
Special Article - Antioxidants in Food
Austin J Nutri Food Sci. 2019; 7(6): 1126.
Antibacterial and Anti-Proliferative Active Compositions of Water Chestunt Peel
Xu XJ, Huang RZ, Du Y and Liao ZX*
Department of Pharmaceutical Engineering, School of Chemistry and Chemical Engineering, Southeast University, PR China
*Corresponding author: Liao ZX, Department of Pharmaceutical Engineering, School of Chemistry and Chemical Engineering, Southeast University, Nanjing 211189, PR China
Received: June 27, 2019; Accepted: July 25, 2019; Published: August 01, 2019
Abstract
Water chestunt (Heleocharis dulcis (Burm. f.) Trin) is a perennial shallow water herb and native fruit of India. In this study, the chemical constituents of water chestunt peel were investgated. The results showed that dry peel of water chestunt is rich in active chemical compositions. Twenty monomeric compounds were isolated. Among them, seven compounds were firstly found from this plant. The results of antibacterial experiments showed that some phenolic acids had better inhibitory effect. Such as ferulic acid (9) and caffeic acid (16) exhibited good inhibitory activity against four bacteria tested. In vitro anti-proliferative activities of seven compounds on four human cancer cell lines were evaluated, the results showed that 17-33 ketone (15) possessed the most potent effects with the IC50 values of 10.20±0.62 μmol·l-1 against T24 cells. The further bioactivity analysis showed that compound 15 induced apoptosis of T24 cells, and altered antiand pro-apoptotic proteins, leading to mitochondrial dysfunction and activations of caspase-3/9 for causing cell apoptosis. The present investigation illustrated compound 15 might be used as a potential antitumor chemotherapy candidate.
Keywords: Water chestunt peel; Chemical constituents; Antibacterial; Antiproliferative activities
Abbreviations
MIC: Minimal Inhibitory Concentration; MHB: Mueller- Hinton Broth; DMSO: Dimethyl Sulfoxide; IC50: 50% Inhibition Concentration; MGC-803: Human Gastric Cancer Cells; SKOV3: Human Ovarian Cancer Cells; T24: Human Bladder Cancer Cells; HepG2: Human Liver Cancer Cells; ROS: Reactive Oxygen Species; DCFH-DA, Dichlorofluorescin Diacetate; DMEM: Dulbecco’s Modified Eagle Medium; PMSF: Phenylmethyl Sulphonylfluoride; RIPA: Radio Immunoprecipitation Assay
Introduction
Edible plants with certain medicinal value generally contain active constituents with antibacterial and anti-tumor effects. Alzoreky and Nakahara [1] studied antibacterial activity of extracts from edible plants (26 species) from China, Japan, Thailand and Yemen, the results showed most of them had strong antimicrobial activity against Bacillus cereus, Staphylococcus aureus, Listeria monocytogenes, Escherichia coli and Salmonella infantis. The anti-tumor activity of 50% ethanol extract from longan (Dimocarpus longan Lour.) pericarp was determined by Prasad et al. [2]. The results showed that it could inhibit the proliferation of SGC-7901 and A549 tumor cells significantly. Allicin in garlic can also induce apoptosis of prostate cancer DU-145 [3] and bladder cancer T24 [4]. Fan et al. [5] found that acidic polysaccharides of Gracilaria lemaneiformis had a significant inhibitory effect on the growth of transplanted H22 hepatocellular carcinoma. Edible plant resources have the characteristics of high safety and low toxicity, and further development and utilization of them will have a broader prospect.
