
Research Article
Austin J Nutri Food Sci. 2017; 5(3): 1093.
Effect of Gamma Irradiation on Phytochemical Content and Antimicrobial Activities of Selected Herbs
Shahzad N¹, Elahi R2,3* and Ali S¹
¹Department of Chemistry, Bacha Khan University, Pakistan
²Nuclear Institute for Food and Agriculture (NIFA), Pakistan
³Laboratory of Food Chemistry and Nutritional Sciences, Institute of Food Science and Technology, Chinese Academy of Agricultural Sciences, China
*Corresponding author: Elahi R, Laboratory of Food Chemistry and Nutritional Sciences, Institute of Food Science and Technology, Chinese Academy of Agricultural Sciences, China
Received: November 20, 2017; Accepted: December 13, 2017; Published: December 20, 2017
Abstract
Seven herbs by irradiating with Cobalt-60 gamma irradiation. The microbial loads i.e., total bacterial count and total fungal count and the phytochemical analysis (total phenol contents, total flavonoids contents and antioxidant activity) of both control and irradiated herbs were determined. The gamma radiation with standard dosages from 1kGy to 7 kGy decreases bacterial heaps of herbal material from 3 log cycles to 6 log cycles. A dosage of 5 kGy decrease the bacterial capacity by 4 log cycles to 7 log cycles in addition a dosage of 7 kGy removed microbial load from the whole herbal medicinal product. The more useful dosages of herbal medicinal product were expected to have in the range from 1 up to 7 kGy. The more effective gamma irradiation between 1 to 7 kGy improved the elimination of microbial load and also improved the quality of the herbal product and also enhanced the shelf life of herbal products for both the local and international market places.
Keywords: Herbal products; Gamma irradiation; Shelf life
Introduction
According to a report of WHO about 80% of world population uses herbal products in their primary form. Even today, herbal medicines have a central role in the medicinal field such as Unani medicine in Pakistan, Ayurvedic medicine in India and traditional Chinese medicine in China [1]. In Pakistan approximately there are 3,600 plant species of which over 2,900 are indigenous has a rich biodiversity. Production of herbal products therefore has a high potential in country in addition to the fact that about 33% medicines worldwide are derivative of lower and higher plants. In Pakistan, herbal medicine has a long history and tradition. The usage of herbal medicinal products is a science based approach for the prevention as well as treatment of diseases the process is called phototherapy [2].
Plant-derived substances have been getting greater attention due to their multipurpose applications in a number of industries. Aromatic and Medicinal Plants (AMPs) are considered as the richest reserves for allopathic drugs, folk medicines, food supplements, nutraceuticals, flavors, fragrances, health beverages, cosmeceuticals, pharmaceutical intermediates and chemical entities for synthetic drugs. Therefore, these are now broadly traded in raw as well as in processed forms all over the world [3].
Herbal plant constituents generally carry an excessive amount of molds and bacteria frequently originated from topsoil. Aerobic sporeforming bacteria commonly prevail whereas a wide variety of fungi as well as bacteria naturally present in the micro flora of herbs [4]. Most of the world pharmacopoeias establish different limits for microbial contamination in medicinal plants. In spite of those different groups, one of the common features is that the presence of Salmonella must not be noticed (none in 10g) [5]. Another recommendation is the detection of aflatoxin, the presence of which can be harmful to health even in small amounts [4].
About medicinal plants, it has been described that exposure to cobalt-60 gamma radiation in the range between 6 kGy to 10kGysare tolerable to sanitize cardamom, cinnamon, turmeric and fennel etc. which doesn’t affect important chemical or sensory changes [6,7]. From Egypt Aziz and coworkers (1997), examining 84 medicinal plants collected in different localities of Cairo markets, revealed that the significant dosage for removal of actynomicets as well as fungi was 5kGy for the whole observed herbal plants.
People have depended on plants as a source of medication as well as food for centuries [8]. Modern research work and practical experience has clearly revealed that treatment using medicinal plant is more valued than using synthetic chemicals for easily available being safe cost effective and for having synergistic effects. Hence, health establishments are looking for nutritional therapies and alternatives approaches. Plants have a wide variety of therapeutic activity such as free radical scavenging, antihypertensive, analgesic, anticholinergic antimicrobial, cardiovascular, anti-fertility antimalarial, stomachic etc., due to the presence of diversity of essential compounds (flavonoids, vitamins, minerals, mucilage’s, glycosides, terpenoids, organic acids, alkaloids, tannins, steroids, etc.) [9-15] in the present work we focused on the evaluation of shelf life of herbal product by irradiating with Co-60.
