
Special Article - Antioxidants in Food
Austin J Nutri Food Sci. 2019; 7(2): 1116.
Spirulina (Arthrospira) Platensis as a Techno-Functional Ingredient in Almond Paste Process Production
Abdelhedi O1,2, Jridi M1,2, Msaddak L3, Belaid A1, Nasri M1, Zouari N1,4*, Fakhfakh N1,4
1Laboratory of Enzyme Engineering and Microbiology, Engineering National School of Sfax (ENIS), University of Sfax, Sfax, Tunisia
2High Institute of Biotechnology of Beja, University of Jendouba, Tunisia
3Gourmandise Company, Tunisia
4High Institute of Applied Biology of Medenine, University of Gabes, Tunisia
*Corresponding author: Zouari N, High Institute of Applied Biology of Medenine, Medenine, University of Gabes, Tunisia
Received: April 18, 2019; Accepted: May 10, 2019; Published: May 17, 2019
Abstract
The purpose of this study was to characterize the Spirulina blue-green alga in terms of functional and chemical properties and to use it in the Kaak Mrachek (almond paste) formulation. Thus, antioxidant and antibacterial activities, total phenolics and flavonoids contents, as well as some techno-functional characteristics of Spirulina were assessed. Moreover, the sensory properties, color, mass loss of almond paste enriched with Spirulina were determined. The Spirulina was found to be a rich source of proteins with very important fat absorption capacity. Spirulina presented relatively interesting antibacterial activity and antioxidant potential evaluated by the ferric (Fe3+) reducing and « DPPH• »-radical scavenging assays. Spirulina was incorporated at three substitution levels (1, 3 and 5%) in almond paste formulation. Obtained results revealed that enrichment with 3% Spirulina did not alter the sensory attributes. Interestingly, Spirulina addition also resulted in the reduction of mass loss of the pastry product during storage. The present study suggests that Spirulina incorporation in almond paste not only added nutraceutical value to food, but also improved its quality (Graphical Abstract).
Keywords: Spirulina; Antioxidant and antibacterial activities; Almond paste; Sensory properties.
Introduction
Among the photosynthetic microorganisms with commercial importance, there are species belonging to the genus Spirulina, now named Arthrospira, which are filamentous cyanobacteria forming spirally coiled uniseriate. Arthrospira platensis is an exceptionally important edible blue-green alga in view of its high nutritional value. This microalga contains up to 70% proteins, many vitamins and minerals especially iron. In addition, it is one of the few sources of dietary γ-linolenic acid and also contains other bioactive substances that have potential health benefits [1-4]. In 1996, the United Nations World Health Organization declared Spirulina as “the best for tomorrow” and as one of the “greatest super food on earth”. Furthermore, Spirulina is a powerful stimulant for the immune system, as shown in animal experiments, by increasing the phagocytic and natural killer activities [5]. In vitro and animal studies have suggested that Spirulina possesses antiviral effects [6]. Moreover, several studies have reported that Spirulina can prevent or inhibit cancers in animals [7,8]. Recently, it is reported that Spirulina has an important antioxidant potential. In fact, Spirulina contains vitamin E, carotenoids, chlorophyll and phycobiliproteins, which are well known to decrease « DPPH• » radicals by their hydrogendonating ability [9]. Besides nutritional value and medicinal properties, Spirulina flour or its protein extract have interesting functional properties with respect to water retention, fat absorption, emulsification capacity and foaming capacity as compared to soybean meal and egg protein [10,11]. Spirulina proteins were able to reduce the interfacial tension at the aqueous/air interface already at relatively lower bulk concentrations than common food proteins. It was also shown that the surface-active components were liked to be protein-pigment complexes rather than individual protein molecules. Moreover, the SDS-PAGE of the Spirulina proteins showed several bands in the range of 14.4-116 kDa, with the most pronounced bands corresponding to molecular masses of 20.1 and 43 kDa [12].
Nowadays there is an increasing consumer demand for more natural food products, having healthy benefits. Microalgae are able to enhance the nutritional content of conventional food and hence to positively affect humans’ health due to their original chemical composition. Relatively little information is available on the effect of Spirulina (Arthrospira platensis) incorporation into pastry products. Thus, the current work reports the characterization of Spirulina as well as the impact of its addition on the firmness, sensory characteristics, and color and oil retention of almond paste.
