
Special Article – Food Safety
Austin J Nutri Food Sci. 2018; 6(1): 1099.
Glycemic and Insulinemic Response to Ingestion of a Novel Food Bar Containing Whey Protein and Isomalto- Oligosaccharides
Grubic TJ¹, Sowinski R1,2, Dalton R¹, Collins PB¹, Reyes AG1,2, Favot CJ¹, Rasmussen C¹, Greenwood M1,2, Murano PS2, Earnest CP1,3 and Kreider RB1,2*
¹Department of Health & Kinesiology, Texas A&M University, USA
²Department of Nutrition and Food Sciences, Texas A&M University, USA
³Nutrabolt, 3891 S. Traditions Drive, USA
*Corresponding author: Kreider RB, Exercise and Sport Nutrition Lab, 118 Human Clinical Research Facility, Texas A&M University, College Station, Texas, 7743-4253 USA
Received: December 20, 2017; Accepted: February 22, 2018; Published: March 01, 2018
Abstract
This study examined the glucose and insulin response of ingesting a novel protein bar using a plant fiber (isomalto-oligosaccharides, IMO) as the carbohydrate source. In a randomized and crossover manner, 20 healthy men and women (Study 1) donated fasting blood samples prior to ingesting a Food Bar (FB) containing 20 g of a whey protein blend, 25 g of carbohydrate (13 g IMO, 4 g sugar, 8 g fiber), and 7 g of fat (1.5g saturated) or 25 g of dextrose (PLA). The experiment was repeated 7 to 10 days later while ingesting the alternative treatment. In study 2, 10 fasted individuals participated in the same experiment while ingesting 2 FB’s or 50 g of dextrose. Blood samples were taken at 10, 20, 30, 60, 90, and 120min post-ingestion while subjective ratings related to appetite and hypoglycemia were obtained at 0, 60 and 120 min. Data were analyzed by general linear model statistics and are presented as mean [95% CI] changes from baseline. Results revealed that the glycemic response to ingestion of the FB was significantly lower during the first 60 min following ingestion in comparison to the dextrose PLA. The glucose integrated AUC (iAUC) change from baseline was significantly lower with FB ingestion (Study 1 FB 60 [CI 48, 71], PLA 160 [134, 186], p<0.001; Study 2 FB 65 [49, 82], PLA 209 [170, 244] mmol-h/L, p<0.001) while no differences were observed between treatments in insulin iAUC responses. In comparison to the dextrose standard, the FB had an iAUC derived glycemic index (GI) of 34 [CI 23, 46] and a Glycemic Load (GL) of 8.5 [CI 5.6, 11.6]. Participants also reported significantly less subjective ratings of appetite and hunger and greater satisfaction from food and feeling of fullness in both studies. No significant differences over time or between treatments were observed in ratings of symptoms of hypoglycemia. Results indicate that ingestion of a whey protein bar using IMO as the source of carbohydrate elicited a low glycemic response in comparison to a reference carbohydrate in healthy individuals. Thus, this FB may serve as a low glycemic food option for individuals on a low glycemic diet and/or athletes interested in optimizing nutrient availability around exercise.
Keywords: Energy bars; Glycemic index; Glycemic load; Glycemic response to food
Introduction
Consumers often ingest carbohydrate and protein energy bars in between meals as snacks or prior to exercise in order to increase amino acid availability and/or maintain blood glucose during exercise [1-4]. However, many energy bars or drinks have a relatively high Glycemic Index (GI) and therefore may not be not suitable for individuals who are glucose intolerant and/or diabetic [3,5]. Additionally, while it is recommended that athletes ingest carbohydrate and protein prior to exercise [1,4], ingesting foods, gels, and/or beverages that have high GI’s may promote hypoglycemia during exercise and thereby hasten fatigue [1,3,4,6,7]. For example, we previously reported that ingestion of moderate to low GI carbohydrate gel during prolonged cycling maintained blood glucose and insulin levels to a greater degree than a higher GI gel [7]. Additionally, that adding different types of carbohydrate with low to high GI’s to whey protein had differential effects on glucose and insulin responses following intense resistanceexercise [6].
Isomalto-oligosaccharides (IMO) are a prebiotic high fiber, low calorie source of carbohydrate that has been used as a functional food and sweetener in Asia for over 3 decades [8-12]. Basic animal studies indicate that IMO’s serve as a soluble dietary fiber and can stimulate activity of the probiotic gut flora, improve gut function, and help manage cholesterol in animals fed on a high fat diet [8,11,13-15]. Given the interest in developing food and energy bars that provide quality protein with a low to moderate glycemic profile, we sought to determine the glycemic and insulinemic responses of ingesting a whey protein food bar with IMO as the source of carbohydrate. Our primary outcome was assessment of the glycemic insulinemic responses to ingesting this Food Bar (FB). The secondary outcome was assessment of how ingestion of this FB affected appetite related variables and subjective ratings of hypoglycemic symptoms. We hypothesized that ingestion of a mixed ingredient food bar containing IMO would promote a low to moderate glycemic response and positively affect perceptions about appetite with no evidence of hypoglycemia.
Methods
Experimental design
This study was conducted with approval by an Institutional Review Board (IRB2016-0830D) and was registered with clinicatrials. gov (#NCT03166514). This study was conducted in two parts at a university-based research setting in randomized, counter-balanced, and crossover manner. In both studies, the independent variable was nutrient intake and dependent variables included blood glucose, insulin, and subjective ratings related to appetite and hypoglycemic side effects.