Materials and Methods
Plant samples
Plant materials such as Nigella sativa (black seed), Zingiber officinal (Ginger), Allium sativa (Garlic) and Carullumatubercullata (Choonga) were collected from local herbal market Peshawar, Pakistan. Similarly lemon juice, apple cider and honey were also collected from the same market. Herbs were sorted and washed thoroughly with tap water followed by drying at 40 °C in hot air oven (??????). After drying, herbs were cut into small pieces, grounded (Retch Muhle-Germany) and passed through the sieves of mesh size 30 mm. The powdered samples were packed in clear polyethylene pouches and sealed with electric sealer PFS 300 (Ladder, China).
Irradiation: Co-60 research gamma radiation source, ISSLEDIOVATE (former USSR), installed at NIFA Peshawar, was used for radiation. The plant samples were irradiated to dose levels of 1, 3, 5 and 7kGy.The irradiation was carried out at ambient conditions (20-35°C, RH 40-85%). The dose rate was 0.76 kGy per hour, as determined with a Fricke dosimeter. The irradiation time varied from 1 to 80 minutes depending upon the dose applied. After irradiation, both radiated and control samples were stored at ambient temperature until the analyses were carried out.
Preparation of extracts: The irradiated and control samples (50 g each) of plant materials were separately extracted in methanol and water 3:150 (v/v) using a Soxhlet extractor. All the extracts were filtered through what man No. 1 filter paper, combined and concentrated to dryness under reduced pressure at 45°C. The dry extracts obtained with each solvent were weighed. Extraction yields for each solvent were calculated by subtracting the dry weight of plant material residue after extraction from the weight of the original plant materials. The extracts were stored at 4°C until further processing. The filtrate was concentrated under vacuum at low temperature using a rotary evaporator.
Determination of Phenolic contents: Using the method of [16]. Method, the total phenolic contents in the extracts were determined using Folin-Ciocaultaeu reagent. The stock solution of sample was prepared by a deliberation of 1.0 mg/ml of which 40 μl were transferred to a test tube and 0.075 ml of Folin-Ciocaultaeu reagent was added that was diluted 10-fold with deionize H2O. The mixture was incubated for 5 minutes at room temperature and then 0.075 ml of 6 % (w/v) of sodium carbonate was added to this mixture. Again the mixture was incubated for 90 minutes at room temperature and the absorption was measured at 750 nm by a spectrophotometer (UVD-2950, labomed. Inc, USA). Gallic acid (0-50 mg/ml) was used as a standard. The total phenol content was said as gallic acid equals in gram each 100 gram of the samples.
Determination of total flavonoids: For total flavonoids estimation the aluminum chloride (AlCl3) colorimetric method [17] was used. Stock solutions of each plant extracts were prepared as 5000 μg/ml of methanol. Of these stock solutions 25 μl were separately mixed with 1975 μl of methanol, 100 μl of 10% aluminum chloride, 100 μl (1M) potassium acetate and 2.8 ml of distilled water to have a final concentration of each sample as 25μg/ml. The reaction mixture was then kept at room temperature for 30 minutes and absorbance was measured at 415 nm with UV-Vis double beam spectrophotometer (UVD-2950, Labomed. Inc, USA). The calibration curve was prepared by using quercetin with final concentrations of 0, 2, 4, 6 and 8 μg/ml-1 and total flavonoids contents were determined as quercetin equivalent (g/100g of the sample).
Determination of DPPH radical scavenging activity: The antioxidant activity of selected herbal ingredients was carried out according to the method of [18]. The DRSA of herbal ingredients extracts were determined in terms of hydrogen donating or radical – scavenging ability using the constant radical (1, 1 diphenyl- 2-picrylhydrazyl (DPPH) (sigma). Selected herbal material was extracted with ethanol; therefore DPPH solution was also prepared in similar solvent. Carefully, 1.0 ml of the ethanol extract (5 mg/20ml) was mixed with 2.0 ml of DPPH solution (0.159 mg/20 ml×4). After incubation (30 minutes) minutes in the dark, the absorption of solution was measured using spectrophotometer at 515 nm at room temperature. The lower absorbance of the reaction indicated lighter radical scavenging activity. Radical scavenging activity was calculated as percentage of DPPH discoloration. The following was used for determination of DPPH radical scavenging activity.
%DPPH-radical-scavenging activity (DRSA) =100 × [1-AE/AD] (1)
Whereas AE represented the absorbance of the test sample while AD represented the absorbance of DPPH solution.
Determination of total bacterial count: Using nutrient agar medium according to method of Bergmann total bacterial count of the four samples were find out by dilution plate method [19].
To perform the total bacterial count of both the non-irradiated (control) and irradiated samples the extracts of the four herbal plants i.e. Carullumatubercullata (choonga), Nigella sativa (kolonji), Zingiber officinal (Ginger) and Allium sativum (Garlic) were taken. A saline solution normally 8.5g/liter was prepared. A dilution of 1:10, 1:100 and 1:1000 was prepared consequently. 1ml of the sample from each dilution was taken with the help of pipette and poured into purified petri dishes. Sterilized nutrient agar was transferred to each petri dish. On solidification the plates were kept at inverted positions in an incubator at a suitable temperature for bacterial growth is 37 °C for 24 hour. Colonies of bacterial formed were calculated with the help of a colony counter and bacterial count per gram of sample was calculated.