Water chestnut is one of edible plants. It is a perennial shallow water herb and native fruit of India usually cultivated all over China and widely distributed around the world, especially in tropical and subtropical regions. It is an excellent fruit and vegetable and can be also used as medicine [6]. According to the Compendium of Materia Medica, water chestunt can help digestion, eliminating phlegm, moistening lung, relieve fever and antialcoholismic. Various medical studies had also shown that water chestunt had functions of antialcoholismic, appetizing, antitumor, antibacterial, preventing respiratory diseases and so on [7-9]. It was also reported that polyphenols in water chestnut have strong antioxidant activity [10]. Luo et al. [11] determined the reducing power and DPPH free radical scavenging rate of the extract of water chestnut peel. The results showed that the extract of water chestnut peel had strong antioxidant activity, indicating that water chestnut peel was an excellent source of natural antioxidants. Zhan et al. [12] studied the antimicrobial activity of the extract of water chestnut peel against Staphylococcus aureus, Escherichia coli and Listeria, the results showed that the ethyl acetate fraction of water chestnut peel extract showed superior antimicrobial activity. At present, there are few reports on the antitumor effect of water chestnut.
The peel of water chestunt was a waste material after processing and eating, most of which was used as household fertilizer usually. However, the active compositions flavonoids, polysaccharides and polyphenols were found in the peel of water chestnut in the literatures [13,14]. Nowadays, there are more and more reports about the resource utilization of the water chestunt peel. Luo et al. [15] developed a healthy beverage which rich in flavonoids with the peel as raw material. Gao et al. [16] used the extract of water chestunt peel, honey and vinegar to make a vinegar beverage. Guo [17] found the good antioxidant activity of pigment of the water chestunt peel and added them into food to prevent the oxidation of lipids and vitamins. The above applications highlight the value of water chestnut peel. In the present study, the biological activities of isolated compounds of Heleocharis dulcis (Burm. f.) Trin peel on diverse bacteria and human cancer cells were investigated. The objective was to document the biochemical composition and the antibacterial and anticancer potentials of the peel extracts.
Materials and Methods
Samples
Dry water chestunt peel was obtained from Guli town, Nanjing, Jiangsu Province, China in 2017.
Extraction and isolation
The dry water chestunt peel (7 kg) was extracted with 95% ethanol (30.0 l×5) at room temperature. After removal of the solvent, the extractum (127.2 g) was suspended in water (2000.0 ml), partitioned sequentially with petroleum ether (PE) (2000 ml×5 times), EtOAc (2000 ml×5 times), n-butanol (1000 ml×5 times) to yield crude PE (25.3 g), EtOAc (40.0 g), n-butanol (25.0 g) extracts respectively. The crude PE extract was decolorized on MCI gel (Mitsubishi Chemical Holoings, Japan), then a sample (13 g) was subjected to silica gel CC (Qingdao Haiyang Chemicals, China) using a gradient system with increasing polarity of PE/EtOAc (from 50:1 to 1:7, v/v) to yield compounds 1, 2, 3 and 4. The EtOAc extract was used the same method to afford compounds 5~14. Due to the high polarity of n-butanol phase compounds, CH2Cl2/CH3OH (30:1/8:1, v/v) system was used as eluent for silica gel column chromatography, then compounds 15~20 were obtained.
MIC determination
The frozen bacterial liquid of 1 ml was added 13 ml broth, the broth was sealed and cultured in a shaking bed (SHZ-82, Changzhou Guohua Electric Appliance Co., Ltd, China) at 37~180 r·min-1 for 18- 24 hours. After the bacteria reached the exponential phase of growth, centrifuged for 5 minutes at 3000 r·min-1, discarded the supernatant, mixed with 4-5 ml PBS solution, centrifuged again under the same conditions, discarded the supernatant again, added 1 ml PBS solution to dilute the bacterial solution to visible turbidity to the naked eye. Then, the 200 μl above-mentioned bacterial solution mixed with 20 ml MHB was stand-by. The 96-well plate was added with MHB, compounds solution, bacterial suspension in sequence. The final volume of each hole was 100 μl and the drug concentration was 512, 256, 128, 64, 32, 16, 8, 4, 2, 1, 0.5 μg·ml-1 in turn. The last hole of each row was growth control without drugs, and the last row was treated with DMSO with the same concentration as the experimental group. The 96-well plate was incubated in incubator for 18-24 h at 37oC. The minimal concentration in the pore without bacterial growth was MIC of the tested drug. All the measurements were repeated three times.
