
Review Article
Austin J Nutri Food Sci. 2019; 7(6): 1128.
Role of Mushrooms in Autism
Bell V1, Ferrão J2, Chaquisse E3, Manuel B4 and Fernandes T5*
1Faculty of Pharmacy, University of Coimbra, Portugal
2Pedagogical University, Mozambique
3National Health Institute, Ministry of Health, Mozambique
4Faculty of Medicine, Eduardo Mondlane University, Mozambique
5Faculty of Veterinary Medicine, Lisbon University, Portugal
*Corresponding author: Fernandes T, Faculty of Veterinary Medicine, Lisbon University, 1300-477 Lisboa, Portugal
Received: August 16, 2019; Accepted: September 24, 2019; Published: October 01, 2019
Abstract
Autism Spectrum Disorder (ASD) is a disorder still very poorly understood first recognized in early childhood in the form of a multi organ system disability caused by impaired neurogenesis and apoptosis, impaired synaptogenesis and synaptic pruning or imbalanced excitatory-inhibition system. Inflammation has been recognised as the pathogenesis of autism but a holistic approach is required. The aetiology is largely unknown and there is no clinical treatment. The gut microbiota may affect symptom manifestation which may benefit from a balanced diet, re-establishment of intestinal permeability, improvement of gut microbiota, raised immunity, supply of antioxidants and detoxification speed. Specific mushrooms may have specific effects on health, well-being, behaviour and fitness and address the potential impact of a dietary mushroom supplement on gastrointestinal inflammation in ASD patients.
Keywords: Autism spectrum disorder; Mushrooms; Nutrition; Gut microbiota
Introduction
Plenty of research has explored the mechanisms behind anxiety, stress, cognition, memory and depression [1]. Many neural system diseases are often neglected and some are hard to understand. Furthermore, there are very few established explanations or choices for intervention or managing some of them; especially the most misconceived ones [2]. Autism spectrum disorder, known since 1938 [3], is a comorbidity (not necessarily implying the presence of multiple diseases) still very poorly understood, typically first recognized in early childhood before the age of 3, and can create challenges throughout a person’s life. It is still very unclear its aetiology and it is probably even more uncertain how it could be supported or remedied in any way. Autism is an example of natural variation also known as Autism Spectrum Disorder (ASD), affecting some 70 million people globally (current estimates are that 1 in 100 people are on the autistic spectrum), dealing with neurological developments in people that interfere with their ability to communicate and socialize with others [4]. Genetic, neuro-immune and environmental factors are connoted [5] despite the fact that each individual case of autism is unique from others [6]. Autism is not anymore considered a comorbidity neither one of five lifelong disorders that were under the umbrella previously designated Pervasive Developmental Disorders (PDD), a category of neuro developmental disabilities. But actually, autism spectrum disorder-ASD means the same thing covering the five conditions. Research has shown that autism tends to run in families being a brain based disorder but not caused by inadequate parenting. With the exception of neuro-inflammatory changes [7], most reported neurobiological abnormalities in ASD are inconsistent [8]. A usual misinterpretation around autism is that it is inevitably linked to intellectual disability [9]. There are no recognised treatments or known medication that can directly cure or lessen the symptoms of autism, some with undesirable side effects or dependency risks. On the other hand, there have been some a few mainstream approaches that have been developed and accepted into conventional medicine that may help with issues associated with or connected to autism [10]. Autism affects all races, ethnic groups, and socioeconomic levels, boys being more prone than girls. The severity of autism conditions may be slowed by behavioural therapy for children, helping people deal with further complications as they grow older [11]. The mainstream treatment of autism has many side effects. Some studies show that uncomplicated diet changes, avoiding potential risk factors and environmental toxins (e.g. heavy metals), incorporating dietary supplements (e.g. vitamins, probiotics), and some medicinal herbs and mushrooms may have some significant beneficial effects [12]. However, the heavy metals from some mushrooms indicate that when consumed high quantity may cause liver or kidney damage and even death may result [13]. The autism spectrum can benefit from a balanced diet including certain foods and supplements, in particular those that curtail inflammation, re-establish intestinal permeability, improve the harmony of gut microbiota, raise immunity, supply antioxidants and speed detoxification [14,15]. Oxidative stress is common to a myriad of neurological diseases [16]. The production of Reactive Oxygen Species (ROS) is a common outcome of normal aerobic cellular metabolism, and determines the cellular redox balance with antioxidants [17]. These protect critical biological targets against [18] therefore, they have been considered as attractive potential beneficial agents to neutralise ROS-mediated neural damage. The key players in oxidative stress outline evidences of their involvement in Multiple Sclerosis, Alzheimer’s, Parkinson’s and Huntington’s diseases [19,20]. Complementary and alternative medical treatments are commonly used for children with autism spectrum disorders [21]. However, most treatments have not been adequately studied and do not have evidence to support their use. This review discusses the existing evidence supporting the administration of mushroom products in ASD patients.