Determination total fungal count: Total fungal counts of all samples were determined using dilution plate method of Bergmann using potato dextrose media [19]. In order to find total fungal count, the control and radiated extracts, 8.5 g each was dissolved in liter of saline water which give 1:10 dilution. Further required dilutions i.e. 1:100 and 1:1000 were prepared accordingly. 1ml of each dilution was poured into sterilized petri dish with the help of a sterilized pipette. Sterilized potato dextrose agar (PDA) 15-20 mL was poured into each petri dish. After cooling, oxytetracyclin was added to the medium to control the bacterial growth. The media plates were incubated at 27- 30°C for 24h. In each samples the fungal colonies were counted, and also fungal colonies per gram of sample were calculated.
Result and Discussion
The selected herbal plants include Carullumatubercullata (choonga), Zingiber officinal (Ginger), Nigella sativa (Black seed) Allium sativum (Garlic), Lemon Juice, apple cider and honey. Products derived from these herbal plants have great importance owing to their versatile applications in a number of industries. These herbal plants have rich reserves for folk medicines, allopathic drugs, nutraceuticals, fragrances, flavors, health beverages, pharmaceutical intermediates and chemical entities. These medicinal plants have a long history and tradition in Pakistan and are used for thousands of years by culture all over the world. These medicinal plants are still a central part of the medical system, such as Unani medicine in Pakistan, Ayurvedic medicine in India and traditional Chinese medicine in China. Keeping in mind the medicinal importance of above mentioned plants, the selected plants were used for the development of Cardio-NIFA.
Determination of total phenolic contents
Phenolic compounds are secondary metabolites that are derivatives of the pentose phosphate, shikimate, and phenyl propanoid pathways in living organisms. They act in defense against pathogens, animal mycophage, or fungivores aggression and as response to various abiotic stress conditions, such as rainfall and ultraviolet radiation Polyphenols have protective activity which has been previously attributed to free radical scavenging, metal chelating properties, capability of inhibiting or reducing different enzymes, such as telomerase, cyclooxygenase or lipoxygenase, and then most importantly as antioxidant compounds with the ability to trap free radicals and thus inhibit the oxidative mechanisms. The total phenolic contents were expressed in milligrams per gram of plant materials. It is evident from the results that irradiating the selected plant materials with gamma radiation of cobalt-60 has greatly affect the total phenol contents compared to control. Irradiation was performed at dose level of 1, 3, 5 and 7 kGy, respectively. The total phenol contents of Allium sativa at 1, 3, 5 and 7 kGy were 0.295 ±, 0.061 ±, 0.063 ± and 0.069 ± mg/g, respectively, while the total phenol contents in control (non-irradiated) was 0.038 ± mg/g. The total phenol contents of the gamma irradiated Zingiber officinal slightly decreased at dose levels of 1, 3, 5 and 7 kGy, respectively. The total phenol contents of Zingiber officinal at 1, 3, 5 and 7 kGy were 1.623 ± 0.005, 1.720 ± 0.006, 1.306 ± 0.008 and 1.532 ± 0.003 mg/g, respectively, while the total phenol contents in control (non-irradiated) was 1.917 ± 0.003 mg/gm. The decrease in total phenol contents of irradiated Nigella sativa extracts was observed as compared to that of the control at dose level of 1, 3, 5 and 7 kGy, respectively. The total phenol contents of Nigella sativa at 1, 3, 5 and 7 kGy were 2.340 ±, 2.243 ±, 2.667 ± and 2.042 ± mg/gram, respectively, while the total phenol contents in control (non-irradiated) was 2.825 mg/gm. similarly the total phenol contents of Carallumatubercullata was slight increased at dose levels of 1 kGy (2.79 ± 0.20) as compared to control and then decreased at high radiation levels of 3 kGy, 5 kGy and 7 kGy i.e. 2.21 ± 0.24, 1.5282 ± 0.09 and 2.127 ± 0.19 mg/g, respectively, while the total phenol contents in control (non-irradiated) was 2.50 ± 0.20 mg/ gram. The effects of cobalt-60 gamma radiation on total phenolic contents of different herbal medicinal plants are illustrated in (Figure 1) respectively. It can be noted that cobalt-60 gamma irradiation with 7.0 kGy caused slight increase in total phenolic contents in Nigella sativa and Carullumatubercullata. However the maximum increase was observed in Carullumatubercullata followed by Nigella sativa. On the other hand gamma irradiation caused reduction in the total phenolic content of Zingiber officinal followed by Allium sativum. The maximum reduction was observed in Allium sativum [20].