Participants
Apparently healthy men and women between the ages 18–35 years with a Body Mass Index (BMI) less than 25 kg/m2 were recruited to participate in this study. Individuals who expressed interest in participating were screened by phone or email to determine if they met initial eligibility to participate in this study. Qualified individuals were invited to attend a familiarization session in which participants received a written and verbal explanation of the study design, testing procedures, and read and signed informed consent statements. Those giving consent completed personal and medical histories and had height, weight, blood pressure, and heart rate determined. There search coordinator reviewed medical history forms, physical examination measurements, and determined eligibility to participate. Participants were excluded from the study if they reported: 1.) any uncontrolled metabolic disorders or cardiovascular disorder, including heart disease, a history of hypertension, diabetes, thyroid disease, hypogonadism; 2.) hepatorenal, musculoskeletal, autoimmune, or neurological disease; 3.) they were currently taking prescribed medication or dietary supplements for thyroid, hyperlipidemia, hypoglycemia, anti-hypertensive, anti-inflammatory, or weight loss (e.g. thermogenic compounds) within three months before the start of this study; or, 4.) Had any known allergies to some of the nutrients contained in the food bar (i.e., almonds, milk, soy, peanuts, tree nuts, egg, and wheat).
Nutritional intervention
In a placebo controlled, counterbalanced, and crossover manner, participants ingested a carbohydrate and protein food bar (FB, FitJoy™, Nutrabolt, Bryan TX) containing 20 g of a whey protein blend, 25 g of carbohydrate (13 g fiber and 4 g of sugar) as IMO plant fiber (VitaFiber™, BioNutra North America, Inc. Edmonton, Alberta, Canada), and 7g of fat (1.5g saturated) or 25 goof dextrose (PLA, Valeant Pharmaceuticals North America LLC, Bridgewater, NJ, USA). After a 7 to 10-day washout period, participants repeated the experiment while ingesting the remaining nutrient. In Study 1, participants ingested one Food Bar (FB) containing 220 calories and one 25 g serving of the PLA providing 100 calories (i.e., typical serving size) while in Study 2 participants ingested two FB’s and two 25 g servings of the dextrose PLA in order to assess the glycemic responses to ingesting a standard oral glucose tolerance test dose (i.e., 50 g). Participants were given as much time as needed to ingest the nutrients but this typically was less than 3-5 minutes.
Testing sequence
Figure 1 presents the general experimental design employed in both studies. For each experiment, participants were instructed to refrain from exercise for 24 h and fast for 10 h prior to reporting to the lab for testing. Once arriving at the lab, body weight was determined, participants completed appetite and hypoglycemia symptom related questionnaires, and they donated a fasting blood sample. Participants then ingested their assigned nutrient and a timer was started. Blood samples were obtained at 10, 20, 30, 60, 90 and 120 min post-ingestion while responses to questionnaires were obtained 60 and 120 minutes after ingestion of the assigned nutrient. Participants observed a 7 to 10-day washout period and then repeated the experiment in a crossover manner while ingesting the remaining nutrient.
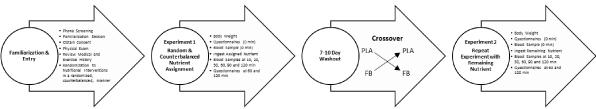
Figure 1: Overview of study design.
Procedures
Anthropometrics
Body weight and height was determined on a Health meter Professional Scale model 500KL (Pelstar LLC, Alsip, IL, USA). Heart rate was taken at the radial artery and systolic and diastolic blood pressure was measured using standard procedures [16].
Blood collection procedures
Venous catheters were placed in the participant’s arm using a BD Incite Auto guard 20 gauge intravenous (IV) catheter (Becton, Dickinson and Company, Franklin Lakes, NJ, USA) using standard procedures [17,18]. Blood samples were collected in 8.5 mL BD Vacutainer® serum separation tubes (Becton, Dickinson and Company, Franklin Lakes, NJ, USA). Samples were left at room temperature for 15 min prior to being centrifuged at 3,500 rpm for 10min using a refrigerated (4°C) Thermo Scientific Heraeus Mega Fuge 40R Centrifuge (Thermo Electron North America LLC, West Palm Beach, FL, USA) [19]. Serum was then aliquot into serum storage containers (Eppendorf North America, Inc., Hauppauge, NY, USA) and frozen at -80°C for subsequent analysis.
Blood chemistry analysis
Blood glucose was analyzed using a Cobas c111 (Roche Diagnostics, Basel, Switzerland) automated clinical chemistry analyzer. Quality control was performed daily to determine whether the system calibrated to acceptable standards using two levels of controls. Serum samples were re-run if values were outside the control values or clinical normality. The test-to-test reliability of performing glucose analysis was 2.3±0.03% with a coefficient of variation (CV) of 1.1%. Insulin was assayed in duplicate by using an Enzyme-Linked Immunosorbent Assay (ELISA) kit (ALPCO, Salem, NH) and assaying samples with a BioTek ELX-808 Ultramicroplate reader set at an optical density of 450 nm with BioTek Gen5 Analysis software (BioTek Instruments Inc., Winooski, VT). The intra-assay CV for insulin ranged from 2.9% to 6.2%.Glycemic Index (GI) was calculated using the integrated area under the curve (iAUC) change from baseline after FB ingestion divided by the iAUC of the dextrose PLA control normalized to 100 [20,21]. Glycemic Load (GL) values were calculated as the product of the amount of available carbohydrate in the FB times the GI value divided by 100 [20,21].
Appetite and hypoglycemia assessment
Participants were asked to subjectively rate appetite, hunger, satisfaction from food, feelings of fullness, and amount of energy using a 0 to 10 Likert scale where 0 was none, 2.5 was low, 5 was moderate, and 7.5 was high, and 10 was severe. Participants were also asked to rank the frequency and severity of their symptoms (i.e., hypoglycemia, dizziness, headache, fatigue, stomach upset) using the following scale: 0 (none), 1-4 (light), 5-6 (mild), 7-9 (severe), or 10 (very severe).
