-Kashmir, India
²Department of Food Engineering & Technology, SLIET, Punjab, India
*Corresponding author: Nayik GA, Department of Food Engineering & Technology, SLIET, Punjab, India
Received: March 03, 2016; Accepted: June 01, 2016; Published: June 07, 2016
Abstract
Edible vaccines offer cost-effective, easily administrable, storable and widely acceptable as bio friendly particularly in developing countries. Oral administration of edible vaccines proves to be promising agents for reducing the incidence of various diseases like hepatitis and diarrhea especially in the developing world, which face the problem of storing and administering vaccines. Edible vaccines are obtained by incorporating a particular gene of interest into the plant, which produces the desirable encoded protein. Edible vaccines are specific to provide mucosal activity along with systemic immunity. Various foods that are used as alternative agents for injectable vaccines include cereals (wheat, rice, corn) fruits (bananas) and vegetables (lettuce, potatoes, tomatoes). Thus, edible vaccines overcome all the problems associated with traditional vaccines and prove to be best substitutes to traditional vaccines.
Keywords: Edible vaccines; Transgenic plant; Traditional vaccines
Introduction
Vaccines have proved to be boon for the prevention of infectious diseases. In spite of the global immunization programme for children against the six devastating diseases, 20% of infants still remain unimmunized which lead to approximately two million unnecessary deaths per annum, particularly in the far flung and poor parts of the world [1]. This is because of the limitations on vaccine production, distribution and delivery. This problem needs to resolve in order to prevent the spread of infections and epidemics by un-immunized populations in the immunized, safe areas [2]. Immunization for certain infectious diseases, either do not exist or they are unreliable or very expensive like; immunization via DNA vaccines is substitute but is an expensive method, along with some undesirable immune responses. Besides being expensive, these vaccines pose the problem of storage and transportation, as many of them require refrigeration. Hence, there is search for easily administrable, storable, fail-safe and widely acceptable bio friendly vaccines and their delivery systems especially in developing countries. Therefore, as substitutes have to be produced for traditional vaccines, it was envisaged that plants could be promising agents for efficient production system for vaccines, which in turn gave rise to the novel concept of edible vaccines.
Concept of Edible Vaccines
Development of edible vaccines involves the process of incorporating the selected desired genes into plants and then enabling these altered plants to produce the encoded proteins. This process is known as transformation, and the altered plants are known as transgenic plants. Edible vaccines like traditional subunit vaccines consist of antigenic proteins and are devoid of pathogenic genes. Despite this advantage, traditional subunit vaccines are unaffordable and technology-intensive, require purification, refrigeration and produce poor mucosal response. Unlikely, edible vaccines would eliminate the need for trained medical personnel required for oral administration particularly in children. Production of edible vaccines is effective process and can be easily scaled up. Edible vaccines offer numerous advantages like they posses good genetic and heat stability and do not need cold-chain maintenance. Edible vaccines can be stored at the site of use thus avoiding long-distance transportation. Syringes and needles are also not required, thus reduces the incidence of various infections [3]. Important advantage of edible vaccines is elimination of contamination with animal viruses-like the mad cow disease, which is a hazard in vaccines developed from cultured mammalian cells, as plant viruses cannot infect humans. Edible vaccines act by stimulating the mucosal as well as systemic immunity, as soon they meet the digestive tract lining. This dual mechanism of action of edible vaccines provide first-line defense against pathogens attacking via mucosa, like Mycobacterium tuberculosis and carriers causing diarrhea, pneumonia, STDs, HIV etc. [1]. Oral administration of edible vaccines to mothers might prove to be useful in immunizing the fetus-in-utero by transplacental movement of maternal antibodies or the infant through breast-feeding. Edible vaccines enable the process of seroconversion in the presence of maternal antibodies, thus playing a possible role in protecting children against diseases like group-B Streptococcus, respiratory syncytial virus (RSV), etc. At present edible vaccines are produced for various human and animal diseases (measles, cholera, foot and mouth disease and hepatitis B, C and E). They can also be used to prevent exceptional diseases like dengue, hookworm, rabies, etc. by combining with other vaccination programmes enabling multiple antigen delivery. Various foods under investigation for use in edible vaccines include banana, potato, tomato, lettuce, rice, etc. [4].