Role of Microbiota
Human life spins around a microbial world and human and animals only exist because they have evolved dealing with microorganism in environment and food. Human metabolism represents a conjugation of microbial and human vital roles namely in health consequences. The microbiome, is a diverse consortium of bacteria, fungi, protozoa, archaea and viruses that inhabit the gut of all mammals, referring to the collection of microbes and their genetic material, confers a variety of physiologic benefits to the host in many key aspects of life as well as being responsible for some diseases. The field is at a stage where more questions than answers are being generated [22,23]. The gut microbiota has been implicated as a potential pathway affecting symptom manifestation in cognitive and neuro developmental disorders, such as anxiety, depression and ASD [24,25] Incredible advances in our understanding of host-microbe interactions, with identification of numerous disease-associated organisms and elucidation of pathogenic mechanisms occurred on the last two decades [26]. The gut microbiota interact with the human body via five communication routes between gut microbiota and brain [27], including immune, endocrine and neural mechanism deeply influencing general growth and development, including development of the nervous system [28]. Our digestive microbiota is a partner of homeostasis directly linked to our brains [29] which is explained by a network of neurons lining our guts that is so extensive some scientists have nicknamed it our “second brain” [30-32]. At present there is a lack of consistent findings relating to the neurobiology of autism and the influence of environment including nutrition [33]. Hormones, some heavy metals and endocrine disrupting compounds (mostly man-made, found in various materials such as pesticides, additives or contaminants in food, and personal care products), have undesired harmful effects on the embryonic and foetal neuro development and in the evolution of ASD [34,35]. The sources of the microorganisms that make up the gut ecosystem, how and why it varies from one person to another, and how the composition of this microbial community influences human digestion, physiology, metabolism, development, and diseases are still poorly understood [36,37]. There is a strong link between ASD and dysbiosis, including high degree of mental distress between these ostensibly contrasting diagnoses [38,39]. Although microbiota is known to alter host immune function including inflammatory cytokine production, the relationship between abnormal microbiota and cytokine production in ASD has been scarce [40,41]. Development of ASD, including autism, is based on a combination of genetic predisposition and environmental factors ASD being among the most heritable of all neuropsychiatric disorders [42,43]. Recent data partly explains the diverse neuro immunological abnormalities in ASD and propose a diverse and complex multifactorial aetiology including a pathogenetic role of intestinal microbiota in autism [44-46]. Abundant research suggests a connection between gut microbiome and autism-like behaviours. Long-term benefit effects of faecal transplant or Microbiota Transfer Therapy (MTT) on autism symptoms and gut health, which persisted long after treatment, was demonstrated on children diagnosed with ASD [47]. The human gut microbiota, through interactions between the microbiome and ASD, may impact on the connection between feeding, nutrition and metabolism with ASD [48,49]. The microbiome being an interface between environmental and genetic risk factors associated with ASD reflect that changes in the microbiome may contribute to symptoms of neuro developmental disease [50,51]. Gastrointestinal comorbidities, including acute and chronic constipation and diarrhoea, occur in children with ASD associated with the harshness of the neuro behavioural disorder [48]. Gut microbial imbalance may correspond with behavioural abnormality in ASD patients. Since the impact of diet on the microbiota composition in children with ASD is still broadly unexplained [52]. Understanding of the relationship among diet, gut flora and host on mitochondrial dysfunction and oxidative stress in the cells, could open up new lines of