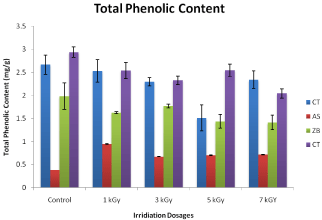
Figure 1: Effect of cobalt-60 Gamma irradiation on total phenolic content of
control and irradiated plants extracts of Carullumatubercullat (CT), Nigella
Sativa (NS), Zingiber Officinal (ZO), Allium Sativa (AS). Each data point
represents mean ± SD of triplicate treatments.
Stated that no significant effect was perceived in total phenolic in radiated tea grasses at 5 kGy; In contrast, [21] found that radiation at 1 kGy or above expressively decreased the phenolic contents in cut Chinese cabbage heads. [22]. Reported a higher content of phenolic in irradiated samples compared to non-radiated mushrooms, similarly [23] found a slight increase in total phenolic content of irradiated almond skin extracts as compared to that of the control at irradiation levels of 7 kGy and above. The dosage of 8 kGy encouraged an increase in the concentration of total phenolic complexes in raw grains from five soybean cultivars, while reduction at dose levels of 2 kGy and 4 kGy was also noticed. For irradiated samples of truffles [24] reported increase in total phenolic content at the dosage leveled of 1 kGy and 7 kGy proposed that through gamma irradiation destructive process of oxidation were capable of breaking the chemical bonds of polyphenols, liberating soluble phenols of lower molecular weight.
Estimation of total flavonoids
Flavonoids are a class of secondary plant metabolites with significant antioxidant and chelating properties. The concentration of flavonoids in plant extracts depends on the polarity of solvents used in the extract preparation. The flavonoid content was expressed in terms of quercetin equivalent in milligrams per gram of plant materials. As it is shown that in case of Nigella sativa, the control has total flavonoids 0.030 mg/g. However the total flavonoids contents were decreased when it was irradiated with Co-60 gamma radiation compared to control the flavonoids contents in N. Sativa were 0.020 ± 0.005, 0.016 ± 0.003, 0.023 ± 0.004 and 0.018 ± 0.001mg/g at 1 kGy, 3 kGy, 5 kGy and 7 kGy, respectively. The total flavonoid contents of non-irradiated (control) of Carullumatubercullata was 0.023 ± 0.003 mg/g. The decrease in total flavonoids contents of irradiated C.tubercullataextracts was observed as compared to that of the control at irradiation level of 1 kGy (0.022 ±), 3 kGy (0.023 ±), 5 kGy (0.020 ±) and 7 kGy (0.016 ±). The Table also shows the increase in total flavonoids contents of Zingiber officinal control was 0.018 mg/g. while the decrease in total flavonoids contents of irradiated Z. officinal extracts was observed as compared to that of the control at irradiation level of 1 kGy (0.011 ± 0.004), 3 kGy (0.013 ± 0.004), 5 kGy (0.012 ± 0.004) and 7 kGy (0.014 ± 0.001). Similarly the total flavonoids content of the non-irradiated (control) Allium Sativum was 0.08 ± 0.003mg/g. The increase in total flavonoids contents of irradiated Allium Sativum extracts was observed as compared to that of the control at irradiation level of 1 kGy (0.09 ±), 3 kGy (0.013 ±), 5 kGy (0.012 ±) and 7 kGy (0.08 ±). Effect of gamma irradiation of cobalt-60 on total flavonoids contents of both irradiated and nonirradiated plant materials are shown in (Figure 2) respectively. The increase in total flavonoid was observed in Zingiber officinal followed by Allium sativum and Nigella sativa and the lowest (activity) one is located in Carullumatubercullata. The result shows that the gamma irradiation has no significant tendency with the increase of total flavonoids [13]. Reported that in herbal medicinal plant soft tissues the phenolic complexes lignin, tannins, flavonoids and antioxidants precursors may conceivably achieved as Reactive Oxygen Species (ROS) scavenging complexes. The increased in total flavonoids contents is found useful for antioxidant characteristics of herbal products because of the polymerization of ingredients of phenolic components and along with the fragmentation and cross linking [25] proved that both the comparatively high scavenging activity and the higher phenolic content can be correlated, which shows good agreement with the reported work of Yang et al. The [26] verified the existing correlation between phenolic contents on the rhizome samples of Nelumbonucifera and scavenging activity. Unlikely we found a negative correlation that has been verified by both [22,27]. Reported that the gamma radiation of mushrooms dosages (freeze dried) among 2.6 ~ 20 kGy, doesn’t show a substantial changes in their anti-oxidant activity. Similarly by UN et al. [26] doesn’t found major variations in the scavenging abilities of the non-irradiated and radiated Chung kookjang and Doenjang at 5, 10 and 20 kGy.