Statistical analysis
Data were analyzed using IBM® SPSS® Version 24 software (IBM Corp., Armonk, NY, USA). The sample size was based on prior research we conducted that indicated an n-size of 10-20 would yield a power of 0.80on changes in glucose and insulin in response to an oral glucose challenge [6,7]. Baseline demographic data were analyzed using one-way ANOVA. Data were analyzed using univariate, multivariate and repeated measures General Linear Models (GLM) with and without gender as a covariate. Since no gender interactions were observed, we report GLM data without the covariate. Wilks’ Lambda multivariate tests are reported to describe overall effects of related variables analyzed. Greenhouse-Geisser univariate tests with least significant difference post-hoc comparisons are presented for individual variables analyzed. Delta changes (post-pre) were calculated and analyzed by one-way ANOVA post-hoc analyses. Data are reported as mean (SD) and mean change from baseline with 95% Confidence Intervals (CI). The integrated area under the curve (iAUC) was used to calculate overall and net change from baseline iAUC values following procedures previously described [22,23]. Data were considered statistically significant when the probability of type I error was 0.05 or less. Mean changes with 95% CI are completely above or below baseline were considered significantly different [24].
Results
Participant demographics
Figure 2 presents a CONSORT diagram for both studies. In study 1, a total of 31 individuals met initial screening criteria and consented to participate in this study. A total of 20 completed the study. In Study 2, a total of 10 individuals met initial screening criteria and consented to participate in this study. A total of 10 completed the study. Table 1 presents participant demographics for the studies. In study 1, participants were 24.3±4.2yr, 73.1±11.4 kg, and had a Body Mass Index (BMI) of 22.6±3.2 kg/m2. Men were significantly taller, heavier, and had a higher BMI. In study 2, participants were 26.3±3.2yr, 73.1±11.4 kg, and had a BMI of 21.8±2.0 kg/m2 with men weighing more and having a higher BMI.
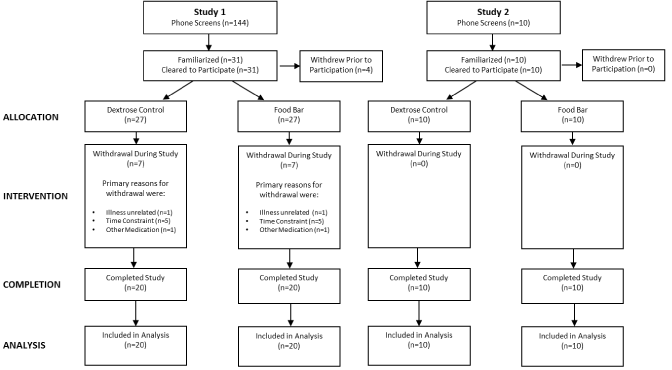
Figure 2: CONSORT diagram.
Study 1
Study 2
Male
Female
Mean
p-Level
Male
Female
Mean
p-Level
N
10
10
6
4
Age (y)
25.1±3.1
23.5±5.0
24.3±4.2
0.230
26.2±4.2
26.4±3.2
26.3±3.2
0.894
Height (m)
1.63±0.04
1.52±0.05
1.57±0.04
0.001
1.73±0.07
1.70±0.08
1.72±0.08
0.417
Weight (kg)
70.9±4.7
60.6±7.8
73.1±11.4
0.001
76.6±9.0
66.9±12.6†
73.1±11.4
0.001
BMI (kg/m2)
23.6±1.3
21.7±1.7
22.6±3.2
0.001
20.8±1.5
22.8±2.2
21.8±2.0
0.023
Table 1: Participant demographics.
Glycemic and insulinemic response
Table 2 presents glucose and insulin data observed by treatment and gender in Study 1 and 2 while Figure 3 shows mean responses to the treatments over time. Multivariate analysis revealed overall Wilks’ Lambda time (p<0.001) and treatment x time (p=0.003) effects in study 1 with no gender effects. Univariate analysis revealed significant time and treatment x time interactions in glucose responses. Posthoc analysis revealed that while blood glucose levels increased in both groups, values in the FB treatment were significantly lower than PLA responses during the first 60 minutes after ingestion. Insulin levels increased over time with no significant differences observed between treatments. In study 2, multivariate analysis revealed overall Wilks’ Lambda time (p=0.001) and treatment x time (p<0.001) effects. In both experiments, glucose and insulin levels peaked 30 minutes after ingestion. Figure 4 presents mean changes with 95% CI’s for both studies. Glucose generally increased to a greater degree and for a longer period of time after ingesting the PLA. Interestingly, FB ingestion was only marginally increased from baseline for the first 30 minutes in Study 1 and 10 minutes in Study 2.
