
Special Article – Anti-cancer Food
Austin J Nutri Food Sci. 2019; 7(3): 1118.
Efficacious Anti-cancer Property of Liver from Peels Extract of Egyptian Citrus reticulata
Selim YA*, Mohamed H and Hussien SY
Faculty of Specific Education, Zagazig University, Egypt
*Corresponding author: Selim YA, Faculty of Specific Education, Zagazig University, Egypt
Received: April 22, 2019; Accepted: May 13, 2019; Published: May 20, 2019
Abstract
Cancer is one of human fatal diseases. Drug development for cancer intervention has many limitations in applications and often associated with serious of side effects. Recent development of natural product based and therapeutically sound anti-cancer agents have gained popularity in the field of functional foods, in which a few have minimal toxicity toward the prevention and treatment of cancer. Their structures were elucidated because of spectroscopic evidence. The cytotoxicity activity of 70% ethanolic extract of Citrus rutaceae peels were tested against colon carcinoma (HCT), human hepatocellular liver carcinoma (HepG2), human breast carcinoma (MCF7) cell lines and mouse fibroblast cell line NHK as normal cells. This assay gave spot on structure Citrus rutaceae peels and derived extract have demonstrated potent efficacious properties against liver cancer due in large part to the rich content of phenolic compounds such as flavonoids and coumarine compounds that, present in citrus peels. This study summarizes the results of currently available data regarding the in vivo anti-cancer activity of citrus peel phenolic compounds and identifies opportunities for subsequent human clinical trials to assess preventive and therapeutic effects in the near future according to Beijing Academy of Food Sciences.
Keywords: Citrus peels; Flavonoids; Antimicrobial activity; Cytotoxic activity
Introduction
Citrus plants belonging to the family Rutaceae which include fruits such as orange, mandarin, lime, lemon, sour orange and grape fruit appear as a well-known promising source of multiple beneficial nutrients for human beings. Processing of citrus byproducts potentially represents a rich source of phenolic compounds and dietary fiber, owing to the large amount of peel produced. These citrus fruit residues, which are generally discarded as waste in the environment, can act as potential nutraceutical resources. Due to their low cost and easy availability, such wastes are capable of offering significant low-cost nutritional dietary supplements. The utilization of these bioactive rich citrus residues can provide an efficient, inexpensive, and environment friendly platform for the production of novel nutraceutical or for the improvement of older ones [1,2]. Citrus is one of the most popular world fruit crops, contains active phytochemicals that can protect health. In addition to this, it provides an ample supply of vitamin C, folic acid, potassium and pectins [3,4]. It has been reported that citrus fruits, citrus fruit extracts and citrus flavonoids exhibit a wide range of promising biological properties due to their phenolic profile and antioxidant properties [5-7]. Early studies of Citrus have a great mission, as they often revealed a rich harvest of biologically active principles including essential oil, flavonoids [8] coumarone [9] and limonoids [10]. In addition, an established use of the citrus peel would also help alleviate pollution problems caused because of the poor disposal of such residues. More research is needed to establish bioavailability and real benefits of these peel extract. Dried peels of mature mandarin Citrus reticulata, was recorded as being appropriate for activation of vital energy and circulation, elimination of phlegm, disperse physical stagnation, etc. The primary active biological constituents of them are adrenergic amines (such as octopamine, and tyramine) [11], flavonoids (flavanones, flavones, and flavonols) and phenolic acids [12]. Due to there is less work on the peels of Citrus reticulata, in this study we investigated the cytotoxic activity of the extract of Citrus reticulata peels.
Experimental
General
Sigma Aldrich Chemical Co., St. Louis, Mo, U.S.A., was the source of the following chemicals. All chemicals and reagents used in this study are of highest analytical grade.
Plant material
The dried peels of Citrus reticulata L. were obtained from local market in Zagazig Government, Sharkia, Egypt and grinded to obtain a fine powder. The plant material was authenticated in Department of Botany, Faculty of Science, Zagazig University, Egypt. A voucher (voucher number: CR-134) sample was deposited for further references
Plant extraction
The dried and grained peels of C. reticulata (500gm) were extracted by 70% ethanol for 3 days x 3 times then filtered and evaporated under reduced pressure in rotary evaporator at 40oC. Almost 55gm of extract was obtained.
Phytochemical screening
Chemical test were carried out for ethanolic extract peels of C. reticulata. For the presence of phytochemical constituents [13].