research on ASD, including potential novel treatment strategies [53-56]. Impairment of physiological regulatory mechanisms governing metabolism, immune response, organ function or unbalance of the gut microbiota are quite complex and critical in gastrointestinal functions and disturbances. The integrity of the gastrointestinal mucosal barrier and the symbiotic relationship with commensal bacteria play a vital role in the gut pathogenesis and is involved in regulating normal functions including motility, permeability, and mucosal immune function [57-59] (Figure 1). Derangements in the gut microbiota in children with ASD have been reported [60] and liaisons between specific microbial genera and some symptoms of ASD have been described [61,62]. The gut microbiota of children with ASD is less diverse, varies between individuals, and exhibits lower levels of Bifidobacterium and Firmicutes and higher levels of Lactobacillus, Clostridium, Bacteroidetes, and Desulfovibrio [63]. It is noticeable that the human body lacks endogenous enzymes to degrade many plant polysaccharides, such as cellulose, hemicellulose (e.g. xylan), complex pectins, and arabinose. In contrast, the human colonic microbiota yields more than 80 different glycosyl hydrolase families [64,65]. In this way, the gut microbiota may have evolved as an adaptation to allow extraction of maximal energy (e.g. short chain fatty acids, acetate, propionate, butyrate and other elements (e.g. serotonin, bile acids, bioactive lipids) from food sources [66] and actually it is widely known that the human body is composed of 10 times more microbial cells than body cells [67]. The plausibility of manipulative procedures to change microbiome evolution establishes the forecast of a spectrum of novel therapeutic paths such as microbiome-mediated therapies, probiotic, antibiotic or dietary administrations that may represent hope to patients and families living with ASD [68]. Gut bacteria are not only critical for regulating gut metabolism, but also important for host immune system [69,70]. The short-chain volatile fatty acids, are produced in the distal colon by microbial fermentation of carbohydrates and endogenous substrates, such as mucus, epithelial cells, and digestive enzymes [71]. This is of great advantage to the host humans since most of the enzymes needed to degrade the polymeric carbohydrate molecules (e.g. cellulose and chitin) present in cell wall of plants are not produced endogenously [72,73]. Still unclear, but most likely, there are effective links between dietary, metabolic, infective-related events, gastrointestinal factors and the behavioural aggravations and exemptions of ASD [7,74]. There is accumulated evidence of an association between specific individual harmful bacteria and symptoms of ASD which is often associated with medical comorbidities and gastrointestinal dysfunction [75]. For example, some nutrient deficiencies, microbiota luxuriance, decreased Bacteroidetes-to-Firmicutes ratio and abundance of Desulfovibrio were related to ASD symptoms [76-78]. However, few studies have assessed dietary intake, namely recommended daily intake of fibre, and microbiota in ASD children [79]. Gut-derived factors, such as dietary or enteric bacterially produced VFA, may therefore be plausible environmental agents that can trigger ASDs. As a critical modulator of enteric and central nervous systems development and function, amygdala dysregulation [80] and serotonin may be the nexus for the microbiota-gut-brain axis in ASD since one of the most prominently established findings in autism is the elevation of serotonin in the brain [81,82]. The idea that systemic bacterial infections play a role in the genesis of symptoms of autism is gaining ground associated with inflammation of the intestinal mucosa leading to the introduction of bacterial components, including neurotoxins, into the bloodstream, creating oxidative stress causing immune dysfunction. The tridirectional interactions between the central nervous system, microbiota and the gastrointestinal tract (microbiotabrain- gut axis) may mediate therapies and be a safe and effective treatment for ASD [83-85].
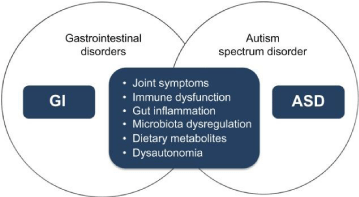
Figure 1: The concept of the overlap syndrome of GI disorders and ASD [57].