Minutes
Variable
Treatment
0
10
20
30
60
90
120
Effect
p-Level
Study 1
Glucose
Time
4.91±0.38
5.63±0.63†
6.29±1.05†
6.56±1.36†
5.36±1.31†
4.7±0.77
4.52±0.40†
Time
0.001
(mmol/L)
FB
4.90±0.36
5.30±0.54†*
5.67±0.71†*
5.61±0.62†*
4.76±0.71*
4.68±0.49
4.61±0.40†
Treatment
0.001
PLA
4.92±0.40
5.79±0.57†
6.92±0.96†
7.51±1.24†
5.96±1.50†
4.71±1.00
4.42±0.39†
Treatment x Time
0.001
Male
5.01±0.43
5.76±0.56
6.60±0.92
6.94±1.37
5.49±1.38
4.63±0.59
4.61±0.35
Gender
0.021
Female
4.81±0.30
5.32±0.57
5.99±1.10
6.18±1.28
5.23±1.26
4.76±0.94
4.42±0.44
FB M
4.97±0.43
5.49±0.47
5.90±0.45
5.82±0.52
4.97±0.80
4.83±0.46
4.80±0.23
Treatment x Gender
0.855
FB F
4.82±0.29
5.11±0.57
5.43±0.85
5.41±0.67
4.54±0.56
4.54±0.49
4.43±0.46
Treatment x Time x Gender
0.247
PLA M
5.05±0.45
6.03±0.53
7.29±0.71
8.06±0.95
6.00±1.67
4.44±0.66
4.42±0.35
PLA F
4.80±0.33
5.54±0.51
6.54±1.06
6.96±1.29
5.92±1.40
4.98±1.22
4.42±0.44
Insulin
Time
7.38±5.18
14.23±9.94†
25.47±16.96†
29.35±17.96†
18.82±12.94†
10.43±9.11†
6.24±4.42
Time
0.001
(μIU/mL)
FB
7.71±4.66
14.03±10.25
27.05±20.32
30.87±20.68
19.92±12.02
12.03±9
7.38±4.95
Treatment
0.453
PLA
7.04±5.76
14.44±9.89
23.89±13.13
27.83±15.17
17.73±14.03
8.83±9.16
5.09±3.59
Treatment x Time
0.833
Male
7.87±4.16
15.70±7.95
28.11±13.60
34.24±15.23
19.75±11.91
9.15±8.39
6.13±2.93
Gender
0.001
Female
6.88±6.11
12.77±11.62
22.83±19.77
24.46±19.49
17.90±14.14
11.71±9.83
6.35±5.62
Time x Gender
0.163
FB M
7.38±2.93
15.95±9.94
28.95±16.41
34.23±16.28
22.80±9.46
11.98±10.54
7.60±3.21
Treatment x Gender
0.928
FB F
8.05±6.09
12.10±10.71
25.15±24.38
27.51±24.75
17.05±14.04
12.09±7.75
7.17±6.43
Treatment x Time x Gender
0.527
PLA M
8.37±5.23
15.44±5.89
27.28±10.93
34.25±15.00
16.71±13.77
6.33±4.44
4.66±1.75
PLA F
5.72±6.22
13.43±13.02
20.51±14.79
21.41±13.00
18.74±14.95
11.32±11.99
5.53±4.87
Study 2
Glucose
Time
4.39±0.42
5.63±0.63†
6.15±1.63†
5.94±1.81†
5.14±1.30†
4.54±0.86
4.31±0.89
Time
0.001
(mmol/L)
FB
4.40±0.42
5.25±0.51†*
4.85±0.87*
4.32±0.79*
4.08±0.38*
4.28±0.49
4.69±0.32
Treatment
0.001
PLA
4.38±0.46
6.02±0.50†
7.44±1.04†
7.57±0.67†
6.19±0.98
4.8±1.08
3.94±1.12
Treatment x Time
0.001
Male
4.56±0.38
5.77±0.70
6.39±1.77
6.08±1.84
5.01±1.25
4.59±1.01
4.27±0.76
Gender
0.334
Female
4.14±0.37
5.43±0.48
5.78±1.40
5.74±1.88
5.32±1.44
4.47±0.6
4.38±1.10
Time x Gender
0.337
FB M
4.57±0.36
5.28±0.57
5.06±1.03
4.49±0.94
4.10±0.37
4.41±0.53
4.75±0.28
Treatment x Gender
0.675
FB F
4.14±0.40
5.20±0.50
4.53±0.52
4.07±0.49
4.06±0.46
4.08±0.39
4.59±0.40
Treatment x Time x Gender
0.697
PLA M
4.54±0.44
6.26±0.42
7.72±1.27
7.66±0.69
5.93±1.13
4.76±1.38
3.79±0.80
PLA F
4.14±0.41
5.65±0.39
7.03±0.42
7.42±0.71
6.58±0.61
4.87±0.52
4.17±1.60
Insulin
Time
8.44±5.96
42.50±25.47†
51.84±25.89†
52.10±22.72†
37.51±19.47†
21.87±13.88†
15.18±12.31†
Time
0.001
(μIU/mL)
FB
7.68±3.01
52.54±31.21
56.69±33.64
52.18±27.96
36.07±20.49
22.67±12.06
16.59±11.47
Treatment
0.509
PLA
9.20±8.05
32.47±13.09
46.99±15.18
52.02±17.55
38.96±19.38
21.07±16.13
13.77±13.56
Treatment x Time
0.41
Male
6.49±4.20
43.15±31.86
52.04±30.89
55.14±22.63
31.98±18.20
20±14.47
11.75±6.97
Gender
0.58
Female
11.36±7.25
41.53±12.77
51.54±17.85
47.53±23.59
45.81±19.41
24.67±13.39
20.32±16.87
Time x Gender
0.485
FB M
7.03±3.72
56.84±39.09
58.10±40.62
55.34±25.24
32.82±20.03
18.29±7.49
13.88±5.75
Treatment x Gender
0.673
FB F
8.65±1.41
46.08±16.81
54.59±25.22
47.44±35.11
40.94±23.21
29.24±15.71
20.65±17.41
Treatment x Time x Gender
0.782
PLA M
5.95±4.92
29.46±15.98
45.99±19.03
54.95±22.13
31.14±18.06
21.71±19.93
9.63±7.94
PLA F
14.07±10.05
36.99±6.56
48.48±9.11
47.62±8.07
50.68±16.65
20.11±10.77
19.99±18.99
Table 2: Glucose and Insulin response to an oral glucose challenge.
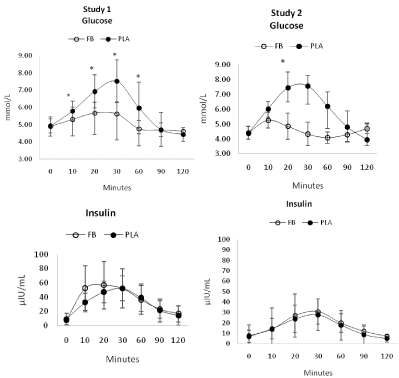
Figure 3: Glucose and insulin values observed in Study 1 and Study 2 for the placebo (PLA) and Food Bar (FB) treatments. *represents p<0.05 difference between
PLA and FB.