In vitro cytotoxicity assay
The cell lines MGC-803 (Human gastric cancer cells), SKOV3 (Human ovarian cancer cells), T24 (Human bladder cancer cells) and HepG2 (Human liver cancer cells) were obtained from the Institute of Biochemistry and Cell Biology, China Academy of Sciences. The tested cell lines were grown on 96-well microtitre plates at a cell density of 10×105 cells well-1 in DMEM medium with 10% FBS. The plates were incubated at 37oC in a humidified atmosphere of 5% CO2/95% air for overnight. Therewith, the cells were exposed to different concentrations of compounds 2, 5, 6, 8, 14, 15 and 17, and incubated for another 48 h. The cells were stained with 10 μl of MTT at incubator for about 4 h. The medium was thrown away and replaced by 100 ml DMSO. The O. D. Value was read at 570/630 nm enzyme labeling instrument. The final IC50 (50% inhibition concentration) values were calculated by the Bliss method (n=5). All the tests were repeated in at least three independent experiments.
Apoptosis analysis
T24 cells were seeded into 6-well plate at a concentration of 2×106 well-1 in 10% FBS DMEM and treated with compound 15 for 24 h. The Cells were rinsed twice with cold Phosphate Buffered Saline (PBS) and then resuspend cells in 1×Binding Buffer (0.1 mol·l-1 Hepes-NaOH (pH 7.4), 1.4 mol·l-1 NaCl, 25 mmol·l-1 CaCl2) at a concentration of 1×106 cells·ml-1, and incubated with 5 μl Annexin V fluorescein isothiocyanate and 5 μl propidium iodide for 0.5 h in dark at 25oC. Then PBS was added and analyzed immediately with the system software (Cell Quest; BD Biosciences).
Hoechst 333258 staining
T24 cells were seeded on a sterile cover slip in 6-well plates were treated with compound 15 for a certain range of time. The culture medium containing compound 15 was removed, and the cells were fixed in 4% paraformaldehyde for 10 min. After twice PBS washes, the cells were stained with 0.5 ml of Hoechst 33258 (Beyotime, Haimen, China) for 5 min and then again rinsed twice with PBS. The stained nuclei were viewed under a Nikon ECLIPSETE2000-S fluorescence microscope using 350 nm excitation and 460 nm emissions.
AO/EB staining
T24 cells were seeded on a sterile cover slip in 6-well tissue culture plates at a concentration of 5×104 cells·ml-1 in a volume of 2 ml. Following appropriate cultivation, the medium was removed and replaced with fresh medium plus 10% foetal bovine serum and supplemented with concentrations of corydalisin C. After the treatment period, the cover slip with a cell monolayer was inverted on a glass slide with 10 μl of AO/EB stain (100 mg·ml-1). Fluorescence was observed on a Nikon ECLIPSETE2000-S fluorescence microscope.
Mitochondrial membrane potential staining
Cationic lipophilic dye JC-1 (Beyotime, Haimen, China) was employed to survey mitochondrial depolarization in T24 cells. Briefly, after the cells were incubated in 6-well plates and subjected to the indicated treatments, they were cultured with an equal volume of JC-1 staining solution (3 mg·ml-1) at 37oC for 20 min and washed twice with PBS. The change in mitochondrial membrane potentials was measured by determining the relative amount of dual emissions from mitochondrial JC-1 monomers or aggregates using flow cytometry. Mitochondrial depolarization was identified by an increase in the green/red fluorescence intensity ratio.
ROS assay
T24 cells were grown on 6-well plates for 24 h and subjected to various treatments. Then, the cells were cultured in a cell-free medium solution containing 10 mmol·l-1 DCFH-DA (Beyotime, Haimen, China) at 37oC for 0.5 h in dark and rinsed three times with PBS. Cellular fluorescence was quantified using flow cytometry at an excitation of 485 nm and an emission of 538 nm.
