
Editorial
Austin J Nutri Food Sci. 2018; 6(2): 1102.
Epigenetic Regulation by Common Risk Factors in Periodontitis and Non-Communicable Diseases
Cho Y, Kim KH, Ryoo H, Lee YM, Ku Y and Seol YJ*
Department of Period Ontology and Molecular Genetics, School of Dentistry and Dental Research Institute, Korea
*Corresponding author: Yang-Jo Seol, Department of Period ontology, School of Dentistry, Seoul National University, Yeongeon-dong, Jongno-gu, 03080, Seoul, Korea
Received: April 16, 2018; Accepted: April 25, 2018; Published: May 02, 2018
Editorial
Chronic diseases occur over longtime-courses, have slow progressions, are not resolved on their own, and are difficult to cure. A Non-Communicable Disease (NCD) is a medical condition that is non-infectious, non-transmissible, and often chronic (lasting three months or longer), and progresses slowly [1]. These chronic NCDs are multi factorial diseases that are affected by environmental factors such as diet and lifestyle, in addition to genetic predisposition. Periodontitis is a chronic dental disease that shares risk factors with other major NCDs including diabetes, cardiovascular disease, cancer, and chronic respiratory diseases [2]. Periodontal disease and NCDs share common risk factors including smoking, alcohol consumption, and poor dietary habits (Figure 1) [3]. These risk factors are mainly behavioral and environmental, have a long-term impact on lifestyle, and can alter the course of a disease through epigenetic rather than genetic modifications.
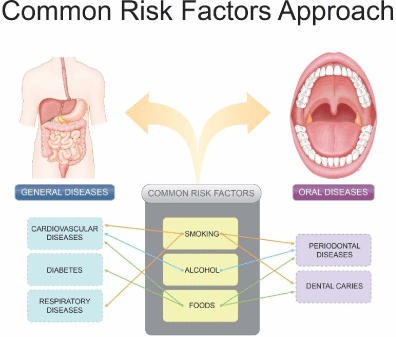
Figure 1: Common risk factors approach in the general and oral diseases.
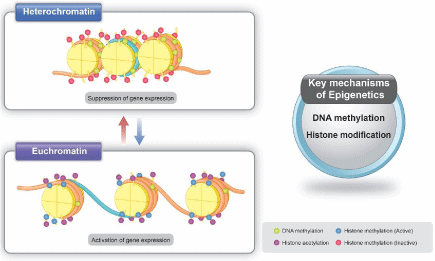
Figure 2: Mechanisms of Epigenetics.
Epigenetic is the study of changes in gene function that does not involve changes in the primary DNA sequence [4].
Epigenetic encompasses an additional layer of genetic regulation, where the Greek prefix “epi” means “around” or “outside of it”. Genetics encompasses DNA mutations that induce changes in gene expression; however, epigenetic refers to the regulation of gene expression without gene mutations. In epigenetic regulation, various environmental factors affect gene expression via the regulation of DNA methylation or his tone modifications [4] (Figure 2). These modifications cause alterations in gene expression without directly altering the DNA sequence. In general, DNA methylation causes a segment of DNA to associate more closely with a tone complex. This prevents transcription factors from binding to a DNA sequence, such as a promoter, resulting in reduced expression of a specific gene. In contrast, his tone acetylating weakens the interaction between the tone complex and DNA, allowing transcription factors to bind to the promoter and increase gene expression.
Epigenetic changes can occur in response to the environment, changes in diet, exposure to pollutants, medication, and even social interactions. Epigenetic changes can alter an individual’s susceptibility to disease, their response to treatment, and their prognosis [5]. Based on the importance of these epigenetic factors, a comprehensive epigenetic analysis of common risk factors affecting period ontitis and other NCDs is required. The growth of epigenetic as an active field of research could provide the answers to how environmental factors regulate chronic diseases. An investigation of diagnostic epigenetic biomarkers related to the treatment of various diseases and common risk factors could provide valuable data and improve quality of life.
In recent years, large data duration via Next-Generation Sequencing (NGS) has been widely used for the comprehensive genetic analysis of many diseases. The completion of the human genome project provided extensive genomic information; however, genome sequence data alone do not fully explain the mechanisms of disease, and the information required to fully understand a disease is still incomplete. To fill this gap in knowledge, it is essential to acquire information on both the genomic sequence as well as the epigenetic status in affected individuals, as it is assumed that epigenetic regulation of gene expression is essential for control of the disease. Previous studies reported that both DNA methylation and his tone modifications occur in periodontal diseases in response to bacterial infection and inflammation [6-8]. Recently, using NGS, we identified that smoking-related changes in DNA methylation patterns and subsequent alterations in the expression of genes are causally related to an increased susceptibility to periodontitis [9]. In this analysis, we found that a disease has different progressions according to the individual’s medical history. Based on these data, we have tried to determine the epigenetic mechanism responsible for these differences. The results of this investigation provide a foundation for further studies using larger cohorts to identify the potential mechanisms underlying the observed patterns related to environmental conditions in order to prevent disease onset or mitigate disease progression.
Epigenomic mapping through system biology is an active and prominent area of international research. It is a field that is creating new technologies and fueling a growing biotechnology industry to achieve the goal of acquiring insights into gene regulation that are not available through classical genetic research. Further NGS studies on the common risk factors of period ontitis and NCDs will be required to develop diagnostic and therapeutic strategies to promote both periodontal health and the overall health of the general population.
Acknowledgement
This research was supported by grants from MSIP/IITP (2017-0- 00398) through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning.
References
- Beagle hole R, Bonita R, Horton R, Ezzati M, BhalaN, Amuyunzu-Nyamongo M, et al. Measuring Progress on Ncds : One Goal and Five Targets. Lancet. 2012; 380: 1283-1285.
- Tonetti MS, Jepsen S, Jin L, Otomo-Corgel J. Impact of the Global Burden of Periodontal Diseases on Health, Nutrition and Wellbeing of Mankind: A Call for Global Action. J Clin Periodontal. 2017; 44: 456-462.
- Petersen PE, Ogawa H. Strengthening the Prevention of Periodontal Disease: The Approach J Periodontal. 2005; 76: 2187-2193.
- DuPont C, Arm ant DR, Brenner CA. Epigenetic: Definition, Mechanisms and Clinical Perspective. Semin Reprod Med. 2009; 27: 351-357.
- Jirtle RL, Skinner MK. Environmental Epigenomics and Disease Susceptibility. Nat Rev Genet. 2007; 8: 253-262.
- Ari G, Cherukuri S, Namasivayam A. Epigenetics and Periodontitis: A Contemporary Review. Journal of clinical and diagnostic research: JCDR. 2016; 10: ZE07-ZE09.
- Gomez RS, Dutra WO, Moreira PR. Epigenetic and Periodontal Disease: Future Perspectives. Inflame Res. 2009; 58: 625-629.
- Grover V, Kapoor A, Malhotra R, Sachdeva S. Epigenetics and Periodontal Disease: Hope to Tame the Untamable. Curr Gene Ther. 2014; 14: 473-481.
- Cho YD, Kim PJ, Kim HG, Seol YJ, Lee YM, Ku Y, et al. Transcriptomics and Methylomics in Chronic Periodontitis with Tobacco Use: A Pilot Study. Clin Epigenetics. 2017; 9: 81.