HPLC analysis of phenolic compounds
This analysis was performed for the detection and quantification of particular phenolic compounds present in aqueous extracts of Carthamus tinctorius L. following a modified method [14]. HPLC analysis was carried out using an Agilent 1260 series. The separation was carried out using C18 column (4.6mm x 250mm i.d., 5μm). The mobile phase consisted of water (A) and acetonitrile (B) at a flow rate 1 ml/min. The mobile phase was programmed consecutively in a linear gradient as follows: 0 min (80% A); 0–5 min (80% A); 5-8 min (40% A); 8-12 min (50% A); 12-14 min (80% A) and 14-16 min (80% A). The multi-wavelength detector was monitored at 280nm. The injection volume was 10μl for each of the sample solutions. The column temperature was maintained at 35°C. HPLC analysis revealed the presence of a number of polyphenols in both extracts of Carthamus tinctorius L.
Biological activities
Antimicrobial activity: Biological activity (Sensitivity tests) was established by Kirby-Bauer Method. Antimicrobial activity of the tested samples was determined using a modified Kirby-Bauer disc diffusion method [15]. Briefly, 100μl of the test bacteria/fungi were grown in 10ml of fresh media until they reached a count of approximately 108 cells/ml for bacteria or 105 cells/ml for fungi [16]. 100μl of microbial suspension was spread onto agar plates corresponding to the broth in which they were maintained. Isolated colonies of each organism that might be playing a pathogenic role should be selected from primary agar plates and tested for susceptibility by disc diffusion method [17,18]. Plates inoculated with filamentous fungi as Candida albicans (ATCC 7102) at 25oC for 48 hours; Gram (+) bacteria as Staphylococcus aureus (ATCC 12600), Bacillus subtilis (ATCC 6051); Gram (-) bacteria as Escherichia coli (ATCC 11775), Pseudomonas aeuroginosa (ATCC 10145). They were incubated at 35-37oC for 24- 48 hours and yeast as Candida albicans incubated at 30oC for 24-48 hours and, then the diameters of the inhibition zones were measured in millimeters [14]. Standard discs of Ampicillin (Antibacterial agent), Amphotericin B (Antifungal agent) served as positive controls for antimicrobial activity but filter discs impregnated with 10μl of solvent (distilled water, chloroform, DMSO) were used as a negative control. Blank paper disks (Schleicher & Schuell, Spain) with a diameter of 8.0mm were impregnated 10μ of tested concentration of the stock solutions. When a filter paper disc impregnated with a tested chemical is placed on agar the chemical will diffuse from the disc into the agar. This diffusion will place the chemical in the agar only around the disc. The solubility of the chemical and its molecular size will determine the size of the area of chemical infiltration around the disc. If an organism is placed on the agar it will not grow in the area around the disc if it is susceptible to the chemical. This area of no growth around the disc is known as a “Zone of inhibition” or” Clear zone”. For the disc diffusion, the zone diameters were measured with slipping calipers of the National Committee for Clinical Laboratory Standards [17]. Agar-based methods such as Etest and disk diffusion can be good alternatives because they are simpler and faster than broth-based methods [19].
Cytotoxic activity
Human tumor cell lines: Human tumor carcinoma cell lines (Liver carcinoma cell line {HEPG2}, Colon carcinoma cell line {HCT} and Breast carcinoma cell line {MCF7}) used in this study were obtained from the American Type Culture Collection (ATCC, Minisota, U.S.A.). The tumor cell lines were maintained at the National Cancer Institute, Cairo, Egypt, by serial sub-culturing.
Drug
Samples were prepared by dissolving 1:1 Stock solution and stored at -20oC in dimethylsulfoxide (DMSO) at 100mM. Different concentrations of the drug were used 5, 12.5, 25, 50μg/ml.