Western blot
From cultured T24 cells after compound treatments as mentioned earlier, total cell lysates were prepared by lysing the cells in ice-cold RIPA buffer (1×PBS, 1% NP-40, 0.5% sodium deoxycholate and 0.1% SDS) containing 100 μg·ml-1 PMSF. After centrifugation at 12,000 r·min-1 for 10 min, the protein in supernatant was analyzed by Bradford method (BIO-RAD) using Multimode various can instrument (Thermo Fischer Scientifics). Thirty micrograms of protein per lane was applied in 12% SDS-PAGE. After electrophoresis, the protein was transferred to polyvinylidinedifluoride (PVDF) membrane (Amersham Biosciences). The membrane was blocked in TBST containing 5% blocking powder (Santacruz) at room temperature for 2 h. The membrane was rinsed with TBST for 5 min, treated with primary antibody and incubated at 4°C overnight (O/N). After three washes in TBST, the membrane was cultivated with corresponding horseradish peroxidase-labeled secondary antibody (1:2000) (Santa Cruz) at room temperature for 1 h. Membranes were rinsed with TBST three times for 15 min and the protein blots were materialized with chemiluminescence reagent (Thermo Fischer Scientifics Ltd.). The X-ray films were developed with developer and fixed with fixer solution.
Statistics
The data were processed by Student’s t-test with the significance level P ‹ 0.05 using SPSS software (17.0; SPSS, Inc., Chicago, IL, USA).
Results and Discussion
Chemical compositions
The structures of these compounds were identified by spectroscopy methods combined with literature data. β-sitosterol (1) [18] and β-daucosterol (4) [19] belong to steroids. Cinnamic acid (7) [20], ferulic acid (9) [21], p-coumaric acid (11) [22] and caffeic acid (16) [23] and p-hydroxybenzoic acid (19) [22] belong to phenolic acids. Betulinic acid (3) [24], (3β)-Lup-20(29)-ene-3,30-diol (6) [25] and betulin (8) [26] belong to triterpenes. Kaempferol (12) [27], quercetin (13) [22] and rutin (17) [22] belong to flavones. Chlorogenic acid (14) [23] belongs to phenylpropanoids. Coumarin (18) [28] belongs to simple coumarin compounds. 16-Hentriacontanone (2) [29], phenol,2,4-bis(1,1-dimethylethyl)-,1,1’,1”-phosphite (5) [30], n-tetratriacont-20,23-dienoic acid (10) [31], 17-33 ketone (15) [32] and n-butyl-β-d-fructofuranoside (20) [33] belong to other classes.
Antibacterial activity
In this paper, the antimicrobial activity of some monomer compounds were evaluated by micro-broth dilution method. The minimal inhibitory concentration of some compounds were shown in (Table 1). The results showed that compounds 3, 9, 15, 16 and 19 had stronger inhibitory effects on Escherichia coli, and their MICs (μg·ml-1) were 32, 64, 64, 64, 64 and 64 respectively. Compounds 9, 12 and 16 had stronger inhibitory effects on Staphylococcus aureus, and their MICs (μg·ml-1) were 64, 16 and 32 respectively. Compounds 14, 16 and 19 had stronger inhibitory effects on Bacillus subtilis, and their MICs (μg·ml-1) were all 32. Compounds 3, 9, 12 and 16 had stronger inhibitory effects on Pseudomonas aeruginosa, and their MICs (μg·ml-1) ranged from 16 to 32.
Compound
Escherichia coli
Staphylococcus aureus
Bacillus subtilis
Pseudomonas aeruginosa
2
128
256
128
64
3
32
256
64
32
5
128
256
128
128
9
64
64
64
32
10
128
128
128
128
11
128
128
128
64
12
128
16
64
32
14
128
128
32
64
15
64
256
128
64
16
64
32
32
16
17
128
128
64
64
19
64
128
32
128
20
128
256
128
256
MHB growth control
+
+
+
+
DMSO solvent control
+
+
+
+
Table 1: Minimal inhibitory concentration [μg·ml-1].
Cytotoxic effects
Some compounds isolated from water chestunt peel against the four cell lines (MGC-803, SKOV3, T24 and HepG2) were evaluated with MTT assay (Table 2). Compared with other compounds, compound 15 showed good inhibitory activity, especially for T24 cancer cell lines, with IC50 values of 10.20±0.62 μM (Table 3). The structure of compound 15 was shown in (Figure 1).