The overall AUC for glucose was significantly lower in FB treatment in Study 1 (FB 599±50, PLA 688±78 mmol-h/L, p<0.001) and Study 2 (530±48, PLA 697±67mmol-h/L, p<0.001). Using the Study 2 values, the FB GI was 76.7±10 with a GL of 19.2±2.5. No significant differences were observed between treatments in the overall insulin AUC (Study 1: FB 2,136±1,073, PLA 1,848±971μIU/ mL-h/L, p=0.38; Study 2: FB 4,185±1,934, PLA 3,888±707μIU/mLh/ L, p=0.65). Figure 5 presents iAUC changes from baseline for glucose and insulin. In both studies, the iAUC change from baseline was significantly greater after PLA ingestion (Study 1 FB 60 [CI 48, 71], 160 [134, 186], p<0.001; Study 2 FB 65 [49, 82], 209 [170, 244] mmol-h/L, p<0.001). No significant differences were observed between treatments in insulin iAUC responses (Study 1: FB 1,436 [1,061, 1,811], PLA 1,302 [1,019, 1,585] μIU/mL-h/L, p=0.55; Study 2: FB 1,434 [917, 1,950], PLA 1,236 [842, 1,630] μIU/mL-h/L, p=0.50). In comparison to consuming 50 g of dextrosenormalized to 100, the FB had an iAUC derived GI of 34 [CI 23, 46] and a GL of 8.5 [CI 5.6, 11.6].
Appetite and hypoglycemia assessment
Table 3 presents responses to eating satisfaction questions. In both experiments, participants reported less subjective ratings of appetite, hunger, and greater satisfaction from food and feeling of fullness. Finally, no significant time, treatment, or time by treatment effects were observed in subjective ratings of hypoglycemia, dizziness, headache, fatigue, or stomach upset.
Minutes
Variable
Treatment
0
60
120
Effect
p-Level
Study 1
Appetite
Time
5.77±2.08
4.87±1.90†
5.40±2.18†
Time
0.013
FB
6.40±1.82
4.55±1.76†
4.65±2.21†*
Treatment
0.001
PLA
5.15±2.18
5.20±2.02
6.15±1.93†
Treatment x Time
0.001
Hunger
Time
5.63±2.11
4.75±2.16†
5.77±1.92
Time
0.006
FB
5.80±2.46
4.05±2.06†*
4.75±1.83†*
Treatment
0.453
PLA
5.45±1.73
5.45±2.06
6.80±1.40†
Treatment x Time
0.002
Satisfaction
Time
0.53±1.99
5.07±2.38†
4.52±2.08†
Time
0.001
FB
0.55±1.76
6.50±1.57†*
5.65±1.50†*
Treatment
0.453
PLA
0.50±2.24
3.65±2.21†
3.40±1.98†
Treatment x Time
0.013
Fullness
Time
2.85±2.08
5.05±2.01†
3.87±2.10†
Time
0.001
FB
2.85±2.11
5.85±2.13†*
5.15±1.76†*
Treatment
0.453
PLA
2.85±2.11
4.25±1.55†
2.60±1.60
Treatment x Time
0.002
Energy
Time
5.72±1.71
6.03±1.63
5.90±1.32
Time
0.420
FB
5.55±1.85
6.30±1.49
6.20±1.40
Treatment
0.077
PLA
5.90±1.59
5.75±1.74
5.60±1.19
Treatment x Time
0.103
Study 2
Appetite
Time
5.80±2.09
3.85±2.32†
5.10±2.63
Time
0.009
FB
6.00±2.71
2.80±2.39†*
3.60±2.22†*
Treatment
0.001
PLA
5.60±1.35
4.90±1.79
6.60±2.17†
Treatment x Time
0.020
Hunger
Time
6.00±1.97
3.75±2.27†
5.15±2.87†
Time
0.002
FB
6.20±2.30
2.50±2.22†*
3.50±2.46†*
Treatment
0.453
PLA
5.80±1.69
5.00±1.56
6.80±2.30†
Treatment x Time
0.009
Satisfaction
Time
0.60±1.43
4.40±2.82†
3.90±2.81
Time
0.001
FB
0.40±1.27
5.00±2.91†
4.90±2.81†
Treatment
0.453
PLA
0.80±1.62
3.80±2.74†
2.90±2.56†
Treatment x Time
0.145
Fullness
Time
1.90±1.83
5.35±0.57†
2.62±3.80†
Time
0.001
FB
1.50±1.72
6.50±0.63†*
2.55±5.10†*
Treatment
0.453
PLA
2.30±1.95
4.20±0.54†
2.25±2.50
Treatment x Time
0.020
Energy
Time
5.85±1.84
6.15±2.23
6.10±1.71
Time
0.632
FB
6.40±1.51
6.90±1.45
6.80±1.32
Treatment
0.077
PLA
5.30±2.06
5.40±2.68
5.40±1.84
Treatment x Time
0.799
Table 3: Eating satisfaction inventory.
Discussion
There is significant interest in developing lowglycemic functional foods for consumers trying to maintain healthy blood glucose levels as well as athletes who want to consume low glycemic protein bars [1- 4]. However, many protein and energy bars contain large amounts of carbohydrate and/or have a relatively high glycemic index, Therefore, these products may not be not suitable for individuals who are glucose intolerant and/or diabetic [3,5] or for athletes who may be susceptible to hypoglycemia [1,3,4,6,7]. Isomalto-oligosaccharides are a prebiotic high fiber, low calorie source of carbohydrate that has been used in functional foods primarily in Asia [8-12]. Reports indicate that IMO serve as a soluble dietary fiber and prebiotic that can promote activity of the probiotic gut flora and improve gut function thereby help manage cholesterol [8,11,13-15]. The purpose of this study was to determine the glycemic and insulinemic response of ingesting a whey protein food bar with IMO as the source of carbohydrate. We hypothesized that ingestion of a mixed ingredient food bar containing IMO would promote a low to moderate glycemic response and positively affect perceptions about appetite with no evidence of hypoglycemia.
Results of this study support this contention. In this regard, we found that the glycemic and insulinemic response of ingesting one and two servings of this FB were much more favorable than ingesting equivalent amounts of reference carbohydrate. Analysis of iAUC changes from baseline which has been suggested to be a more accurate assessment of glycemic response to ingesting food [25,26] indicated that the FB study had a low glycemic index (34 [CI 23, 46]) and glycemic load 8.5 [CI 5.6, 11.6] [25] when normalized to the dextrose reference. Glucose levels increased less than 15% from fasting values after FB ingestion compared to an increase of up to 73% with dextrose. Additionally, although the treatments differed in energy content and sweetness which influence perceptions about appetite, hunger, and satiety [27]; ingestion of the energy/food bar also decreased perceptions of appetite and hunger and increased feelings of fullness with no symptoms associated with hypoglycemia. These findings indicate that the food bar studied may be a good food choice for individuals on low glycemic diets and/or trying to manage weight [28-35].