Cells and culture conditions
RPMI-1640 medium was used for culturing and maintenance of the human tumor cell lines. The medium was supplied in a powder form. The working solution was prepared by dissolving 10.4gm powder and 2gm sodium bicarbonate dissolved in 1 L distilled water. The medium was then sterilized by filtration in a Millipore bacterial filter (0.22μm). The prepared medium was kept in a refrigerator (4°C). Before use the medium was warmed at 37°C in a water bath and the supplemented with 1% penicillin/streptomycin and 10% fetal bovine serum. Trypan blue dye: 0.05% of the dye was prepared and used for viability counting. Fetal Bovine Serum (FBS): 10% concentration was prepared and used for supplementation of RPMI1640 medium prior to use Penicillin/ Streptomycin: 100 units/ml Penicillin/2mg/ ml Streptomycin were used for the supplementation of RPMI-1640 medium prior to use. Trypsin-EDTA: 0.25% solution containing 2.5g Pocrine trypsin was used for the harvesting of cells. A cryotube containing frozen cells was taken out of the liquid nitrogen container and then thawed in a water bath at 37°C. The cryotube was opened under strict aseptic conditions and its contents were supplied by 5ml supplemented medium drop by drop in a 50ml sterile falcon tubes. The tube was incubated for 2 hours then centrifuged at 1200rpm for 10 minutes and the supernatant was discarded. The supernatant was discarded and the cell pellet was suspended and Seeded in 5ml supplemented medium in T25 Nunclon sterile tissue culture flasks. The cell suspension was incubated and followed up daily the supplemented medium was replaced every 2- 3 days. Incubation was continued until a confluent growth was achieved and the cells were freshly subculture before each experiment to be in the exponential phase of growth. The medium was discarded. The monolayer cell was washed twice with 5ml phosphate buffered saline. All the adherent cells were dispersed from their monolayer by the addition of 1ml trypsin solution (0.25% trypsin w/v) for 2 minutes. 50μl of 0.05% trypan blue solution was added to 50μl of the single cell suspension. The cells were examined under the inverted microscope using the haemocytometer. Non-stained (viable) cells were counted and the following equation was used to calculate the cell count /ml of cell suspension.
Viable cells /ml = number of cells in 4 quarters X 2 (dilution factor) X 104
The cells were then diluted to give the required cell number for each experiment. To avoid the loss of the cell line, excess cells were preserved in liquid nitrogen as follows: Equal parts of the cell suspension and freezing medium (10% DMSO in supplemented medium) were dispersed to cryotubes. The cryotubes were racked in appropriately labeled polystyrene boxes, gradually cooled till reaching -80oC. Then the cryotubes were stored in a liquid nitrogen (-180oC) till use.
Determination of potential cytotoxicity of drug on human cancer cell line
Principle: The cytotoxicity was carried out using Sulphorhodamine-B (SRB) assay following the method. Nat. Protoc. 2006:1, 1112-1116. SRB is a bright pink aminoxanthrene dye with two sulphonic groups. It is a protein stain that binds to the amino groups of intracellular proteins under mildly acidic conditions to provide a sensitive index of cellular protein content [20].
Reagants and buffers: Glacial acetic acid: 1% was used for dissolving the unbound SRB dye. Sulphorhodamine-B (SRB): 0.4% concentration was dissolved in 1% acetic acid was used as a protein dye. Trichloroacetic acid (TCA): 50% stock solution was prepared, 10% solution was used for protein precipitation. Tris base, 10mM, (pH 10.5) was used for SRB dye solubilization. It was prepared by dissolving 121.1gm of tris base in 1000ml distilled water and pH was adjusted by 2M HCl.
Procedure
Cells were seeded in 96-well microliter plates at initial concentration of 3x103 cell/well in a 150μl fresh medium and left for 24 hours to attach to the plates. Different concentrations 0, 12.5, 25, 50 and 100μg/ml of drug was added. For each drug concentration, 3 wells were used. The plates were incubated for 48 hours. The cells were fixed with 50μl cold trichloroacetic acid 10% final concentration for 1 hour at 40C. The plates were washed with distilled water using (automatic washer Tecan, Germany) and stained with 50μl 0.4% SRB dissolved in 1% acetic acid for 30 minutes at room temperature. The plates were washed with 1% acetic acid and air-dried. The dye was solubilized with 100μl/well of 10M tris base (pH 10.5) and optical density (O.D.) of each well was measured spectrophotometric ally at 570nm with an ELISA microplate reader (Sunrise Tecan reader, Germany). The mean background absorbance was automatically subtracted and means values of each drug concentration was calculated. The experiment was repeated 3 times.
Calculation
The percentage of cell survival was calculated as follows:
Surviving fraction = O.D. (treated cells)/ O.D. (control cells).
The IC50 values (the concentrations of resveratrol required to produce 50% inhibition of cell growth) were also calculated.
Results and Discussion
Phytochemical study
All results of phytochemical analysis are showed in the Table 1. In the present study, the 70% ethanolic extract of peels of showed positive results for triterpenes and/or steroids as measured by the Liebermann-Burchard reaction. It was found that D. tortuosa aqueous extract contained polyphenols and flavonoids, which may be responsible for the biological activities found.