Role of Mushrooms
Mushrooms are rich in bioactive metabolites, including polysaccharides, enzymes (e.g. superoxide dismutase, ribonucleases, laccases), proteins (ribosome inactivating proteins), dietary fibres (β-Glucans), and many other biomolecules (secondary metabolites, lectins, antifungal proteins, ubiquitin-like proteins, protease inhibitors), which have been shown to be successful in the prevention and treatment of several human health hazards [86-88] Macrofungi are both food and a significant nutritional supplement for humans. However, several fungus species accumulate both important nutritional elements and heavy metals in their fruit bodies [89]. Although living organisms need certain elements such as iron, cobalt, copper, manganese, chrome and zinc in trace amounts, excessive amounts of these elements may create toxic effects on these living organisms [90]. Many people on the spectrum take multiple medications, which can lead to serious side effects and may not even be effective. Families of children with autism and related disorders, due to anxiety, may turn to therapies that are not based in the realm of conventional medical or psychological practice, but this matter is quite complex and findings should not be generalized [91]. The most effective treatment is a combination of specialized and supportive educational programming, communication training, social skills support and behavioural intervention [92]. Treatments do not address the core symptoms of the disorder and there are no medications that cure ASD, but it can help provide some control over aggression, mood problems, rigid behaviour, and attention deficits. Associated problems such as seizures, disrupted sleep patterns, gastrointestinal problems or dietary imbalances should have medical care and often use complementary and alternative medicine [93,94]. The use of more than one antipsychotic medication in the treatment of child and adolescent psychiatric conditions has increased over the last decade which is concerning, considering the risks of adverse effects associated with these medications [95]. Complementary and Alternative Medical (CAM) treatments are commonly used for children with ASD including mind and body practices, energy medicine, biomedical treatments and natural products [96]. ASD being multifactorial demands collaborative intervention of health professionals. Gluten- and casein-free diet in children showed little evidence of beneficial effects for the symptoms of ASD [97]. Mushroom nutrition may come into play in the association between dietary factors and microbiota composition as mushrooms we have shown to increase Lipoxin A4 and having a high content in superoxide dismutase that can cross the blood brain barrier [98]. The most commonly used mushrooms as potent healthboosters include Chaga (Inonotus obliquus), Reishi (Ganoderma lucidum), Turkey Tail (Trametes versicolor, Coriolus versicolor), Shiitake (Lentinula edodes), Lion’s Mane (Hericium erinaceus), and Cordyceps (Cordyceps sinensis). The connection between mushroom nutrition and immune system is well recognised [99], however the true integration of research between mushroom nutrition, animal energy status and immune function is still far from clear. There are multiple recognised clinical and immune modulating uses of mushrooms due to their content in β-glucans [30,100]. Mushrooms contain immunostimulants (β-glucans, lipopolysaccharide, and polyinosinic: polycytidylic acid) that can strengthen and increase immune system activity. Some contain immunomodulators that can adjust the level of function in the individual’s immune system [101] and their effects on various cancers have been well documented [102] while the folate in mushrooms plays an important role in DNA synthesis and repair [103]. If a person is fungiphobic, mushrooms are not a path of nutritional recovery for ASD. For people with ASD specific mushrooms (Reishi, Maitake, Shiitake, and Cordyceps) may have specific effects on health, well-being, behaviour and fitness. Cordyceps, a combination from a caterpillar and a fungus, is not a mushroom but a parasitic fungus infecting insects at different phases of their evolution and it is being cultivated for its outstanding health benefits. Cordycepin, rich in some 20 nucleosides and their related compounds, affects the synergistic actions of immune cells and increases the cytokine network contributing to the increase of cell receptors and control of cellular and humoral acquired immunity [104,105]. For children with ASD who enjoy eating common mushrooms (white button, shiitake, and oyster), there is a matter of chance for repairing functional activities. People on the autism spectrum can over-respond too many challenges such as toxins and allergens, and under-react to a seemingly harmless foreign substance such as viruses, yeast, and intracellular bacteria. These responses require a specific type of immune system response, which can be effective using mushrooms to successfully induct the Th2 cells which secrete cytokines [106,107].