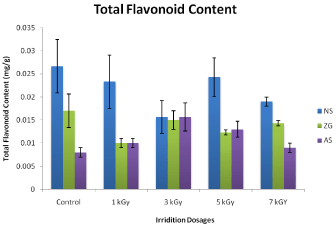
Figure 2: Effect of cobalt-60 Gamma irradiation on total flavonoids content
of control and irradiated plants extracts of Carullumatubercullat (CT), Nigella
Sativa (NS), Zingiber Officinal (ZO), Allium Sativa (AS). Each data point
represents mean ± SD of triplicate treatments.
DPPH-Radical-Scavenging Activity (DRSA)
Free radical scavenging is one of the mechanisms in inhibiting lipid oxidation commonly used to estimate antioxidant activity. The extraction pattern was consistent with total phenolic and flavonoid. The DPPH free radical scavenging activity was expressed in milligrams (mg) per gram (g) of the herbal material. The percent scavenging ability of Allium sativum non-irradiated control was 41.67 %. ± 0.73 The increase in scavenging ability of irradiated A. sativum extracts was observed as compared to that of the control at irradiation level of 1 kGy (42.42% ± 0.31) and 3 kGy (45.68% ± 0.99) respectively. Similarly a slight decrease as compared to the A. sativum control at radiation levels of 5 kGy (37.92% ± 0.25) and 7 kGy (40.67% ± 1.04) occur, respectively. The anti-oxidant ability of Zingiber officinal non-irradiated control was observed (93.11 %.± 0.61). The scavenging ability of radiated Z. official at radiation level of 1 kGy and 3 kGy was (94.61% ± 0.23) and (95.74% ± 0.79) respectively, which was increased compared to control. Similarly the scavenging percent ability of Z. officinal was decreased at radiation level of 3 kGy (92.24% ± 0.79) as compared to control of Z. officinal. It was observed that at 7 kGy (94.24% ± 0.72) again the increase in percent scavenging of Z. officinal as compared to control occurs. The effects of gamma irradiation on free radical scavenging activity of herbal plants constituents are shown in (Figures 3-5), respectively. It was observed that Zingiber officinal, Carullumatubercullata and Nigella sativa have highest anti-oxidative activity whereas the Allium sativum has lowest anti-oxidative activity. The gamma irradiation tends to decrease the anti-oxidant activity of the various deliberate methanolic extracts except the Carullumatubercullata, which is in agreement with reported work of [28], who discovered important reduction in anti-oxidant activity of dark pepper radiated at dosages 5, 7.5, 10, 20 and 30 kGy [13]. Stated that there is increase in the scavenging activity of N. sativa seeds when irradiated with gamma radiation. There are various compounds of phenol in plant tissues which are potential antioxidant, flavonoids, tannins and lignin precursor may act as ROS (reactive oxygen species) scavenging compounds [25]. Described that increase in concentration of total phenolic is favorable for antioxidant characteristics of herbal products which is attributed to the polymerization and fragmentation of consistent phenolic compounds. One can correlate the high scavenging activity herbal products to the higher phenolic content, which is in fair agreement with [26] work. They confirmed a positive correlation between phenolic contents and scavenging activity of the rhizome samples of Nelumbonucifera. On the other hand, [27] reported a negative correlation [22]. Reported that there is no significant modification in scavenging activity of irradiation of freeze dried mushrooms at doses between 2.5 and 20 kGy, respectively [7]. Observed that there is no noteworthy variation in the scavenging capabilities of control and irradiated samples at 5.0, 10 and 20 kGy, respectively, it low gamma radiation doses.
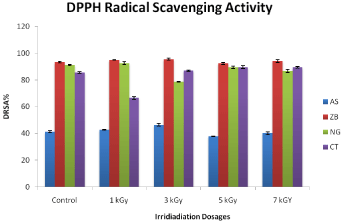
Figure 3: Effect of cobalt-60 gamma irradiation on DPPH radical scavenging
activity of control and irradiated plants extracts of Carullumatubercullat (CT),
Nigella Sativa (NS), Zingiber Officinal (ZO), Allium Sativa (AS). Each data
point represents mean ± SD of triplicate treatments.
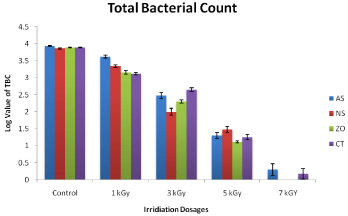
Figure 4: Effect of cobalt-60 Gamma irradiation on Total Fungal Count
(TBC) of Carullumatubercullat (CT), Nigella Sativa (NS), Zingiber Officinal
(ZO), Allium Sativa (AS). Each data point represents mean ± SD of triplicate
treatments.