Test
Result
Alkaloids
++
Anthraquinone
+
Coumarins
++
Flavonoids
+++
Glycosides
+++
Saponins
-
Steroids
++
Tannins
++
Terpenoids
++
Highly positive ‘+++’, Moderate ‘++’, Negative ‘-’
Table 1: Phytochemical screening of D. tortuosa extract.
Total phenolic compounds
HPLC analysis indicate the presence of Gallic acid, Chlorogenic acid, Catechin, Caffeine, Coffeic acid, Syringic acid, Rutin, Ellagic acid, Coumaric acid, Vanillin, Ferulic acid, Naringenin, Propyl Gallate, 4`.7-Dihydroxyisoflavone, Querectin and Cinnamic acid (Figure 1 & Table 2) that might have been responsible for their therapeutic potential. The amounts of polyphenols are shown in Table 1.
Polyphenolic compound
Conc. (μg/g)
Gallic acid
Chlorogenic acid
Catechin
Caffeine
Coffeic acid
Syringic acid
Rutin
Ellagic acid
Coumaric acid
Vanillin
Ferulic acid
Naringenin
Propyl Gallate
4`.7-DihydroxyisoFlavone
Querectin
Cinnamic acid
344.49
144.10
0.00
11.06
66.78
76.11
2.75
17.59
87.46
68.17
0.00
50.94
72.78
932.33
365.25
3.65
Table 2: Polyphenolic compounds of safflower flowers.
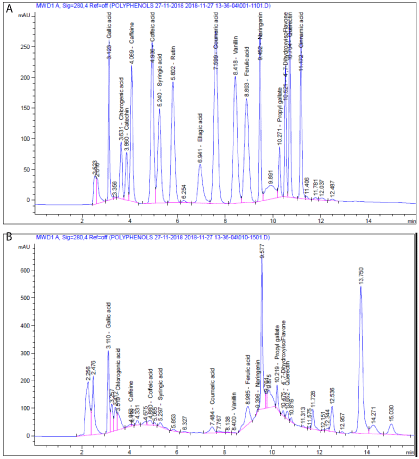
Figure 1: HPLC chromatogram: A) Standard mixture of polyphenolic compounds; B) ethanoic extract of safflower flowers.
Biological activities
Antimicrobial activity: Before discovered the existence of microbes, certain plants had healing potential, indeed, that they contained what we would currently characterize as antimicrobial principles was accepted. In recent years, emergences of bacterial resistance against multiple antibiotics have accelerated and are multi-drug resistant bacteria. So, there has been growing interest in substitution of synthetic antimicrobial agents by natural compounds has fostered research on vegetable sources and screening of plant materials in order to identify new compounds or test natural chemicals already known for important activities [21]. It found that all the 70% ethanolic extract of Citrus reticulate possess very high antimicrobial activity against gram negative bacteria as shown in Table 3. On the other hand the peels of citrus possess moderate antimicrobial activity against gram positive bacteria. These results may be attributed to the high poly phenolic contents which were observed from phytochemical screening and HPLC chart [22]. Also, citrus peels showed no an effect on the different strains of fungi.
Sample
Inhibition zone diameter(mm/mg sample)
Bacterial species
Fungi
(G+)
(G-)
Bacillus subtilis
Staphylococcus aureus
Escherichia
coli
Pseudomonas aeruginosa
Aspergillus flavus
Candida albicans
Control:
DMSO
0.0
0.0
0.0
0.0
0.0
0.0
Ethanolic 70% extract of peels of Citrus reticulata L.
50mg
0.0
0.0
11.0
11.0R
0.0
0.0
100mg
11.00
0.0
11.0
09.00
0.0
0.0
G: Gram reaction; R: Incomplete inhibition
Table 3: Antimicrobial activity of ethanoic extract of peels of Citrus on different cell lines.