Autism, Neuroimmunity and Environmental Factors
Autism is a severe neurological condition and one of the most mysterious and challenging lifelong developmental disability of present medicine. Contrary to popular opinion, autism is not just the congenital condition once assumed to be and results from the combination of genetic and environmental factors being preventable, and even treatable, once understood the underlying causes [108,109]. Parental age, perinatal risks, medication, smoking and alcohol abuse, nutrition deficiencies, some vaccinations, teratogenic compounds, toxic exposures, and even extreme psychosocial factors may constitute risk factors for adverse outcomes. Several other factors may reduce the risk of a child to develop ASD such an adequate vitamin D during pregnancy and lactation, avoidance of environmental toxins (e.g. heavy metals, pesticides, alcohol) [110]. Several herbal medicine products were found positive on ADHD (attention deficithyperactivity disorder) cases and the most promised were mushrooms and a plant named Bacopa monnieri which may be a helpful natural remedy for autism [111,112]. The culinary medicinal fungus lion’s mane Hericium erinaceus (Figure 2) showed some scientific reason to believe that could be of some marginal help on these. Other studies have documented that this mushroom and oyster mushroom (Pleurotus giganteous) could be of benefit to symptoms relating to anxiety and depression, which may relate to both autism and ADHD. Daily consumption of these mushrooms may keep people away from several life-threatening disorders. However still needs further scientific validation to consider these mushrooms as useful in the prevention or treatment of dementia and cognitive dysfunction [113,114]. Cordyceps (Cordyceps militaris) (Figure 3) is considered to be neuroprotective to the highest degree. No direct research has been undertaken to manifest a solid link between cordyceps and ADHD or autism, though the makings of a link are already clear [115,116]. Globally, more than 300 million people of all ages suffer from depression, resulting from a complex interaction of social, psychological and biological factors. Therefore, there is an interest in finding a safer compound with robust and rapid antidepressant effect namely on mild cases and for children and adolescents. Cordyceps promotes strong anti-depressant potential [117], which could be an ally to those with autism and which may benefit ADHD [118,119]. Reishi (Ganoderma lucidum) mushroom could also be supportive to both ADHD and autism. Some studies suggest it could have antidepressant- like effects and neuroprotective activity [120] but extracts of G. lucidum should be used with caution as there appears to be potential for toxicity [121]. Therefore, researchers suggest that reishi should be administered in combination with traditional treatment rather than replacing it [122]. Psilocybin, the psychoactive compound in magic mushrooms, is proving a prodigious treatment for anxiety, depression, addiction, and one study even found it might lead to neurogenesis, or the regrowth of brain cells, acting as serotonin agonists [123]. But there are only few small studies indicating that psilocybin could produce great results and pharmaceutical companies are not interested in researching an inexpensive substance [124]. Safety is clearly one issue that is important to discuss when contemplating the use of psychedelic drugs as medical or therapeutic treatments [125,126].

Figure 2: Fruiting body and mycelium of Pleurotus giganteous (left) and
Hericium erinaceus (right).

Figure 1: Image of Cordyceps sinensis (a genus of Ascomycete fungi).
Children and Ecosystems
The interaction between three sources of information, genetic, past experience and present environment, from conception onwards, governs the physical and emotional state of humans, the homeostasis of the natural human systems [127]. Deficient nutrition and an imbalanced inner ecosystem is most plausible connected to autism. For example, zinc plays a role on neuronal depolarization at synapses and its deficiency or disrupted zinc dynamics might be linked to individuals with ASDs [128]. Enabling an efficient child’s immune system and ability to metabolise nutrients and remain free of toxins, is a condition from birth. Subsequently, after a typical programme of vaccination, the child’s levels of mercury and aluminium from vaccine adjuvants crosses increase and may cause the child’s brain and nervous systems not to function as they should [129]. Two subtypes of ASD, with or without gastrointestinal disorders, have been identified, possibly reflecting different grades of inflammation and mucus production, which has impact on the implementation of diet or dietary supplements different for the subtypes [130]. To balance the inner ecosystem may improve autism showing that diet is indeed key to treat autism and its