Material and Methods
Spirulina
Spirulina powder was purchased from the “Spiruline BioMed” farm (Medenine, Tunisia).
Chemical analysis
Moisture, protein, fat and ash contents of Spirulina were determined according to AOAC [13]. Total phenolics and flavonoids contents were measured as previously described [14,15]. Total phenolics content was expressed as mg gallic acid equivalents (GAE)/100g of Spirulina. Flavonoids content was expressed as mg quercetin equivalents (QE)/100g of Spirulina.
Techno-functional characteristics
The water holding capacity (WHC) was expressed as g of water bound per 100g of Spirulina and Fat Absorption Capacity (FAC) was expressed as g of oil bound per 100g of Spirulina [16].
Color measurement
Color measurement parameters (Lightness L*, redness a* and yellowness b*) were carried out using a color flex spectrocolorimeter (Hunter Associates Laboratory Inc., Reston, USA). The L* value indicates the lightness, 0-100 representing dark to light, b* value indicates the degree of the blue-yellow color, with a higher positive b* value indicating more yellow and a* value gives the degree of the green-red color, with a higher positive a* value indicating more red.
Evaluation of antioxidant activities
Preparation of ethanolic extract: The Spirulina (25 g) was Soxhlet-extracted using 300ml of ethanol during 6h. The average yield of the Spirulina extract was found to be 3.26% (m/m). The solvent was then evaporated under vacuum and the residual solvent was removed by flushing with nitrogen. Finally, the obtained extract was kept in the dark at 4°C until further analysis.
Free radical scavenging activity on 1, 1-diphenyl-2- picrylhydrazyl (DPPH): The « DPPH• »-radical scavenging activity was determined as described previously by Bersuder et al, [17]. Extract solutions were prepared at different concentrations from 0.5 to 6mg/ ml. In each tube, 500μl of the sample solution at all concentration was allowed to react with 375μl of ethanol solution and 125μl of 0.02% « DPPH• ». The reaction mixtures were incubated for 60 min in the dark at room temperature and the reduction of « DPPH• » radical was measured at 517nm. The « DPPH• »-radical scavenging activity was calculated as follows:
Scavenging activity (%) = [(ODC - ODS)/ODC] × 100
Where ODC, and ODS represent the absorbance’s of the control and the sample reaction tubes, respectively. Extract concentration (mg/ml) providing 50% inhibition (IC50) was calculated from the graph plotting scavenging activity against extract concentration. All tests were carried out for three sample replications and the results were averaged.
Ferric (Fe3+) reducing antioxidant power: The ability of sample to reduce iron was determined according to the method of Yildirim et al, [18] with slight modifications. A volume of 0.5ml of each sample, at different concentrations (0.1-2mg/ml), was mixed with 1.25ml of potassium phosphate buffer (0.2 M, pH 6.6) and 1.25ml of 1% potassium ferricyanide solution. The reaction mixtures were incubated for 20 min at 50°C. After incubation, 0.5ml of 10% trichloroacetic acid (TCA) was added and the reaction mixtures were then centrifuged for 10 min at 3000rpm. Finally, 1.25ml of the supernatant solution from each sample mixture was mixed with 1.25ml of distilled water and then 0.25ml of 0.1% ferric chloride was added. The absorbance of the resulting solutions was measured at 700nm after 10 min of incubation. The extract concentration providing 0.5 of absorbance (EC50) was calculated from the graph of absorbance at 700nm against extract concentration. All tests were carried out for three sample replications and the results were averaged.
Antibacterial activity
Microbial strains: Six pathogenic bacterial strains were used for antibacterial screening of Spirulina. Four Gram-positive bacteria: Micrococcus luteus (ATCC 4698), Staphylococcus aureus (ATCC 25923), Bacillus cereus (ATCC 11778) and Listeria monocytogenes sp, and two Gram-negative bacteria: Salmonella typhi (ATCC 19430) and Pseudomonas aeruginosa (ATCC 27853) were tested.