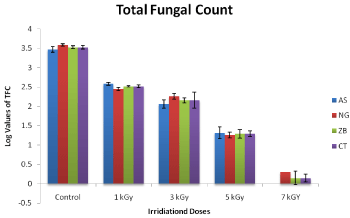
Figure 5: Effect of cobalt-60 Gamma irradiation on Total Fungal Count
(TFC) of Carullumatubercullat (CT), Nigella Sativa (NS), Zingiber Officinal
(ZO), Allium Sativa (AS). Each data point represents mean ± SD of triplicate
treatments.
Total Bacterial Counts (TBC)
The Total Bacterial Count (TBC) for the raw or un- irradiated herbal products were extremely high. The log values for Allium sativum (control) was 3.9685 which was very high. After the radiation absorbed at the level of 1kGy the log values decreased to 3.6021 which showed slight decrease in TBC as compared to control N. sativa. The log values for N. sativa decreased constantly at further radiation levels of 3kGy, 5 kGy and 7kGy as 2.3010 ±, 1.477 ± and 0.3010 ±, respectively which showed that increasing the irradiated dose level could decrease TBC significantly. Similarly in case of Nigella sativa, total bacterial count of control was extremely high. The log value for N. sativa (control) was 3.8751 which were very high. After treatment with Co-60 gamma irradiation at dose 1kGy, 3kGy, 5kGy and 7kGy the total bacterial count was significantly reduced and their log values were 3.3010 ±, 2.1761 ±, 1.3010 ± and 0.0000 respectively. Similarly in case of Zingiber officinal, total bacterial count of control was extremely high. The log value for Z. officinal (control) was 3.9081 which were very high. After treatment with Co-60 gamma irradiation at dose 1 kGy, 3 kGy, 5 kGy and 7 kGy the total bacterial count was significantly reduced and their log values were 3.176 ±, 3.3973 ±, 1.1761 ± and 0.301 ±, respectively.
Total Fungal Count (TFC)
Spoilage of herbal products by various types of fungi is also a serious storage issue which needs proper consideration. Gamma radiations of Co-60 origin are used to reduce the total fungal count in herbal products. The total fungal count was extremely high for un-radiate sample in case of Allium sativum the log value of TFC was 3.4771, which was extremely high. After irradiating with Co-60 gamma radiation the TFC values were significantly decreased. The log value at 1kGy (A. sativum was 2.6020 ± 0.001which were constantly decreased with increase in radiation dosage. At 3kGy and 5kGy the TFC of A. sativum were 2.0000 ± 0.003 and 1.761 ± 0.001, respectively, while at 7kGy the log value was 0.000. These results showed that irradiating with gamma radiation can significantly decrease the TFC of sample.
Similarly in case of N. sativa, total fungal count of control was extremely high. The log value for N. sativa (control) was 3.6021 ± 0.005 which were very high. After treatment with Co-60 gamma irradiation at dose 1kGy, 3kGy, 5kGy and 7kGy the total fungal count was significantly reduced and their log values were 2.4771 ± 0.005, 2.310 ± 0.003, 1.3010 ± 0.004 and 0.3010 ± 0.001 respectively. These results showed that irradiating with gamma radiation can significantly decrease the TFC of sample.
The Total Fungal Count (TFC) for the raw or un- irradiated herbal products were extremely high. The log value for Zingiber officinal control was 3.5441 ± 0.003 which were very high. After the radiation absorbed at the level of 1kGy the log values decreased to 2.9031 ± 0.001 which showed slight decrease in TFC as compared to control Z. officinal. The log values for Z. officinal decreased constantly at further radiation levels of 3kGy, 5 kGy and 7 kGy as 2.5441 ± 0.002, 1.379 ± 0.002 and 0.3010 ± 0.005, respectively, which showed that increasing the irradiated dose level could decrease TFC significantly.
Similarly in case of Carullumatubercullata, total fungal count of control was extremely high. The log value for C. tubercullata (control) was 3.5798 which were very high. After treatment with Co-60 gamma irradiation at dose 1kGy, 3kGy, 5kGy and 7kGy the total fungal count was significantly reduced and their log values were 2.9542 ±, 2.3979 ±, 1.3010 ± and 0.0000, respectively. These results showed that irradiating with gamma radiation can significantly decrease the TFC of sample.