milder forms in the subtype with bowel dysfunctions, and this balance may be achieved by adding probioticsrich diets (fermented foods and beverages) or prebiotics (mushroom fibres). These fermented foods and mushrooms build strong, healthy immune and digestive systems. Soon after incorporating some of these into their diets, autistic children show improvements on digesting high-quality lipids rich in raw, unsaturated fatty acids essential to healing [131]. Epidemiological evidence shows also a clear association between gut problems and skin disorders [132] although autism’s co-morbidity with hypomelanosis is justified by genetic and epigenetic variants that protect against vitamin-D deficiency. Children with reduction or absence of the pigment melanin have increased autism risk because parents tend to reduce sun exposure being aware of photosensitivity and skin-cancer [133]. Most research comprises epidemiological studies detailing age of parents, use of antidepressants, mothers with diabetes and other situations which do not demonstrate cause and effect and originate conflicting conclusions. Some studies suggest that taking vitamin D and vitamin B9 (folate), supplements during pregnancy can decrease the baby’s autism risk. But the evidence is not definitive [134]. Folate and folic acid are different since folic acid is a synthetic form of vitamin B9 and the majority of folic acid is not converted to the active form of vitamin. The un-metabolized folic acid may build up and be associated with several health problems. It is difficult to link autism to environmental ecosystem and besides pollutants and chemicals anything altering the likelihood of having a condition and not encoded in an individual’s DNA is a risk factor. Folate has a crucial role in cell growth and the formation of DNA. Periconceptional folic acid intake may reduce ASD risk in those with high prenatal air pollution exposure [135]. Vitamin-D enhancement may aid treatment and prevention of autism in children however; intervention must start long before on nutritional status of mothers before and during pregnancy, thereby allowing changes to be performed. The mechanisms of how these processes occur are not fully understood [136,137].
Concluding Remarks
Nature functions in an infinitely more complex manner than we can understand using our simplified models of reality. The impact of dietary intake on the gut microbiota composition was not systematically investigated although alterations in its profile have been confirmed in children with ASD [138-140]. Each person with ASD is unique, and intervention plans must be individualized based on the needs of the individual and family. Recommended services should be based on proven interventions with a strong evidence base emphasizing a holistic approach to health maintenance. Dietary therapy is insufficient to effectively treat autism, but it can be used as a complement to medical and psychological interventions. The use of some mushrooms may reduce the severity of issues springing from autism or ADHD, however, being a delicate collection or network of causes, lifestyle is very important and mushrooms may have only peripheral benefit. Some treatments have been unsuccessful, some have undesirable side effects, and others require more research.
Conflict of Interest
The authors declare that the work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. No research involving humans or animals, therefore informed consent not necessary and all ethical issues were taken into consideration.
References
- Mandolesi L, Polverino A, Montuori S, Foti F, Ferraioli G, Sorrentino P, et al. Effects of Physical Exercise on Cognitive Functioning and Wellbeing: Biological and Psychological Benefits. Front Psychol. 2018; 9: 509.
- Lee SW. A Copernican Approach to Brain Advancement: The Paradigm of Allostatic Orchestration. Frontiers in Human Neuroscience. 2019; 13.
- Kanner L. Autistic disturbances of affective contact. Acta Paedopsychiatr. 1968; 35: 100-136.
- Murphy C, Wilson CE, Robertson DM, Ecker C, Daly EM, Hammond N, et al. Autism spectrum disorder in adults: diagnosis, management, and health services development. Neuropsychiatr Dis Treat. 2016; 12: 1669-1686.
- Kalkbrenner AE, Schmidt RJ, Penlesky AC. Environmental Chemical Exposures and Autism Spectrum Disorders: A Review of the Epidemiological Evidence Curr Probl Pediatr Adolesc Health Care. 2014; 44: 277-318.
- Jick H, Kaye JA. Epidemiology and possible causes of autism. Pharmacotherapy. 2003; 23: 1524-1530.
- Madore C, Leyrolle Q, Lacabanne C, Benmamar-Badel A, Joffre C, Nadjar A, et al. Neuroinflammation in Autism: Plausible Role of Maternal Inflammation, Dietary Omega 3, and Microbiota. Neural Plast. 2016; 1-15.
- Amaral DG. Examining the Causes of Autism. Cerebrum. 2017; 1-17.
- Ripamonti L. Disability, Diversity, and Autism: Philosophical Perspectives on Health. New Bioeth. 2016; 22: 56-70.