Agar diffusion method: The antibacterial activity assay was performed referring to the method described by Berghe and Vlietinck [19]. Culture suspensions (200μl) of the microorganisms (106 colony-forming units (cfu/ml) of bacterial cells were spread on Luria-Bertani (LB) agar. Then, 50μl of Spirulina ethanolic extract (150μg/ml) were loaded into wells punched in the agar layer. Before incubation, all plates were stored in the dark at 4°C for 2h, to allow the extract diffusion. At the end of incubation time (24h at 37°C for bacteria strains) positive antibacterial activities were established by the presence of measurable inhibition zones. Negative controls were prepared using sterile water. Gentamycin (10μg/well) was used as positive standards. Antimicrobial activity was evaluated by measuring the growth inhibition zone (diameter expressed in millimeters) around the wells.
Pastry product preparation and quality characterization
Almond paste preparation: Almond pastes (Kaak Mrachek) were prepared in a local pastry industry (Gourmandise, Sfax, Tunisia) and the standard formulation was composed by: almond fine powder (53.5g); sugar (28.4g); glucose (9g) and egg white (9.1g). The ingredients were mixed until a homogeneous texture paste was obtained. Then, the paste was cooked at 100°C during 10 min before being shaped manually. After that, cooking was continued at 50°C for 5 hours in a hot chamber. The finished product was finally packaged stored at 12°C, until analysis. Spirulina was incorporated at three levels (1, 3 and 5%) in the formulation of almond pastes, by substituting almond powder.
Mass loss and textural parameters analysis: Almond pastes (almost 100g) were placed in an enclosed area on several layers of Whatman papers, which absorb oil and water exudates. Temperature (29°C) and humidity (57% RH) were measured by a Kistock datalogger (Kimo, Montpon, France) and they were constant during the storage period (30 days). Mass loss was measured as the percentage loss of product mass in comparison with the initial mass. Hardness and elasticity of almond pastes were measured using a texturometer (Lloyd Instruments Ltd., West Sussex, UK) as previously described by Ayadi et al, [16].
Sensory evaluation: The sensory properties (color, odor, taste, texture, masticability and overall acceptability) were evaluated according to the method of Murray et al. (2001) by thirty panelists. A five-point hedonic scale was used, where 5: I like extremely; 4: I like moderately; 3: neither like nor dislike; 2: I dislike moderately; and 1: I dislike extremely for each attribute.
Statistical analysis
All analytical determinations were performed in triplicate. Oneway analysis of variance was conducted using SPSS software, 18.0. A difference was considered statistically significant at p‹0.05.
Results and Discussion
Spirulina characterization
Chemical and techno-functional properties: Table 1 presents mean values of a gross chemical composition of Spirulina used in the pasta formulations. This blue-green alga is exceptionally characterized by its high protein content (56.58g/100g of Spirulina), which is one of the main reasons to consider them as an unconventional source of proteins [20]. The chemical composition of microalgae is not an intrinsic constant factor but varies over a wide range. Environmental factors, such as temperature, illumination, pH-value, mineral contents, CO2 supply, algal density and growth phase can modify chemical composition of biomass [21]. Proteins are recognized by their interesting techno-functional properties that justify their incorporation into food products. Therefore, Water Holding Capacity (WHC) and Fat Absorption Capacity (FAC) were also assessed (Table 1). Spirulina used in this study showed interesting WHC (373.7g water/100g of Spirulina) that was comparable to that of soy proteins [22]. This ability to absorb water is influenced by the presence of ionizable groups, pH and the presence of salts. The amino acid composition also influences the ability of proteins to absorb water. In fact, the polar groups of proteins tend to bind the molecules of water easily. This property of fixing water by proteins is very important in many foods since it plays a determining role in the texture of these foods. Alongside its hydration property, Spirulina also possessed an important capacity to hold oil with an estimated FAC of 647.3g oil/100g of Spirulina, which is twice as high as that of soy protein [22]. This can be explained by the amphiphilic nature of the proteins that can effectively stabilize the oil/water phases of an emulsion. In particular, fat absorption property could be exploited in some food products to enhance their retention of fat and flavor, and to increase the technological yield [23].