Developing an Edible Vaccine
The selected gene obtained from the microbes encoding specific antigen can be handled in two different ways:
- Suitable plant virus is genetically engineered to produce the desired peptides/proteins. The recombinant virus is then incorporated into the plant, which enables it to produce a huge number of new plants from which chimeric virions are isolated and purified. The consequential edible plant vaccine can then be used for immunological applications.
- In another method, the desirable gene is incorporated with plant vector by transformation. Many other approaches have been utilized which can be categorized into following groups:
Agrobacterium mediated gene transfer
In this method, the suitable gene (recombinant DNA) is incorporated into the T-region of a disarmed Ti plasmid of Agrobacterium; a plant pathogen, which is co-cultured with the plant cells, or tissues that needs to be transformed (Figure 1). This approach is slow with lower yield however; it showed satisfactory results in dicotelydenous plants like potato, tomato and tobacco. Researches in some fields have proven this approach good in expressing the desirable traits by selected genes in several experimental animals and plants [5,6].
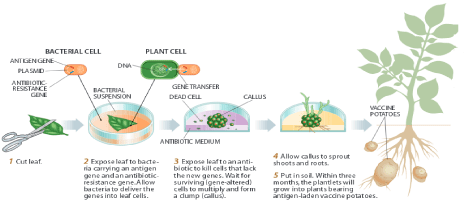
Figure 1: Development of edible vaccines from potato.
Biolistic method
This sophisticated method involves the use of gene gun that fires the gene containing DNA coated metal (e.g. gold, tungsten) particles at the plant cells [7]. Plant cells are then permitted to grow in new plants, which are later on cloned to produce ample number of crop with similar genetic composition. This approach is highly attractive due to its undependability on regeneration ability of the species as DNA is directly incorporated into cells of plant. However, requirement of expensive device particle gun adds to the major drawback to this method.
Electroporation
In this method DNA is inserted into the cells after which they are exposed to high voltage electrical pulse which is believed to produce transient pores within the plasma lemma. This approach requires the additional effort of weakening the cell wall as it acts as an effective barrier against entry of DNA into cell cytoplasm hence, it requires mild enzymatic treatment.
Mechanism of Action
Since almost all human pathogens invade at mucosal surfaces via urogenital, respiratory and gastrointestinal tracts as their leading path of entry into the body. Thus, foremost and prime line of the defense mechanism is mucosal immunity [8]. The most efficient path of mucosal immunization is oral route because oral vaccines are able to produce mucosal immunity, antibody mediated immune response and cell mediated immune response. As an advantage orally administered antigen containing plant vaccine do not get hydrolysed by gastric enzymes due to tough outer wall of the plant cell. Transgenic plants containing antigens act by the process of bioencapsulation i.e., outer rigid cell wall and are finally hydrolysed and released in the intestines. The released antigens are taken up by M cells in the intestinal lining that are placed on Payer’s patches and gut-associated lymphoid tissue (GALT). These are further passed on to macrophages and locallymphocyte populations, producing serum IgG, IgE responses, local IgA response and memory cells, that rapidly counterbalance the attack by the real infectious agent [1] (Figure 2).
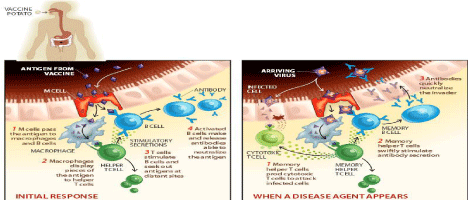
Figure 2: Mechanism action of edible vaccines produced from potato.
Advantages of Edible Vaccines
- Edible vaccines have efficient mode of action for immunization, as they do not require subsidiary elements to stimulate immune response.
- Edible vaccine unlike traditional vaccines brings forth mucosal immunity.
- Edible vaccines are comparatively cost effective, as they do not require cold chain storage like traditional vaccines [9].
- Edible vaccines offer greater storage opportunities as they seeds of transgenic plants contain lesser moisture content and can be easily dried. In addition, plants with oil or their aqueous extracts possess more storage opportunities [10].