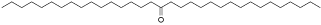
Figure 1: Structure of compound 15.
Compound
Inhibition rate at 50 μM concentration [%]
MGC-803
SKOV3
T24
HepG2
2
50.01
51.9
52.08
59.04
5
18.49
37.53
29.55
27.83
6
23.03
30.8
32.37
35.52
8
44.87
10.39
34.09
30.69
14
26.79
41.75
28.17
21.77
15
72.54
69.21
81.06
63.59
17
37.67
44.87
26.54
39.33
Table 2: Inhibition rate at 50 μM concentration [%].
Compound
MGC-803 [μM]
SKOV3 [μM]
T24 [μM]
HepG2 [μM]
2
48.09±1.19
46.67±1.25
45.15±0.79
41.04±0.83
15
20.02±1.47
25.65±1.13
10.20±0.62
35.37±0.77
5-Fu
45.57±1.78
42.03±1.49
38.46±0.86
30.96±1.27
Table 3: Cytotoxicity (IC50, μM) of compound 2, 15 and 5-Fu (5-fluorouracil).
Effects of compound 15 on the induction of apoptosis
In order to confirm whether compound 15 induced reduction in cell viability was responsible for the induction of apoptosis, T24 cells were co-stained with PI and Annexin-V FITC, and the number of apoptotic cells was estimated by flow cytometry. T24 cells were exposed to compound 15 for 24 h, and there was an obvious apoptotic performance between cells treated with experimental groups and the vehicle control group. As shown in (Figure 2), few (5.45%) apoptotic cells were present in the control panel; in contrast, the population rose to 12.88% at the concentration of 10 μM after treatment with compound 15 for 24 h. Further increase to 35.25% occurred after treatment with 15 at the concentration of 40 μM. The results clearly confirmed that compound 15 triggered apoptosis on T24 cells in dose-dependent apoptotic features from 0 to 40 μM.
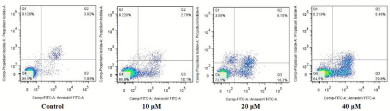
Figure 2: Apoptosis Annexin V-FITC/PI.
Morphological characterization of cell apoptosis of T24 cells by Hoechst 33258 staining
In order to further validate the cell apoptosis upon treatment of compound 15, T24 cells treated with compound 15 for 24 h were stained with Hoechst 33258. Our experimental observation showed that, in the control group, most of the cells exhibited the weak blue fluorescence of normal cells (Figure 3). After the treatment of compound 15, some cells emitted brilliant blue fluorescence, and nuclei of more T24 cells appeared hyper condensed (brightly stained). Remarkably, the numbers of apoptotic nuclei containing condensed chromatin increased significantly after the T24 cells were treated with compound 15 for 24 h, indicating that apoptosis of the T24 cells was induced by compound 15 in a concentration-dependent manner.
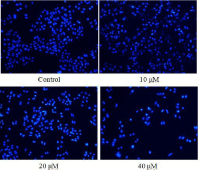
Figure 3: Hoechst 33258 staining of T24 cells treated with compound 15.
Morphological characterization of cell apoptosis of T24 cells by AO/EB staining
Apoptosis was further evaluated using acridine orange/ethidium bromide (AO/EB) double staining, which differentiates between necrosis and apoptosis by the difference in membrane integrity. AO can pass through cell membranes of living or early apoptotic cells, while staining by EB indicates a loss of membrane integrity. The cytotoxicity of compound 15 for 24 h at the concentrations of 10, 20 and 40 μM was detected by AO/EB staining; T24 cells not treated with compound 15 for 24 h were used as controls. The results are shown in (Figure 4). The normal cells were stained only by AO and were bright green, while apoptotic cells stained by AO and EB were red-orange. These findings also confirmed that compound 15 was able to induce apoptosis.
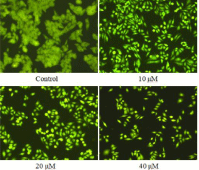
Figure 4: AO/EB staining of T24 cells treated with compound 15.