Chemical composition
Moisture
13.25±0.10
Proteina
56.58±0.96
Fata
3.51±0.35
Asha
9.68±0.50
Total phenolicsb
173.28±3.21
Flavonoidsc
27.87±2.54
Antioxidant activities
DPPH• Scavenging activity (IC50, mg/ml)
0.30±0.05
Ferric (Fe3+) reducing power (EC50, mg/ml)
0.56±0.04
Techno-functional characteristics
Water holding capacitya
373.70±21
Fat absorption capacitya
647.30±34
ag/100 g of Spirulina; b mg gallic acid equivalents (GAE)/100 g of Spirulina; c mg quercetin equivalents (QE)/100 g of Spirulina.
Table 1: Chemical, antioxidant and techno-functional properties of Spirulina.
Phenolic compounds are primarily responsible for antioxidant properties. Thus, the total phenolics and flavonoids contents of Spirulina extract were determined (Table 1). The obtained results showed that total phenolics and flavonoids contents were 173.28mg GAE/100g of Spirulina and 27.87mg QE/100g of Spirulina, which contribute to the antioxidant potential of Spirulina in addition to proteins.
Antioxidant and antimicrobial activities: The antioxidant activities of the Spirulina were investigated by using Fe3+ reducing and « DPPH• » radical-scavenging assays (Table 1). Ferric reducing antioxidant power measures the reducing ability against ferric ion (Fe3+), which indicates the ability of compounds to give an electron to the ion [24]. Based on absorbance values, Spirulina was relatively an effective reducing agent with an EC50 value of 0.56mg/ml. Besides, the scavenging effect of Spirulina on « DPPH• » radicals was evaluated and the IC50 value was found to be 0.30mg/ml (Table 1). Nevertheless, the reducing power or the scavenging activity of Spirulina remained significant lower (p‹0.05) than those of the standard butylhydroxyanisol (BHA) (data not shown). Although the standard BHA has shown higher antioxidant activity than Spirulina, natural antioxidants were more interesting as compared to artificial ones that were suspected of having negative health effects on consumer health [25].
The antimicrobial activities of Spirulina against four Grampositive and two Gram-negative bacteria species were evaluated by determining the inhibition zones (mm) on solid medium (Table 2). The Spirulina presented relatively an interesting antibacterial potential against all investigated micro-organisms. In fact, the values of the inhibition zones of the tested strains varied between between 9.50 and 13.0mm.
Strains
Inhibition zone diameters (mm)
Spirulina
extract
Gentamycin
Gram -
P. aeruginosa
9.50±0.50
25±1
S. typhi
13.00±0.50
15±2
Gram +
M. luteus
10.50±0.70
18±1
S. aureus
10.00±0.50
37±1
B. cereus
9.50±0.70
22±2
L. monocytogenes
13.00±0.50
19±1
Table 2: Antimicrobial activities of Spirulina ethanolic extract against Grampositive and Gram-negative bacteria.
Effect of Spirulina on the Kaak Mrachek (almond paste) quality
Spirulina was used as a new functional ingredient to improve the nutraceutical and technological properties of Kaak Mrachek that is widely distributed among Tunisian consumers. The Kaak Mrachek, inspired by the traditional Kaak with its ring shape, is a confectionary product prepared from an almond paste topped with broken pistachios.
Therefore, 4 formulations were prepared: F0 (control formulation), F1 (formulation enriched with 1% of Spirulina), F2 (formulation enriched with 3% of Spirulina) and F3 (formulation enriched with 5% of Spirulina). A study of the texture, color, mass loss (Table 3) and sensory properties (Figure 1) of the prepared products was carried out.
Graphical Abstract:
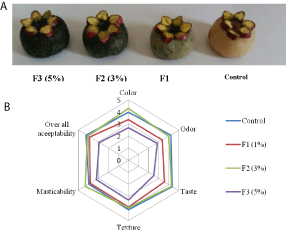
Figure 1: (A) Kaak Mrachek prepared with 1% (F1), 3% (F2) and 5% (F3) of
Spirulina. The control represents the product without enrichment. (B) Sensory
evaluation of the formulated products. The attributes were evaluated using
a five-point hedonic scale, where 5: I like extremely; 4: I like moderately; 3:
neither like nor dislike; 2: I dislike moderately; and 1: I dislike extremely for
each attribute.