Interestingly, even though glucose levels were only modestly increased following FB ingestion, insulin levels increased in both groups with values generally higher following FB ingestion. There are several possible reasons for this finding. First, there is some evidence that amino acid ingestion can modestly increase insulin levels and that ingestion of protein or amino acids with carbohydrate may promote a greater effect [36-39]. So, since the FB treatment contained 20 g of whey protein, this may have contributed to this finding. Second, although IMO is a prebiotic, it is a type of oligosaccharide that has been reported to stimulate growth of “friendly” bacteria and thereby promote activity of the probiotic gut flora and improve gut function [11,40-42]. Therefore, it is possible that intestinal absorption of glucose was enhanced thereby serving to help maintain blood glucose levels to a greater degree while the increased availability of amino acids served to stimulate insulin levels. Additional research should examine potential mechanisms associated with these findings.
It is also important to note that changes in blood glucose and insulin, macronutrient content of a food, portion size, perceptions about sweetness, and energy content of a food affect subjective ratings of satiety as well as secretion of appetite-related hormones [43,44]. Generally, hypoglycemia stimulates appetite and hunger while increases in blood glucose and insulin after consuming food reduce appetite and hunger. In this study, perceptions about appetite and hunger decreased while satisfaction with food and feelings of fullness increased to a greater degree with FB treatment despite blood glucose levels increasing by less than 15%. While this may simply be related to these other factors [43], it is interesting that these findings were observed with only a modest increase in blood glucose. Additional research is needed to examine how IMO and foods using IMO as a carbohydrate source influence satiety.
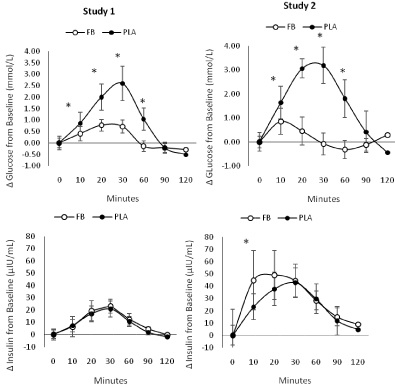
Figure 4: Mean changes with 95% CI’s in glucose (top panel) and insulin (bottom panel) during Study 1 and Study 2 for the placebo (PLA) and Food Bar (FB)
treatments. Confidence intervals crossing zero are statistically significant (p<0.05). *represents p<0.05 difference between PLA and FB.
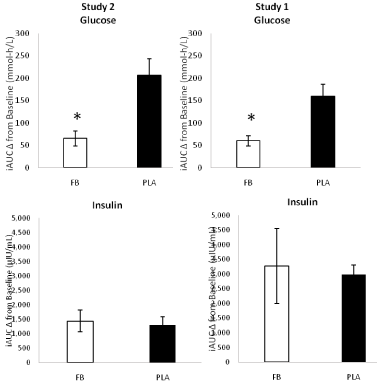
Figure 5: Integrated area under the curve (iAUC) change from baseline for glucose and insulin observed in Study 1 and Study 2 for the placebo (PLA) and Food
Bar (FB) treatments. *represents p<0.05 difference between PLA and FB.
The maintenance of blood glucose while observing a similar or greater increase in insulin also has some potential applications for individuals involved in exercise training. It is recommended that athletes consume low to moderate sources of carbohydrate with 10 to 20 g of high quality protein prior to intense and prolonged exercise in order to maintain blood glucose availability, prevent hypoglycemia, minimize exercise induced protein degradation during exercise, and stimulate protein synthesis [1,2,4,6]. However most commercially available energy/food bars contain large amounts of high glycemic carbohydrate and/or low amounts of quality protein which may not be optimal for athletes to ingest prior to exercise. Additionally, they are typically marketed as in-between meal snacks or meal replacements rather than to optimize nutrient availability around exercise [45]. The energy/food bar studied contains a low glycemic source of carbohydrate (IMO plant fiber) and 20 g of high quality whey protein that would provide more than 6 g of Essential Amino Acids (EAA). We found that this energy/food bar has a low GI, elicited only a modest increase in blood glucose levels, yet promoted a similar increase in insulin as compared to a high GI carbohydrate (dextrose). Theoretically, this may serve as an optimal pre-exercise source of carbohydrate for active individuals because in can provide a more sustained release of glucose while stimulating insulin and thereby lessening exercise-induced catabolism during exercise [1,2,4,6]. Additional research should evaluate whether ingestion of this energy/ food bar prior to, during, and/or following intense exercise can help maintain blood glucose level, reduce markers of catabolism, and/or promote recovery.
In conclusion, using IMO as a carbohydrate source in a protein energy/food bar promoted a significantly lower glycemic response while still stimulating insulin release. The protein/food bar had a low glycemic index (34 [CI 23, 46]) and glycemic load 8.5 [CI 5.6, 11.6] [25] when normalized to the dextrose reference. It also reduced perceptions related to appetite with no effect on hypoglycemia related symptoms. Thus, this protein/food bar may serve as a low glycemic food option for individuals on a low glycemic diet or trying to maintain weight and/or athletes interested in optimizing nutrient availability around exercise. Additional research should evaluate the potential benefits of using IMO as a carbohydrate source in functional foods as well as other potential health effects of increasing dietary availability of IMO.
Acknowledgment
We would like to thank all individuals who participated in this study; Susannah Williamson and Victoria Pizzitola for assisting with data collection; and, Dr. J.P. Bramhall for serving as medical director for this study. This study was supported by Nutrabolt (Bryan, TX, USA) through an unrestricted research grant provided to Texas A & M University. However, the sponsor was not involved in data collection or data entry and there were no restrictions on publication of the data or preparation of this paper. As stated below, competing interests were supervised and managed by a university approved management plan to insure that data were accurately reported.