- Edible vaccines do not need sophisticated equipments and machines as they could be easily grown on rich soils and the method is economical compared to cell culture grown in fermenters.
- Edible vaccines are widely accepted as they are orally administered unlike traditional vaccines that are injectable. Thus, they eliminate the requirement of trained medical personnel and the risk of contamination is reduced as they do not need premises and manufacturing area to be sterilized [11].
- Edible vaccines offer greater opportunity for second-generation vaccines by integrating numerous antigens, which approach M cells simultaneously
- Edible vaccines are safe as they do not contain heat-killed pathogens and hence do not present any risk of proteins to reform into infectious organism.
- Edible vaccine production process can be scaled up rapidly by breeding.
Limitations of Edible Vaccines
Following are some major drawbacks of edible vaccines,
- Individual may develop immune tolerance to the particular vaccine protein or peptide.
- Dosage required varies from generation to generation and, plant to plant, protein content, patient is age, weight, ripeness of the fruit and quantity of the food eaten.
- Edible vaccine administration requires methods for standardization of plant material/product as low doses may produce lesser number of antibodies and high doses are responsible immune tolerance.
- Edible vaccines are dependent on plant stability as certain foods cannot be eaten raw (e.g. potato) and needs cooking that cause denaturation or weaken the protein present in it [12].
- Edible vaccines are prone to get microbial infestation e.g. potatoes containing vaccine can last long if stored at 4°C while a tomato cannot last long.
- Proper demarcation line is necessary y between ‘vaccine fruit’ and ‘normal fruit’ to avoid misadministration of vaccine, which can lead to vaccine tolerance.
- Edible vaccine function can be hampered due to vast differences in the glycosylation pattern of plants and humans.
- Langridge WH. Edible vaccines. Sci Am. 2000; 283: 66-71.
- Ramsay AJ, Kent SJ, Strugnell RA, Suhrbier A, Thomson SA, Ramshaw IA. Genetic vaccination strategies for enhanced cellular, humoral and mucosal immunity. Immunol Rev. 1999; 17: 27-44.
- Webster DE, Thomas MC, Strugnell RA, Dry IB, Wesselingh SL. Appetising solutions: an edible vaccine for measles. Med J Aust. 2002; 176: 434-437.
- Giddings G, Allison G, Brooks D, Carter A. Transgenic plants as factories for biopharmaceuticals. Nat Biotechnol. 2000; 18: 1151-1155.
- Mercenier A, Wiedermann U, Breiteneder H. Edible genetically modified microorganisms and plants for improved health. Curr Opin Biotechnol. 2001; 12: 510-515.
- Chikwamba R, Cunnic J, Hathway D, McMurary J, Mason H. A functional antigen in a practical crop: LT-B producing maize protects mice against E.coli heat liable enterotoxin (LT) andchorea toxin (CT). Transl Res. 2002; 11: 479-493.
- Taylor NJ, Fauquet CM. Microparticle bombardment as a tool in plant science and agricultural biotechnology. DNA Cell Biol. 2002; 21: 963-977.
- Arakawa T, Yu J, Chong DK, Hough J, Engen PC, Langridge WH. A plant based cholera toxin B subunit-insulin fusion protein protects against the development of autoimmune diabetes. Nat Biotechnol. 1998: 16; 934-938.
- Nochi T, Takagi H, Yuki Y, Yang L, Masumura T, Mejima M, et al. Rice-based mucosal vaccine as a global strategy for cold-chain- and needle-free vaccination. Proc Natl Acad Sci U S A. 2007; 104: 10986-10991.
- Pascual DW. Vaccines are for dinner. Proc Natl Acad Sci U S A. 2007; 104: 10757-10758.
- Streatfield SJ, Jilka JM, Hood EE, Turner DD, Bailey MR, Mayor JM, et al. Plant-based vaccines: unique advantages. Vaccine. 2001; 19: 2742–2748.
- Moss WJ, Cutts F, Griffin DE. Implications of the human immunodeficiency virus epidemic for control and eradication of measles. Clin Infect Dis. 1999; 29: 106-112.