Addition level
(g/100 g of product)
Control (0)
F1 (1%)
F2 (3%)
F3 (5%)
Texture parameters
Hardness (N)
3.70±0.25a
3.88±0.33a
4.01±0.25b
5.95±0.31c
Elasticity (mm)
6.35±0.41a
5.22±0.24a
4.38± 0.31b
2.27± 0.35c
Color parameters
L*
60.25±0.85a
47.53±0.60b
36.45±0.55c
32.15±0.25d
a*
4.10±0.04a
-1.10±0.23b
-1.57±0.16b
-3.85±0.18c
b*
23.45±0.87a
12.50±0.56b
5.82±0.42c
2.22±0.32d
Mass loss (%)
11.03±0.50a
9.66±0.60b
7.28±0.80c
5.52±0.60d
a,b,c,d Values with same superscript letters in the same line are non-significant at p<0.05.
Table 3: Texture and color parameters and mass loss of almond paste enriched with Spirulina.
Results of instrumental texture analysis of formulated products showed that hardness, expressing the maximum energy necessary for the destruction of the product structure, increased significantly (p‹0.05) with increasing Spirulina level at 3 or 5% (Table 3). In addition, Spirulina enrichment at 3 or 5% decreased significantly (p‹0.05) the elasticity of the final product. The increase in F2 or F3 hardness can be explained by the effect of proteins that enhance the product structure. Table 3 also shows that Spirulina proteins, presenting an important fat absorption capacity, reduced significantly (p‹0.05) the mass loss of the product after 1-month storage. The almond paste color is an important parameter to determine its acceptability. The effect of adding Spirulina on the color characteristics of Kaak Mrachek is also presented in Table 3. The obtained results showed that color parameters (L*, a* and b*) significantly (p‹0.05) decreased with the increase in blue-green algae level. The enriched products were darker than the control, which explains the significant decrease (p‹0.05) in L* values. Indeed, the L* value decreased from 60.25 (control product) to 32.15 for F3 formulation. As the level of enrichment with Spirulina increased, the product become more greenish (Figure 1A). This could also be explained by the richness of Spirulina in pigments, in particular phycobiliproteins.
Sensory analysis was performed by evaluating the color, odor, taste, texture (firmness), mastic ability and overall acceptability of fresh formulated almond paste (Figure 1). The Kaak Mrachek color was an important parameter affecting its acceptability. Interestingly, the phycobiliprotein-rich Spirulina gave a green color to the product, which reminds to that of pistachio-based Kaak Mrachek. However, the obtained results showed that the increase of Spirulina level at 5% decreased the trend of the average scores for the most analyzed attributes. Regarding the overall acceptability, almond paste supplemented with 3% of Spirulina (F2) remained acceptable, since the average score obtained for overall acceptability was 4.10 (Figure 1B).
Conclusion
Obtained results suggest that Kaak Mrachek (almond paste) enriched with 3% of Spirulina showed no undesirable organoleptic response and the product remained acceptable. The combination of the exceptional nutraceutical value of microalgae with coloring properties, associated with an increase demand in natural products, make microalgae worth exploring for utilization in food industry, comparing with the traditional ingredients. In perspective, it is interesting to study the microbiological and oxidative stability of the formulated product.
References
- Kapoor R, Mehta U. Utilization of beta-carotene from Spirulina platensis by rats. Plant Food Hum Nutr. 1993; 43: 1–7.
- Simpore J, Kabore F, Zongo F, Dansou D, Bere A, Pignatelli S, et al. Nutrition rehabilitation of undernourished children utilizing Spiruline and Misola. Nutr J. 2006; 5: 3–7.
- Sharma MK, Sharma A, Kumar A, Kumar M. Spirulina fusiformis provides protection against mercuric chloride induced oxidative stress in Swiss albinomice. Food Chem Toxicol. 2007; 45: 2412–2419.
- Gouveia L, Batista AP, Sousa I, Raymundo A, Bandarra NM. Microalgae in novel food products. In: Papadopoulos KN, ed. Food Chemistry Research Developments. 2008; Nova Science Publishers, Inc.
- Qureshi MA, Ali RA. Spirulina platensis exposure enhances macrophage phagocytic function in cats. Immunopharm Immunot. 1996; 18: 457–463.