Cytotoxic activity: The majority of the cancer treatments are accompanied by a degree of herbal supplements. There is advantageous effect of medicinal plants on cancer. Several therapies include herbal remedies to improve the quality of life. Plant derived natural products such as flavonoids, terpenes, alkaloids etc. have received wide attention in recent years due to their diverse pharmacological properties including cytotoxic and cancer chemo preventive effects [23]. The look for anti-cancer agents from plant sources started in the 1950s with the discovery and development of the vinca alkaloids like vincristine and vinblastine, and the isolation of the cytotoxic podophyllotoxines. Natural products discovered from medicinal plants have played a vital role in the management of cancer. Natural products or natural product derivatives consist of 14 of top 35 drugs in 2000 based on worldwide sales [24]. Plant based medication has definitely found a role in cancer healing (chemotherapy), and the mechanism of interaction between many phytochemicals and cancer cells has been studied extensively. In particular, there is growing interest in the pharmacological estimation of various plants used in Indian tradition system of medicine. There are more than 2,70,000 higher plants existing on this planet. But only a small portion has been surveyed phyto chemically. So, it is anticipated that plants can provide potential bioactive compounds for the development of new leads to combat cancer diseases [25]. The phytochemical screening of HEBE showed presence of chemical compounds such as alkaloids, flavonoids, glycosides, phenolics, steroids, tannins and terpenoids, whereas saponins is absent in HEBE. Medicinal plants may contain various kinds of chemical components and their biological activities are not generally attributable to a single moiety [26]. The present study was undertaken to assess the cytotoxic activity of HEBE and AGSE. The cytotoxic result obtained in the present study demonstrates for the first time, to the best of our knowledge, that HEBE and AGSE caused a dose-dependent growth inhibitory effect. The cytotoxicity of ethanolic extract of peels of Citrus were tested against all cell lines colon carcinoma (HCT), human hepatocellular liver carcinoma (HepG2), human breast carcinoma (MCF7) and mouse fibroblast cell line NHK as normal cells. This assay gave spot overview on the cytotoxic activity of peels extract and total phenolic content relationship. IC50 of each cell line was then used to establish the toxicity of it. In general, cytotoxicity of 70% peels extract against colon carcinoma (HCT) and human breast carcinoma (MCF7) cell lines were no effect as IC50›15. On the other hand the same extract had high cytotoxic against human hepatocellular liver carcinoma (HepG2, IC50 9.9 μg/mL) and had no toxic effect on normal cell. In conclusion, high content of total phenolic especially, flavonoids more potent cytotoxic activity (Figure 2 & Table 4).
Conc.(μg/ml)
MCF7
HEPG2
HCT
BHK
0
1
1.568
1
1
12.5
0.868
0.6
0.615
0.891
25
0.653
0.495
0.57
0.55
50
0.242
0.425
0.245
0.175
100
0.232
0.522
0.26
0.091
IC50
34
9.9
30
28.5
Table 4: % cell survival of ethanolic extract of peels of Citruson different cell lines.
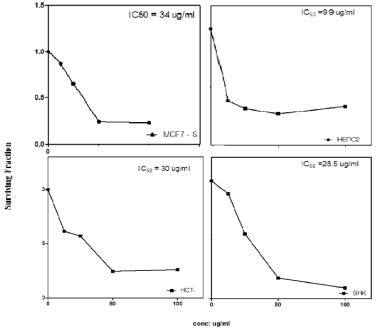
Figure 2: % cell survival of ethanoic extract of peels of Citrus on different
cell lines.
Conclusion
In this study, 70% ethanolic extract from peels of Citrus reticulata L. The antibacterial activity of all the citrus peels may be due to the high content of antioxidant components in its peels. Therefore, by future studies, it is possible to clear out the exact chemical nature of the compounds responsible for their action against the respective bacteria. If this goal is achieved soon, we can design great accuracy of specific chemical compounds from this plant part. Citrus peels extract have demonstrated potent activity against liver cancer due in large part to the rich content of phenolic compounds such as flavonoids and coumarone compounds.
References
- Shafiya R, Rajkumari K, Sofia SA, Nadia B, Fiza N, and Gulzar AN, Citrus peel as a source of functional ingredient: A review.Journal of the Saudi Society of Agricultural Sciences. 2018; 17: 351-358.
- Moore GA. Oranges and lemons: Clues to the taxonomy of Citrus from molecular markers. Trends in Genetics. 2001; 17: 536-540.
- Proteggente A, Saija A, De Pasquale CA. Rice-Evans The compositional characterisation and antioxidant activity of fresh juices from Sicilian sweet orange (Citrus sinensis L. Osbeck) varieties. Free Radic Res. 2003; 37: 681- 687.
- Guimarães R, Barros L, Barreira JCM, Sousa MJ, Carvalho AM. I.C.F.R. Ferreira Targeting excessive free radicals with peels and juices of citrus fruits: grapefruit, lemon, lime and orange. Food Chem Toxicol. 2009; 48: 99-106.