Compound 15 induced loss of Δψm in T24 cells
The loss of Δψm is regarded as a limiting factor in the apoptotic pathway. To further investigate the apoptosis-inducing effect of compound 15, mitochondrial membrane potential changes were assayed using the fluorescent probe JC-1. T24 cells treated with compound 15 for 24 h at concentrations of 10, 20 and 40 μM were stained with JC-1, while cells not treated with compound 15 were used as controls. Our results indicated that treatment with compound 15 led to the loss of Δψm in T24 cells (Figure 5). After T24 cells were exposed to 10, 20 and 40 μM of compound 15 for 24 h, Δψm was reduced to 74.0%, 42.9% and 28.0% of the control, respectively, suggesting the occurrence of depolarization of mitochondria by compound 15.
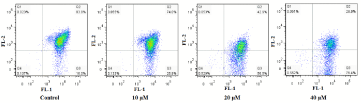
Figure 5: Effects of compound 15 on Δψm level of T24 cells.
Decrease of intracellular ROS level in T24 cells induced by compound 15
It is well known that an increase in intracellular ROS can lead to apoptosis, whereas a decrease in ROS can also ruin the stability of mitochondria, which is followed by a loss of mitochondrial transmembrane potential, release of cytochrome c into cytosol, and cascade activation of caspases [34,35]. The results are shown in (Figure 6). Compared with the control group, the curve of the treated group shifted to the right. With the increase of drug concentration, the right shift increased further, indicating that compound 15 can promote the production of ROS in cells.
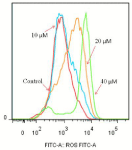
Figure 6: Effects of compound 15 on the intracellular ROS level of T24 cells.
Compound 15 induced expression of pro- and antiapoptotic proteins
The Bcl-2 family members are important regulators of the mitochondrial apoptotic pathway. Two most important members of Bcl-2 family, the anti-apoptotic protein Bcl-2 and the pro-apoptotic protein Bax, are key regulators of this progress [36]. The effects of compound 15 on the constitutive levels of Bax, Bcl-2 and caspase-3 in T24 cells are given in (Figure 7), western blotting testing revealed that elevation of Bax expression, compared with control group, whereas the protein expression level of Bcl-2 was decreased in a timedependent manner and in a dose-dependent manner.
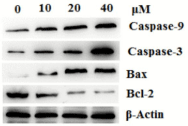
Figure 7: Effects of compound 15 on major protein markers in mitochondrial
pathway.
Conclusion
In this study, we investigated the chemical compositions of water chestnut peel. The results showed that dry peel of water chestnut is rich in chemical compositions. Twenty monomer compounds, including steroids, flavones, triterpenes and phenolic acids were isolated from water chestnut peel. In addition, some compounds in water chestnut peel had significant inhibitory activity against Escherichia coli, Staphylococcus aureus, Bacillus subtilis and Pseudomonas aeruginosa. Compound 15 demonstrated potent cytotoxic activities against the T24 cancer cell lines. The further bioactivity analysis showed that compound 15 induced apoptosis of T24 cell lines. Therefore, the peel of water chestunt has a rich prospect of food and medical applications. This study provided a basic knowledge for future development and utilization of the peel of water chestnut.
References
- Alzoreky NS, Nakahara K. Antibacterial activity of extracts from some edible plants commonly consumed in Asia. International Journal of Food Microbiology. 2003; 80: 223-230.
- Prasad KN, Hao J, Shi J, Liu T, Li J, Wei X, et al. Antioxidant and anticancer activities of high pressure-assisted extract of longan (Dimocarpus longan lour). Fruit pericarp. Innovative Food Science and Emerging Technologies. 2009; 10: 413-419.
- Herman-Antosiewicz A, Kim YA, Kim SH, Xiao D, Singh SV. Diallyl trisulfideinduced G2/M phase cell cycle arrest in DU145 cells is associated with delayed nuclear translocation of cyclin-dependent kinase 1. Pharmaceutical Research. 2010; 27: 1072-1079.