- Shih SR, Tsai KN, Li YS, Chueh CC, Chan EC. Inhibition of enterovirus 71–induced apoptosis by allophycocyanin isolated from a blue-green alga Spirulina platensis. J Med Virol. 2003; 70: 119–125.
- Mohan IK, Khan M, Shobha JC, Naidu MU, Prayag A, Kuppusamy P, et al. Protection against cisplatin-induced nephrotoxicity by Spirulina in rats. Cancer Chemoth Pharm. 2006; 58: 802–808.
- Roy KR, Arunasree KM, Reddy NP, Dheeraj B, Reddy GV, Reddanna P. Alteration of mitochondrial membrane potential by Spirulina platensis C-phycocyanin induces apoptosis in the doxorubicin-resistant human hepatocellular–carcinoma cell line HepG2. Biotechnol Appl Bioc. 2007; 47: 159–167.
- Gad AS, Khadrawy YA, El-Nekeety AA, Mohamed SR, Hassan NS, Abdel- Wahhab MA. Antioxidant activity and hepatoprotective effects of whey protein and Spirulina in rats. Nutrition. 2010.
- Anusuya Devi M, Venkataraman LV. Functional properties of protein products of mass cultivated blue-green alga Spirulina platensis. J Food Sci. 1984; 49: 24–27.
- Nirmala C, Prakash V, Venkatarman LV. Physicochemical and functional properties of proteins from spray dried algae (Spirulina platensis). Nahrung. 1992; 36: 569–577.
- Chronakis IS, Galatanu AN, Nylander T, Lindman B. The behavior of protein preparations from blue-green algae (Spirulina platensis strain Pacifica) at the air/water interface. Colloid Surf A. 2000; 173: 181–192.
- AOAC. Official methods of analysis. Association of Official Analytical Chemists, AOAC, Washington D.C. 1995; 87-90.
- Sun B, Richardo-da-Silvia JM, Spranger I. Critical factors of vanillin assay for catechins and proanthocyanidins. J Agric Food Chem. 1998; 46: 4267–4274.
- Dewanto V, Wu X, Adom KK, Liu RH. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J Agric Food Chem. 2002; 50: 3010–3014.
- Ayadi MA, Abdelmaksoud W, Ennouri M, Attia H. Cladodes from Opuntia ficus indica as a source of dietary fiber : Effect on dough characteristics and cake making. Ind Crop Prod. 2009; 30: 40–47.
- Bersuder P, Hole M, Smith G. Antioxidants from a heated histidine–glucose model system. Investigation of the antioxidant role of histidine and isolation of antioxidants by high performance liquid chromatography. J Am Oil Chem Soc. 1998; 75: 181–187.
- Yildirim A, Mavi A, Kara AA. Determination of antioxidant and antimicrobial activities of Rumex crispus L. extracts. J Agric Food Chem. 2001; 49: 4083– 4089.
- Berghe VA, Vlieinck AJ. Screening methods for antibacterial and antiviral agents from higher plants, Methods. Plant Biochem. 1991; 6: 47–48.
- Soletto D, Binaghi L, Lodi A, Carvalho JCM, Converti A. Batch and fed batch cultivations of Spirulina platensis using ammonium sulphate and urea as nitrogen sources. Aquaculture. 2005; 243: 217–224.
- Affan MA, Lee DW, Al-Harbi SM, Kim HJ, Abdulwassi NI, Heo SJ, et al. Variation of Spirulina maxima biomass production in different depths of ureaused culture medium. Braz J Microbiol. 2015; 46: 991-1000.
- Wong PYY, Kitts DD. Comparison of the Buttermilk Solids Functional Properties to Nonfat Dried Milk, Soy Protein Isolate, Dried Egg White, and Egg Yolk Powders. J Dairy Sci. 2003; 86: 746–754.
- Thebaudin JY, Lefebvre AC, Harrington M, Bourgeois CM. Dietary fibers: nutritional and technological interest. Trends Food Sci Technol. 1997; 8: 41–48.
- Khantaphant S, Benjakul S. Comparative study on the proteases from fish pyloric caeca and the use for production of gelatin hydrolysate with antioxidative activity. Comp Biochem Physiol. 2008; 151:410–419.
- Namiki M. Antioxidants/antimutagenics in food. Cri. Re. Food Sci Nutr. 1990; 29: 273–300.s