- Ruf S, Hermann M, Berger IJ, Carrer H, Bock R. Stable genetic transformation of tomato plastids & expression of a foreign protein in fruit. Nat Biotechnol. 2001; 19: 870-875.
- Nemchinov LG, Liang TJ, Rifaat MM, Mazyad HM, Hadidi A, Keith JM. Development of a plant-derived subunit vaccine candidate against hepatitis C virus. Arch Virol. 2000; 145: 2557-2573.
- Modelska A, Dietzschold B, Sleysh N, Fu ZF, Steplewski K, Hooper DC, et al. Immunization against rabies with plant-derived antigen. Proc Natl Acad Sci U S A. 1998; 95: 2481-2485.
- Yusibov V, Hopper DC, Spitsin SV, Fleysh N, Kean RB. Expression in plant and immunogenicity of plant virus based experimental rabies vaccines. Vaccine. 2002; 20: 3155-3164.
- Richter LJ, Thanavala Y, Arntzen CJ, Mason HS. Production of hepatitis B surface antigen in transgenic plants for oral immunization. Nat Biotechnol. 2000; 18: 1167-1171.
- Moffat AS. Exploring transgenic plants as a new vaccine source. Science. 1995; 268: 658, 660.
- Daniell H, Khan MS, Allison L. Milestones in chloroplast genetic engineering: an environmentally friendly era in biotechnology. Trends Plant Sci. 2002; 7: 84-91.
- Prakash CS. Edible vaccines and antibody producing plants. Biotechnol Develop Mon. 1996; 27: 10-13.
Challenges
General challenges
Many different challenges are confronted before developing a plant-based vaccine. However, it has been proved in three successful human clinical trials that sufficient doses of antigen can be achieved with plant-based vaccines [11]. But, to determine dose, following considerations need to be born in mind viz. person’s weight, age; fruit/plants size, ripeness and protein content. The quantity to be eaten is important, particularly in infants, who might spit it, eat only a part or eat it whole and throw it up afterwards. Lesser dosage fails to produce sufficient antibodies, and higher dosage may lead to tolerance. Practically it would be more appropriate to concentrate the vaccine into a teaspoonful of baby food rather than incorporating it in a whole fruit. The transgenic plants can further be made available in various shapes like; pills, puddings, chips, etc. Trials to enhance the quantity of antigens produced present a challenge in the form of under developed growth of plants and reduced tuber/fruit formation, because many m-RNA from the transgene lead to gene-silencing in plant genome.
One of the approaches to overcome above mentioned challenges is listed as follows:
Expressing the plant nuclear genetic material in; plastids [13] foreign genes [14] fused protein coats [15] and by standardizing and optimizing the coding sequence of bacterial/viral genes.
Non scientific challenges
Albeit, edible vaccine production is focused in the developing countries, which is basic, reason of poor research in this field because smaller organizations invest in it as larger companies are engaged in livestock market than human application. Also very, few number of international and local government organizations support which mostly remains underfunded. Many of the organizations have lost interest in edible vaccines research due to unavailability of investors, assurance in returns on investments, grants, research aid and financial support. Also the already available inject able vaccines for diseases like tetanus, diphtheria etc. provide lesser opportunity to develop edible vaccines for them as recombinant vaccines are so cheap now.
Examples of Edible Vaccines
Transgenic potatoes for diarrhea
The first successful human trial for an edible vaccine was conducted in year 1997 in which volunteers were fed transgenic potatoes, which possessed the b-subunit of the E. coli heat-labile toxin, responsible for diarrhea. A 4-fold increase in serum antibodies 1999 was manifested in ten out of the 11 volunteers [1]. Next clinical trial took place at the Boyce Thompson Institute at Cornell University, USA, in which 20 volunteers ate the potatoes containing the Norwalk virus (responsible for vomiting and diarrhea), out of which19 showed an immune response [1]. Potato-based edible vaccine has a major drawback that it needs to be eaten as raw because cooking causes denaturation of protein and makes it uneffective.
Transgenic tomatoes against diarrhea
Transgenic tomatoes were produced at the Cornell University, in the US, against the Norwalk virus, responsible agent for severe diarrhea. The transgenic tomatoes are capable to produce surface protein specific to the virus and it has been shown that mice fed with transgenic tomatoes showed an immune response towards the virus.