- Chandrashekhar S, Bommangoudar JS. Management of Autistic Patients in Dental Office: A Clinical Update. Int J Clin Pediatr Dent. 2018; 11: 219-227.
- Levy SE, Hyman SL. Complementary and Alternative Medicine Treatments for Children with Autism Spectrum Disorders. Child Adolesc Psychiatr Clin N Am. 2008; 17: 803-820.
- Thapar A, Cooper M, Eyre O, LangleyK. What have we learnt about the causes of ADHD? J Child Psychol Psychiatry. 2013; 54: 3-16.
- Okwulehie IC, Ogoke JA. Bioactive, nutritional and heavy metal constituents of some edible mushrooms found in Abia State of Nigeria. International Journal of Applied Microbiology and Biotechnology Research. 2013; 1: 7-15.
- Carvalho AN, Firuzi O, Gama MJ, van Horssen J, Saso L. Oxidative Stress and Antioxidants in Neurological Diseases: Is There Still Hope? Curr Drug Targets. 2017; 18: 705-718.
- Siniscalco D, Schultz S, Brigida A, Antonucci N. Inflammation and Neuro- Immune Dysregulations in Autism Spectrum Disorders. Pharmaceuticals (Basel). 2018; 11: 56.
- Engwa GA. Free Radicals and the Role of Plant Phytochemicals as Antioxidants Against Oxidative Stress-Related Diseases. In T. Asao & M. Asaduzzaman (Eds.), Phytochemicals - Source of Antioxidants and Role in Disease Prevention. InTech. 2018.
- Uttara B, Singh AV, Zamboni P, Mahajan RT. Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol. 2009; 7: 65-74.
- Mason RP. Imaging free radicals in organelles, cells, tissue, and in vivo with immuno-spin trapping. Redox Biol. 2016; 8: 422-429.
- Mulle JG, Sharp WG, Cubells JF. The Gut Microbiome: A New Frontier in Autism Research. Current Psychiatry Reports. 2013; 15: 337.
- Dicks LMT, Geldenhuys J, Mikkelsen LS, Brandsborg E, Marcotte H. Our gut microbiota: a long walk to homeostasis. Beneficial Microbes. 2018; 9: 3-20.
- Catinean A, Neag MA, Muntean DM, Bocsan IC, Buzoianu ADX. An overview on the interplay between nutraceuticals and gut microbiota. Peer J. 2018; 6: e4465.
- Kang DW, Adams JB, Gregory AC, Borody T, Chittick L, Fasano A, Khoruts A, et al. Microbiota Transfer Therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: an open-label study. Microbiome. 2017; 5: 10.
- Cope EK. Host-Microbe Interactions in Airway Disease: toward Disease Mechanisms and Novel Therapeutic Strategies. mSystems. 2018; 3: e00158-17.
- Li Q, Han Y, Dy ABC, Hagerman RJ. The Gut Microbiota and Autism Spectrum Disorders. Front Cell Neurosci. 2017; 11: 120.
- Ignatova V. Influence of Gut Microbiota on Behavior and Its Disturbances. In Behavioral Neuroscience. Intech Open. 2019.
- Zhao L, Zhang F, Ding X, Wu G, Lam YY, Wang X, et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science. 2018; 359: 1151-1156.
- Wang HX, Wang YP. Gut Microbiota-brain Axis. Chin Med J (Engl). 2016; 129: 2373-2380.
- Coretti L, Paparo L, Riccio MP, Amato F, Cuomo M, Natale A, et al. Gut Microbiota Features in Young Children With Autism Spectrum Disorders. Front Microbiol. 2018; 9: 3146.
- Sharon G, Sampson TR, Geschwind DH, Mazmanian S. K. The Central Nervous System and the Gut Microbiome. Cell. 2016; 167: 915-932.
- Bell V, Ferrão J, Chaquisse E, Fernandes T. Host-Microbial Gut Interactions and Mushroom Nutrition. Journal of Food and Nutrition Research. 2018; 6: 576-583.
- Martin CR, Osadchiy V, Kalani A, Mayer EA. The Brain-Gut-Microbiome Axis. Cell Mol Gastroenterol Hepatol. 2018; 6: 133-148.