- Montanari A, Chen J, and Widmer W. Citrus flavonoids: a review of past biological activity against disease. J.A. Manthey, B.S. Buslig (Eds.), Flavonoids in the Living System, Plenum Press, New York. 1998; 103-113.
- Yamamoto ALM, Ayala E, Katrich ST. Characterization of antioxidant compounds in Jaffa sweeties and white grapefruits. Food Chem. 2004; 84: 503-510.
- Boskou D. Radical scavenging activity of various extracts and fractions of sweet orange flavedo (Citrus sinensis). Food Chem. 2006; 94: 19-25.
- Gattuso G, Barreca D, Gargiulli C, Leuzzi U, and Caristi C. Flavonoid Composition of Citrus Juices. Molecules. 2007; 12: 1641-1673.
- El-Shafae AM, Soliman AS. A pyranocoumarin and two alkaloids (one with antispasmodic effect) from Citrus deliciosa. Pharmazie. 1998; 53: 640-643.
- Khalil AT, Maatooq GT, El Sayed KA. Limonoids from Citrus reticulata. Z Nature for sch. 2003; 58: 165-170.
- Avula B, Joshi VC, Weerasooriya A, and Khan IA. Liquid chromatography for separation and quantitative determination of adrenergic amines and flavonoids from Poncirus trifoliatus Raf. Fruits at different stages of growth. Chromatographia. 2005; 62: 379-383.
- Xu GH, Ye XQ, Chen JC, Liu DH. Effect of heat treatment on the phenolic compounds and antioxidant capacity of citrus peel extract. J Agric Food Chem. 2017; 55: 330-335.
- Raad NH, Munim RA, Shakier SM, Khudhair AM, Moayad SH, Kadum YA. Anti-bacterial activity of aqueous and alcoholic extracts of Capsella Bursa against selected pathogenicbacteria. Amer J Bio Sci. 2013; 1: 6-10.
- Bauer AW, Kirby WM, Sherris JC, and Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1996; 45: 493-496.
- Shuangchan W, Yuan Y, Hui T, Zhike L, Xiaofei L, Wei H, and Hong D. Carthamus red from Punica granatum L. exerts antioxidant and hepatoprotective effect against ccl4-induced liver damage in rats via the Nrf2 pathway. J of Ethnopharmacology. 2013: 148: 570-578.
- Pfaller MA, Diekema DJ. Epidemiology of Invasive Candidiasis: a Persistent Public Health Problem. Clin Microbiol Rev. 2007; 20: 133–163.
- NCCLS. National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial disk susceptibility tests. Approved standard. Document M2-A5. Wayne, Pa: National Committee for Clinical Laboratory Standards. 1993.
- NCCLS. National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial disk susceptibility tests. Approved standard M2- A6. Wayne, Pa: National Committee for Clinical Laboratory Standards. 1997.
- Matar E, Shine JM, Naismith SL, Lewis SJ. Using virtual reality to explore the role of conflict resolution and environmental salience in Freezing of Gait in Parkinson’s disease. Parkinsonism Relat Disord. 2013; 19: 937–942.
- Vichai V, Kirtikara K, Sulforhodamine B. colorimetric assay for cytotoxicity screening. Nat Protoc. 2006; 1: 1112-1116.
- Abdel-Salam AF, and Mostafa FAA. Evaluation of antimicrobial activity and phytochmical analysis of citrus lemon, mandarin, and orange peel. J Agric Food Chem. 2004; 5: 43-56.
- Ehigbai IO, Ehimwenma SO, Faith EO, and Kelly O. Phytochemical, antimicrobial, and antioxidant activities of different citrus juice concentrates. Food Sci Nutr. 2016; 4: 103-109.
- Babu BH, Shylesh BS, and Padikkala J. Tumour reducing and anticarcinogenic activity of Acanthus ilicifolius inmice. J Ethno Pharmacol. 2002; 79: 27–33.
- Butlet MS. The role of natural product chemistry in drug discovery. J Nat Prod. 2004; 67: 2141-2153.
- Shoeb M. Anticancer agents from medicinal plants. Bang J Pharmacol. 2006; 1: 35-41.
- Cho EJ, Yokozawa T, Rhyu DY, Kim SC, Shibahara N, Park JC. Study on the inhibitory effects of Koreanmedicinal plants and their main compounds on the 1, 1- diphenyl-2- picrylhydrazyl radical. Phytomedicine. 2003; 10: 544-551.