Author Contributions
T.J.G served as study coordinator and assisted with data collection, data analysis, and manuscript preparation. R.J.S., R.D., P.B.C., A.G.R. and C.J. F assisted in data collection and analysis. C.R. served as lab coordinator and project manager for the study coordinator. C.P.E. served as a scientific liaison to the sponsor, assisted in study design, data analysis and interpretation, and provided comments on the manuscript. However, C.P.E. was not involved in data collection or data entry and there were no restrictions on publication of the data or preparation of this paper. M.G. assisted in study oversight, data analysis, and manuscript review. R.B.K. obtained the grant, served as study Principal Investigator and assisted in the design of the study, data analysis, and manuscript preparation. All authors read and approved the final manuscript.
Conflicts of Interest
C.P.E. serves as a paid consultant for Nutrabolt and is a Research Associate in the ESNL. Further, he holds scientific consultancies with Naturally Slim (Dallas, TX, USA) and Catapult Health (Dallas, TX, USA). R.B.K. serves as a university approved scientific advisor for Nutrabolt. P.S.M. served as quality assurance supervisor in accordance to a conflict of interest management plan that was approved by the university’s research and compliance office, the internal review board, and office of grants and contracts and monitored by research compliance. Remaining investigators have no competing interests to declare. The results from this study do not constitute endorsement by the authors and/or the institution concerning the nutrients investigated.
References
- Kerksick CM, Arent S, Schoenfeld BJ, Stout JR, Campbell B, Wilborn CD, et al. International society of sports nutrition position stand: Nutrient timing. J Int Soc Sports Nutr. 2017; 14: 33.
- Jager R, Kerksick CM, Campbell BI, Cribb PJ, Wells SD, Skwiat TM, et al. International society of sports nutrition position stand: Protein and exercise. J Int Soc Sports Nutr. 2017; 14: 20.
- Campbell B, Wilborn C, La Bounty P, Taylor L, Nelson MT, Greenwood M, et al. International society of sports nutrition position stand: Energy drinks. J Int Soc Sports Nutr. 2013; 10: 1.
- Kreider RB, Wilborn CD, Taylor L, Campbell B, Almada AL, Collins R, et al. Issn exercise & sport nutrition review: Research & recommendations. J Int Soc Sports Nutr. 2010; 7: 7.
- Hertzler S. Glycemic index of "energy" snack bars in normal volunteers. J Am Diet Assoc. 2000; 100: 97-100.
- Kreider RB, Earnest CP, Lundberg J, Rasmussen C, Greenwood M, Cowan P, et al. Effects of ingesting protein with various forms of carbohydrate following resistance-exercise on substrate availability and markers of anabolism, catabolism, and immunity. J Int Soc Sports Nutr. 2007; 4: 18.
- Earnest CP, Lancaster SL, Rasmussen CJ, Kerksick CM, Lucia A, Greenwood MC, et al. Low vs. High glycemic index carbohydrate gel ingestion during simulated 64-km cycling time trial performance. J Strength Cond Res. 2004; 18: 466-472.
- Goffin D, Delzenne N, Blecker C, Hanon E, Deroanne C, Paquot M. Will isomalto-oligosaccharides, a well-established functional food in asia, break through the european and american market? The status of knowledge on these prebiotics. Crit Rev Food Sci Nutr. 2011; 51: 394-409.
- Wang W, Xin H, Fang X, Dou H, Liu F, Huang D, Han S, et al. Isomalto-oligosaccharides ameliorate visceral hyperalgesia with repair damage of ileal epithelial ultrastructure in rats. PLoS One. 2017; 12: e0175276.
- Singh DP, Singh J, Boparai RK, Zhu J, Mantri S, Khare P, et al. Isomalto-oligosaccharides, a prebiotic, functionally augment green tea effects against high fat diet-induced metabolic alterations via preventing gut dysbacteriosis in mice. Pharmacol Res. 2017; 123: 103-113.
- Singh DP, Khare P, Zhu J, Kondepudi KK, Singh J, Baboota RK, et al. A novel cobiotic-based preventive approach against high-fat diet-induced adiposity, nonalcoholic fatty liver and gut derangement in mice. Int J Obes (Lond). 2016; 40: 487-496.
- Mookiah S, Sieo CC, Ramasamy K, Abdullah N, Ho YW. Effects of dietary prebiotics, probiotic and synbiotics on performance, caecal bacterial populations and caecal fermentation concentrations of broiler chickens. J Sci Food Agric. 2014; 94: 341-348.
- Singh DP, Singh S, Bijalwan V, Kumar V, Khare P, Baboota RK, et al. Co-supplementation of isomalto-oligosaccharides potentiates metabolic health benefits of polyphenol-rich cranberry extract in high fat diet-fed mice via enhanced gut butyrate production. Eur J Nutr. 2017.
- Singh DP, Khare P, Bijalwan V, Baboota RK, Singh J, Kondepudi KK, et al. Coadministration of isomalto-oligosaccharides augments metabolic health benefits of cinnamaldehyde in high fat diet fed mice. Biofactors. 2017; 43: 821-835.
- Kalra PA. Introducing iron isomaltoside 1000 (monofer(r))-development rationale and clinical experience. NDT Plus. 2011; 4: i10-i13.
- Medicine ACoS. Acsm's guidelines for exercise testing and prescription. Lippincott Williams & Wilkins. 2013.
- Bowling JL, Katayev A. An evaluation of the roche cobas c 111. Laboratory Medicine. 2010; 41: 398-402.
- Jenkins DJ, Wolever TM, Taylor RH, Barker H, Fielden H, Baldwin JM, et al. Glycemic index of foods: A physiological basis for carbohydrate exchange. Am J Clin Nutr. 1981; 34: 362-366.
- Bush VJ, Janu MR, Bathur F, Wells A, Dasgupta A. Comparison of bd vacutainer sst™ plus tubes with bd sst™ ii plus tubes for common analytes. Clinica chimica acta. 2001; 306: 139-143.