- Wang YB, Qin J, Zheng XY, Bai Y, Yang K, Xie LP. Diallyl trisulfide induces Bcl-2 and caspase-3-dependent apoptosis via downregulation of Akt phosphorylation in human T24 bladder cancer cells. Phytomedicine. 2010; 17: 363-368.
- Fan Y, Wang W, Song W, Chen H, Teng A, Liu A. Partial characterization and anti-tumor activity of an acidic polysaccharide from Gracilaria lemaneiformis. Carbohydrate Polymers. 2012; 88: 1313-1318.
- Kosuge T, Yokota M, Sugiyama K, Yamamoto T, Ni M, Yan S. Studies on antitumor activities and antitumor principles of Chinese herbs. i. antitumor activities of Chinese herbs. YAKUGAKU ZASSHI. 1985; 105: 791-795.
- Cai J. Nutritional Health Care and Processing Utilization of Water chestnut. Food and Nutrition in China. 2005; 2: 40-42.
- Gao MP, Jiang W, Wei SL, Lin ZC, Cai BH, Yang L, et al. High-efficiency propagation of Chinese water chestnut [Eleocharis dulcis (Burm. f.) Trin. ex Hensch] using a temporary immersion bioreactor system. Plant Cell Tissue and Organ Culture. 2015; 121: 761-772.
- Sun J, You YL, Garcia-Garcia E, Long X, Wang JB. Biochemical properties and potential endogenous substrates of polyphenoloxidase from chufa (Eleocharis tuberosa) corms. Food Chemistry. 2010; 118: 799-803.
- Parr AJ, Waldron KW, Ng A, Parker ML. The wall-bound phenolics of Chinese water chestnut (Eleocharis dulcis). Journal of the Science of Food & Agriculture. 1996; 71: 501-507.
- Luo Y, Li X, He J, Su J, Peng L, Wu X, et al. Isolation, characterization, and antioxidant activities of flavonoids from chufa (Eleocharis tuberosa) peels. Food Chemistry. 2014; 164: 30-35.
- Zhan G, Pan LQ, Mao SB, Zhang W, Wei YY, Tu K. Study on antibacterial properties and major bioactive constituents of Chinese water chestnut (Eleocharis dulcis) peels extracts/fractions. European Food Research and Technology. 2014; 238: 789-796.
- Grassby T, Jay AJ, Merali Z, Parker ML, Parr AJ, Faulds CB, et al. Compositional analysis of Chinese water chestnut (Eleocharis dulcis) cellwall material from parenchyma, epidermis, and subepidermal tissues. Journal of Agricultural and Food Chemistry. 2013; 61: 9680-9688.
- You Y, Duan X, Wei X, Su X, Zhao M, Sun J, et al. Identification of major phenolic compounds of Chinese water chestnut and their antioxidant activity. Molecules. 2007; 12: 842-852.
- Luo YH, Gao ZM, Chen L, Wei XF, Liu YQ. Development of water chestunt beverage containing flavonoids. Food Research and Development. 2008; 29: 60-63.
- Gao ZM, Luo YH, Chen ZL, Pan ZT. Development of Eleocharis tuberose peel fruit vinegar beverages. China Brewing. 2010; 5: 164-166.
- Study on the extraction and stability of eleocharis turerosa peel pigment. Food Science. 2004; 25: 115-121.
- Li XQ, Gao K, Jia ZJ. Eremophilenolides and other constituents from the roots of Ligularia sagitta. Planta Medica. 2003; 69: 356-360.
- Yan HJ, Gao SS, Li CS, Li XM, Wang BG. Chemical constituents of a marinederived endophytic fungus Penicillium commune G2M. Molecules. 2010; 15: 3270-3275.
- Wang JH, Ji MH, Shu HM, Chen GY, Song XP, Wang J. Chemical constituents from the roots of Polyalthia obliqua. Chinese Journal of Natural Medicines. 2012; 10: 303-306.