Other transgenic plants
Presently, banana is being exploited as a good source for edible vaccine production because of it’s two major advantages it does not require cooking and is locally grown plant. However, the protein expression in transgenic banana is tissue specific promoter dependent. Several other examples involve rabies glycoprotein expressed by viral vectors in spinach [16] and hepatitis B surface antigen in case of lettuce and potato [17].
Applications
Cancer therapy
Several plants have been successfully engineered to generate monoclonal antibodies that have been verified as effective cancer therapy agents. One example is that of monoclonal body in case of soyabean (BR-96) is an efficient agent that attacks doxorubicin responsible for breast cancer, ovarian cancer, colon cancer and lung tumors [18].
Birth control
Administration of TMV produces protein that is found in Mousezona pellucida (ZB3 protein) and is capable of preventing fertilization of eggs in mice due to resulting antibodies [18].
Chloroplast transformation
As the chloroplast genome cannot be transmitted with in crops via usual cross pollination due to its nature of maternal inheritance [19]. It may contribute to its transmission as well as accumulation in ample quantities in the form of transgenic protein.
Role in autoimmune diseases
In concern with autoimmune diseases, scaling up of self- antigen production in plants is underway in it’s developmental stage. Few of the diseases that are under study include; multiple sclerosis, rheumatoid arthritis, lupus and transplant rejection. In one clinical study strain of mouse susceptible to diabetes were fed with potatoes capable of expressing insulin and a protein called GAD (glutamic acid decarboxylase), linked to CT-B subunit. It has been found out that the protein proved successful in suppressing immune attack and delayed the onset of high blood sugar level [8].
Recombinant drugs/proteins
Besides, being major producers of vaccines and antibodies, plant compositions are altered by engineered viral inoculations to produce enzymes; drugs (albumin, serum protease and interferon) e.g. glucocerebrosidase (hGC) production in tobacco plants for treating Gaucher’s disease, Interleukin-10 to treat Crohn’s disease This method of production is quite cheaper and reduces the cost by thousand-fold [20]. The process of recombinant therapeutic protein production from plants has been commercialized as hirudin which is an antithrombin-anti-viral protein that inhibits the HIV virus in vitro, trichosanthin(ribosome in activator) and angiotensin-I (antihypersensitive drug) [20].
The Future of Edible Vaccines
Resistance towards GM foods presents a threat to the rising future of edible vaccines. Transgenic contamination is also a major concern which need to be addressed properly as it led United States to pay off approximately $12 billion. Edible vaccines before launching in market for human applications require certification by WHO in terms of it’s quality, efficiency and environmental effect. Despite above concerns the future of edible vaccines is reflected by enormous increase in land area used for cultivation of transgenic crops from 1.7 to 44.2 million hectares from 1996 to 2000. Also the number of countries growing them increased from 6 to 13 which predicted that transgenic crops gained wide acceptance industrially as well as in developing countries. Edible vaccines present good economical and technological benefits as more than 350 genetically engineered products are presently in progress in the United States and Canada. In the near future edible vaccine against smallpox, anthrax, plague, etc can be produced on a large scale (upto millions of doses) within a short span of time.
Conclusion
Edible plant-derived vaccines present a better possibility of safer and more efficient immunization in the future. Limitations linked with traditional vaccines, like production, distribution and delivery can be eliminated by the use of edible vaccines through various immunization programmes. Edible vaccines successfully embraced the obstacles encountered in rising vaccine technology. Despite restricted global access to health care and much attention still being paid towards complex diseases like HIV, malaria, etc. The time is not so far when there is need for an economical, safer and efficient delivery system to be developed at a larger scale in the form of edible vaccines The ray of hope is based on assumption that edible vaccines may be grown mostly in the developing countries which is basically a fact as in reality they would be used in these countries. Hence, edible vaccines provide a greater opportunity in the near future when no longer injectable needles be used but a fruitful path may be available where an individual get protected from diseases by simply eating a fruit.
Acknowledgement
The first author is very much thankful to Ishrat Majid doctoral fellow at Department of Food Engineering & Technology, SLIET Longowal, Punjab, India for helping in writing this article.
References