Effect of irradiation on the microbial load
The control samples of Nigella sativa (black seed), Zingiber officinal, (Ginger), Allium sativa (Garlic) and Carullumatubercullata (Choonga) were contaminated by microbes. In 1998 the WHO reported the maximum permissible total count level of 1.0×104cfu/g. This high contamination level is because of great expected micro vegetation of the aromatic plant along with the common circumstances throughout their agronomy, processing, drying, harvesting, handling, storage, sales and supply. However, it has been testified that during processing the bacterial position of the herbal medicinal plants constituents is not so far affected by lesser impurity, however possibly will mostly owing to the microbial flora which herbal medicinal plants ensure [29]. This total microscopic load decreased linearly with absorbed radiation dose as shown in figures closed agreement with the reported results of [30], who described that 5 kGy radiation leads to decrease the desired microbial reduction. At 5kGy the uppermost compassion of microorganisms to cobalt-60 gamma irradiation were detected in Carullumatubercullata, whereas the lowest sensitivity was detected in Allium sativum. However at 7 kGy the micro-organisms were absent. The gamma radiation at a dosage level of 5 kGy decreased the total microbial load of herbal extracts by 98.5%, and it was totally removed at a dosage level of 7 kGy [31]. To completely decontaminate the product and to obtain the required standard the use of gamma irradiation helped it by chance. To achieve the highest standard of medicinal herbal product and food through the destruction of microorganism gamma radiation is one of the hardly any processes that allow it. Gamma irradiation kills the microbes by excruciating the molecules of water into hydrogen, hydroxyl and oxygen radicals is the primary mechanism involve these radicals combine with the microbial components like DNA and destroy or deactivate them so a result they stop their function [32]. Gamma irradiation has the ability to extend shelf life and improve the hygienic quality of the product and also increase their effectiveness on both export markets and on domestic levels. Consequences of this study has revealed that gamma radiation of the herbal products with medium to high doses significantly decrease their bacteriological loads to standard domestic and worldwide values in contrast to control or un radiated samples. To completely decontaminate the herbal product to acceptable standard effective gamma irradiation dosage from 3 kGy to 7 kGy was required. This observation might be due to non-uniformity of the microbial loads and that underlines the need for Good Manufacturing Practices (GMPs) in production protocols to ensure low microbial load of products and subsequently the use of low (effective) irradiation doses. A dose of 7 kGy was significant to remove all polluting micro flora in all the herbal products. Further deep study at 7 kGy of all the samples indicate that microbial load reduced to the level of < 103cfu/g and the examination at 5 kGy reduced the microbial load the level of < 104cfu/g. in other hand in a related examination gamma radiation amongst 1kGy-7 kGy decrease the microbial load of the herbal medicinal product less than 103cfu/g without causing any useful variations in composition and quantity of the product [33,34]. Further study of the raw herbal products at 7 kGy decreases the microbial load from 6 log cycles to 8.6 × 10 cfu/g. In the same way the microbial load of some local herbal teas decreases by 4 to 5 log cycles at 2kGy to 3kGy.
Conclusion
In Pakistan approximately there are 3,600 plant species of which over 2,900 are indigenous has a rich biodiversity. Production of herbal products therefore has a high potential in country in addition to the fact that about 33% medicines worldwide are derivative of lower and higher plants [35]. It is important to improve and modernize the herbal medicine industry for the country to break into the global market. Through the use of gamma radiation herbal product production of high quality assurance the state a vast trade marketplace like in most Asian countries. It is suggested that upcoming educations should examine the radio sensitivities of the various fungal and bacterial isolates of medicinal herbal yields with the view to their removal with low gamma radiation doses.
References
- Evans WC. Trease and Evans’ Pharmacognosy, 15th edn. London: WB Saunders, 2001.
- Joanne Barnes. Herbal Medicine, 3rdedn. Pharmaceutical Press. RPS Publishing London, UK. 2007.
- Saeed M, Muhammad N, Khan H. Assessment of Heavy Metal Content of Branded Pakistani Herbal Products. Trop J Pharm Res. 2011; 10: 499-506.
- World Health Organization. Quality control methods for medicinal plant materials. Geneva: World Health Organization. 1998.
- FarmacopeaUfficiale Della RepublicaItaliana. Drogue vegetali e preparazioni, IX. Ed. Roma: IstitutoPoligrafico e ZeccadelloStato. 1991.
- Sing L., Mohan MS., Sankarann R., Sharma TR. The use of gamma irradiation for improving quality of spices. J Food Sci Technol. 1988; 25: 357-360.
- Toofonian F, Stegeman H. Comparative effect of ethylene oxide and gamma irradiation on the chemical, sensory and microbial quality of ginger, cinnamon, fennel and fenugreek. Acta Alimentaria. 1988; 17: 271-281.
- Bach E, Flower Remedies. Alternative Medicine, The Burton Goldberg Group. Future Medicine Publishing, Inc. Will behington, USA. 1994.
- Hameed HA. The Complete Book of Home Remedies. Orient Paperbacks. New Delhi, India. 1982.
- Atta-ur-Rahman, Khattak K F, Nighat F, Shabbir M, Hemalal KD, Tillekeratne LM, et al. Dimerictropane alkaloids from Erythroxylummoonii. Phytochemistry.1998; 48: 377-383.
- Khattak KF, Atta-ur-Rahman, Choudhary MI, Hemalal KD, Tillekeratne LM, New Tropane Alkaloids from Erythroxylummoonii. Journal of Natural Products. 2002; 65: 929-931.