- Kuo CC, Chiang W, Liu GP, Chien YL, Chang JY, Lee CK, et al. 2,2’-Diphenyl- 1-picrylhydrazyl radical-scavenging active components from adlay (Coix lachryma-jobi L. var. ma-yuen Stapf) hulls. Journal of Agricultural & Food Chemistry. 2002; 50: 5850-5855.
- Céspedes CL, Valdez-Morales M, Avila JG, El-Hafidi M, Alarcón J, Paredes- López O. Phytochemical profile and the antioxidant activity of Chilean wild black-berry fruits, Aristotelia chilensis (Mol) Stuntz (Elaeocarpaceae). Food Chemistry. 2010; 119: 886-895.
- Tang KS, Konczak I, Zhao J. Identification and quantification of phenolics in Australian native mint (Mentha australis R. Br.). Food Chemistry. 2016; 192: 698-705.
- Lee SM, Min BS, Lee CG, Kim KS, Kho YH. Cytotoxic triterpenoids from the fruits of Zizyphus jujuba. Planta Medica. 2003; 69: 1051-1054.
- Ribeiro de Souza e Silva S, Divina de Fátima Silva G, Cláudio de Almeida Barbosa L, Duarte LP, Vieira Filho SA. Lupane Pentacyclic Triterpenes Isolated from Stems and Branches of Maytenus imbricata (Celastraceae). Helvetica Chimica Acta. 2005; 88: 1102-1109.
- Gu Q, Wang RR, Zhang XM, Wang YH, Chen JJ. A new benzofuranone and anti-hiv constituents from the stems of Rhus chinensis. Planta Medica. 2007; 73: 279-282.
- Abd-Alla HI, Shaaban M, Shaaban KA, Abu-Gabal NS, Laatsch H. New bioactive compounds from Aloe hijazensis. Natural product research. 2009; 23: 1035-1049.
- Ernst L. 13C NMR spectroscopy of polycyclic aromatics. VI. Coumarin and the methylcoumarins. Journal of Magnetic Resonance. 1976; 21: 241-246.
- González-Trujano ME, Navarrete A, Reyes B, Cedillo-Portugal E, Hong E. Anticonvulsant Properties and Bio-Guided Isolation of Palmitone from Leaves of Annona diversifolia. Planta Medica. 2001; 67: 136-141.
- Lee S. (Ed.).: Phenol,2,4-Bis(1,1-Dimethylethyl)-,1,1’,1”-Phosphite. In: Encyclopedia of Reagents for Organic Synthesis, John Wiley & Sons, Ltd. 2010.
- Qu C, Yue SJ, Lin H, Ka J. Chemical constituents of Carthamus tinctorius. Chinese Traditional and Herbal Drugs. 2015; 46: 1872-1876.
- Yang J, Cao AC, Zhou DX, Zhang DC, He L. Study on the Liposoluble Chemical Constituents of Eupatorium adenophorum Spreng. Chinese Traditional and Herbal Drugs. 2006; 37: 0-31.
- Zhang CZ, Xu XZ, Li C. Fructosides from Cynomorium songaricum. Phytochemistry. 1996; 41: 975.
- Wang XH, Jia DZ, Liang YJ, Yan SL, Ding Y, Chen LM, et al. Lgf-YL-9 induces apoptosis in human epidermoid carcinoma KB cells and multidrug resistant KBv200 cells via reactive oxygen species-independent mitochondrial pathway. Cancer Letters. 2007; 249: 256-270.
- Zhang JY, Wu HY, Xia XK, Liang YJ, Yan YY, She ZG, et al. Anthracenedione derivative 1403P-3 induces apoptosis in KB and KBv200 cells via reactive oxygen species independent mitochondrial pathway and death receptor pathway. Cancer Biology & Therapy. 2007; 6: 1413-1421.
- Zhang SS, Nie SP, Huang DF, Feng YL, Xie MY. A Novel Polysaccharide from Ganoderma atrum Exerts Antitumor Activity by Activating Mitochondria- Mediated Apoptotic Pathway and Boosting the Immune System. Journal of Agricultural and Food Chemistry. 2014; 62: 1581-1589.