- Brand-Miller J, Holt S. Testing the glycaemic index of foods: In vivo, not in vitro. Eur J Clin Nutr. 2004; 58: 700-701.
- Foster-Powell K, Holt SH, Brand-Miller JC. International table of glycemic index and glycemic load values: 2002. Am J Clin Nutr. 2002; 76: 5-56.
- Zhang Y, Huo M, Zhou J, Xie S. Pksolver: An add-in program for pharmacokinetic and pharmacodynamic data analysis in microsoft excel. Comput Methods Programs Biomed. 2010; 99: 306-314.
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003; 28: 916-931.
- Page P. Beyond statistical significance: Clinical interpretation of rehabilitation research literature. Int J Sports Phys Ther. 2014; 9: 726-736.
- Wallace AJ, Monro JA, Brown RC, Frampton CM. A glucose reference curve is the optimum method to determine the glycemic glucose equivalent values of foods in humans. Nutr Res. 2008; 28: 753-759.
- Wolever TM. Effect of blood sampling schedule and method of calculating the area under the curve on validity and precision of glycaemic index values. Br J Nutr. 2004; 91: 295-301.
- Sorensen LB, Moller P, Flint A, Martens M, Raben A. Effect of sensory perception of foods on appetite and food intake: A review of studies on humans. Int J Obes Relat Metab Disord. 2003; 27: 1152-1166.
- Barazzoni R, Deutz NEP, Biolo G, Bischoff S, Boirie Y, Cederholm T, et al. Carbohydrates and insulin resistance in clinical nutrition: Recommendations from the espen expert group. Clin Nutr. 2017; 36: 355-363.
- Sandouk Z, Lansang MC. Diabetes with obesity--is there an ideal diet?. Cleve Clin J Med. 2017; 84: S4-S14.
- Baetge C, Earnest CP, Lockard B, Coletta AM, Galvan E, Rasmussen C, et al. Efficacy of a randomized trial examining commercial weight loss programs and exercise on metabolic syndrome in overweight and obese women. Appl Physiol Nutr Metab. 2017; 42: 216-227.
- Kerksick CM, Wismann-Bunn J, Fogt D, Thomas AR, Taylor L, Campbell BI, et al. Changes in weight loss, body composition and cardiovascular disease risk after altering macronutrient distributions during a regular exercise program in obese women. Nutr J. 2010; 9: 59.
- Kreider RB, Rasmussen C, Kerksick CM, Wilborn C, Taylor Lt, Campbell B, et al. A carbohydrate-restricted diet during resistance training promotes more favorable changes in body composition and markers of health in obese women with and without insulin resistance. Phys Sportsmed. 2011; 39: 27-40.
- Kreider RB, Serra M, Beavers KM, Moreillon J, Kresta JY, Byrd M, et al. A structured diet and exercise program promotes favorable changes in weight loss, body composition, and weight maintenance. J Am Diet Assoc. 2011; 111: 828-843.
- Lockard B, Earnest CP, Oliver J, Goodenough C, Rasmussen C, Greenwood M, et al. Retrospective analysis of protein- and carbohydrate-focused diets combined with exercise on metabolic syndrome prevalence in overweight and obese women. Metab Syndr Relat Disord. 2016; 14: 228-237.
- Wilborn C, Beckham J, Campbell B, Harvey T, Galbreath M, La Bounty P, et al. Obesity: Prevalence, theories, medical consequences, management, and research directions. J Int Soc Sports Nutr. 2005; 2: 4-31.
- Gunnerud UJ, Ostman EM, Bjorck IM. Effects of whey proteins on glycaemia and insulinaemia to an oral glucose load in healthy adults; a dose-response study. Eur J Clin Nutr. 2013; 67: 749-753.
- Notomi T, Karasaki I, Okazaki Y, Okimoto N, Kato Y, Ohura K, et al. Insulinogenic sucrose+amino acid mixture ingestion immediately after resistance exercise has an anabolic effect on bone compared with non-insulinogenic fructose+amino acid mixture in growing rats. Bone. 2014; 65: 42-48.
- Salehi A, Gunnerud U, Muhammed SJ, Ostman E, Holst JJ, Bjorck I, et al. The insulinogenic effect of whey protein is partially mediated by a direct effect of amino acids and gip on beta-cells. Nutr Metab (Lond). 2012; 9: 48.
- Schmid R, Schusdziarra V, Schulte-Frohlinde E, Maier V, Classen M. Role of amino acids in stimulation of postprandial insulin, glucagon, and pancreatic polypeptide in humans. Pancreas. 1989; 4: 305-314.
- Mizubuchi H, Yajima T, Aoi N, Tomita T, Yoshikai Y. Isomalto-oligosaccharides polarize th1-like responses in intestinal and systemic immunity in mice. J Nutr. 2005; 135: 2857-2861.
- Kihara M, Sakata T. Production of short-chain fatty acids and gas from various oligosaccharides by gut microbes of carp (cyprinus carpio l.) in micro-scale batch culture. Comp Biochem Physiol A Mol Integr Physiol. 2002; 132: 333-340.
- Rycroft CE, Jones MR, Gibson GR, Rastall RA. A comparative in vitro evaluation of the fermentation properties of prebiotic oligosaccharides. J Appl Microbiol. 2001; 91: 878-887.
- Reynolds RC, Stockmann KS, Atkinson FS, Denyer GS, Brand-Miller JC. Effect of the glycemic index of carbohydrates on day-long (10 h) profiles of plasma glucose, insulin, cholecystokinin and ghrelin. Eur J Clin Nutr. 2009; 63: 872-878.
- Speechly D, Rogers GG, Buffenstein R. Acute appetite reduction associated with an increased frequency of eating in obese males. Int J Obes Relat Metab Disord. 1999; 23: 1151-1159.
- Painter JE, Prisecaru VI. The effects of various protein and carbohydrate ingredients in energy bars on blood glucose levels in humans. Cereal foods world. 2002; 47: 236.