- Khattak KF, Atta-ur-Rahman, Choudhary MI, Nortropane Alkaloids from Erythroxylummoonii, Heterocycles. 2003; 60: 917-924.
- Khattak K F, Ihasnullah, Simpson T J. Effect of gamma irradiation on the antioxidants properties of Nigella sativa. Food Chemistry. 2008; 110: 967- 972.
- Khattak KF, Ihasnullah, Simpson TJ. Effect of gamma irradiation on the antioxidants properties of Nelumbunucifera. Radiat Phys and Chem. 2009; 78: 206-212.
- Khattak KF, Simpson TJ. Effect of gamma irradiation on the antimicrobial and free radical scavenging activities of Glycyrrhizaglabra root. Radiat Phys and Chem. 2010; 79: 507-512.
- Velioglu YSJ. Antioxidant Activity and Total Phenolics in Selected Fruits, Vegetables, and Grain Products. Food Chem. 1998; 46: 4113-4117.
- Bag GC, Grihanjali Devi P, Bhaigyabati Th. Assessment of Total Flavonoid Content and Antioxidant Activity of Methanolic Rhizome Extract of Three Hedychium Species of Manipur Valley. Int J Pharm Sci Rev Res. 2015; 30: 154-159.
- Abbasi AM, Shah MH, Li T, Fu X, Guo X, Liu RH. Ethnomedicinal values, phenolic contents and antioxidant properties of wild culinary vegetables. Ethnopharmacol. 2015; 162: 333-345.
- Bergmann U, Horne CR, Collins TJ, Workman JM, Cramer SP. Chemical dependence of interatomic X-ray transition energies and intensities – a study of Mn Kβ”and Kβ2,5 spectra. Chem Phys Lett. 1999; 302: 119-124.
- Mishra BB, Gautam S, Sharma A. Microbial decontamination of tea (Camellasinensis) by gamma radiation. J Food Sci. 2006; 71: 151 -156.
- Kim AH, Kim JH, Kim JK, Yook DH, and Byun M, Hyun JA, et al. Combined effects of irradiation and modified atmosphere packaging on minimally processed Chinese cabbage (Brassica rapa L). Food Chem. 2005; 89: 589- 597.
- Huang S, Mau J, Tsai Y. Antioxidant properties of hot water extracts from GanodermatsugaeMurrill. J Food Chem. 2007; 105: 1702-1710.
- Harrison K, Were LM. Effect of gamma irradiation on total phenolic content yield and antioxidant capacity of Almond skin extracts. Food Chem. 2007; 102: 932-937.
- Adamo M, Capitani D, Mannina L, Cristinzio M, Ragani P, Tata A, et al. Radiat Phys Chem. 2001; 71: 165-168.
- Stajner D, Milosevic M, Popvic BM. Irradiation Effects on Phenolic Content, Lipid and Protein Oxidation and Scavenger Ability of Soybean Seeds. Int J Mol Sci. 2007; 8: 618-627.
- Yang D, Wang Q, Jiang J, Ying T. Antioxidant activities of various extracts of lotus (Nelumbonuficera Gaertn) rhizome. Asia Pac. J Clin Nutr. 2007; 16: 158-163.
- Zielinski H, Kozlowska H. Antioxidant Activity and Total Phenolics in Selected Cereal Grains and Their Different Morphological Fractions. Journal of Agric Food Chem. 2000; 48: 2008-2016.
- Suhaj M, Racova J, Polovka M, Brezova V. Effect of gamma irradiation on antioxidant activity of black pepper (Piper nigrumL). Food Chem. 2006; 97: 696-704.
- AbouDonia MA. Microbiological quality and aflatoxinogenesis of Egyptian spices and medicinal plants. GlobalVeterinaria. 2008; 2: 175-181.
- Nemtanu MR, Brasoveanu M, Minea R, Grecu MN, Alblescu M, Mirtu E. Micro-biodecontamination of Spirulinaplatensis and green coffee using accelerated electron beams. Romanian J Biophys. 2006; 16:141-148.
- Karem AH, Attia SH, Doaa A. Improving microbiological quality of certain medicinal plants by using gamma irradiation. Egypt J Rad Sci Applic. 2002; 15: 81-96.
- Niemira BA, Sommers CH. New Applications in Food Irradiation. In Heldman DR. Encyclopedia of Agricultural, Food and Biological Engineering. 2006.
- Farkas J. Irradiation of Dry Food Ingredients. CRC Press Inc. Boca Raton Florida. 1988; 39-44: 67-69.
- Migdal W, Owczarczcyk B. The Effect of Ionizing Radiation on Microbiological Decontamination of Medical Herbs and Biologically Active Compounds. Radiat Phys Chem. 1998; 52: 91-94.
- Sofowora A, Ogunbodede E, Onayade A. The Role and Place of Medicinal Plants in the Strategies for Disease Prevention. Afr J Tradit Complement Altern Med. 2013; 10: 210-229.