- Baj A, Moro E, Bistoletti M, Orlandi V, Crema F, Giaroni C. Glutamatergic Signaling Along The Microbiota-Gut-Brain Axis. Int J Mol Sci. 2019; 20: 1482.
- Gialloreti LE, Mazzone L, Benvenuto A, Fasano A, Alcon AG, Kraneveld A, et al. Risk and Protective Environmental Factors Associated with Autism Spectrum Disorder: Evidence-Based Principles and Recommendations. J Clin Med. 2019; 8: 217.
- Marí-Bauset S, Donat-Vargas C, Llópis-González A, Marí-Sanchis A, Peraita-Costa I, Llopis-Morales J, et al. Endocrine Disruptors and Autism Spectrum Disorder in Pregnancy: A Review and Evaluation of the Quality of the Epidemiological Evidence. Children (Basel). 2018.
- Long M, Ghisari M, Kjeldsen L, Wielsøe M, Nørgaard-Pedersen B, Mortensen EL, et al. Autism spectrum disorders, endocrine disrupting compounds, and heavy metals in amniotic fluid: a case-control study. Mol Autism. 2019; 10: 1.
- Shkoporov AN, Ryan FJ, Draper LA, Forde A, Stockdale SR, Daly KM, et al. Reproducible protocols for metagenomic analysis of human faecal phageomes. Microbiome. 2018; 6: 68.
- Dominguez-Bello MG, Godoy-Vitorino F, Knight R, Blaser MJ. Role of the microbiome in human development. Gut. 2019; 68: 1108-1114.
- Hsiao EY. Gastrointestinal Issues in Autism Spectrum Disorder. Harv Rev Psychiatry. 2014; 22: 104-111.
- DeFilippis M. Depression in Children and Adolescents with Autism Spectrum Disorder. Children (Basel). 2018; 5: 112.
- Schirmer M, Smeekens SP, Vlamakis H, Jaeger M, Oosting M, Franzosa EA, et al. Linking the Human Gut Microbiome to Inflammatory Cytokine Production Capacity. Cell. 2016; 167: 1125-1136.
- Bastiaanssen TFS, Cowan CSM, Claesson MJ, Dinan TG, Cryan JF. Making Sense of the Microbiome in Psychiatry. International Journal of Neuropsychopharmacology. 2019; 22: 37-52.
- Colonetti K, Roesch LF, Schwartz IVD. The microbiome and inborn errors of metabolism: Why we should look carefully at their interplay? Genetics and Molecular Biology. 2018; 41: 515-532.
- Almandil N, Alkuroud D, AbdulAzeez S, AlSulaiman A, Elaissari A, Borgio JF. Environmental and Genetic Factors in Autism Spectrum Disorders: Special Emphasis on Data from Arabian Studies. International Journal of Environmental Research and Public Health. 2019; 16: 658.
- Berding K, Donovan SM. Diet Can Impact Microbiota Composition in Children With Autism Spectrum Disorder. Frontiers in Neuroscience. 2018; 12: 515.
- Sanctuary MR, Kain JN, Angkustsiri K, German JB. Dietary Considerations in Autism Spectrum Disorders: The Potential Role of Protein Digestion and Microbial Putrefaction in the Gut-Brain Axis. Frontiers in Nutrition. 2018; 5: 40.
- Ma B, Liang J, Dai M, Wang J, Luo J, Zhang Z, et al. Altered Gut Microbiota in Chinese Children With Autism Spectrum Disorders. Frontiers in cellular and infection microbiology. 2019; 9: 40.
- Kang DW, Adams JB, Coleman DM, Pollard EL, Maldonado J, McDonough- Means S, et al. Long-term benefit of Microbiota Transfer Therapy on autism symptoms and gut microbiota. Scientific Reports. 2019; 9: 5821.
- Liu F, Li J, Wu F, Zheng H, Peng Q, Zhou H. Altered composition and function of intestinal microbiota in autism spectrum disorders: a systematic review. Translational Psychiatry. 2019; 9: 43.
- Plaza-Díaz J, Gómez-Fernández A, Chueca N, Torre-Aguilar M, Gil Á, Perez-Navero JL, et al. Autism Spectrum Disorder (ASD) with and without Mental Regression is Associated with Changes in the Fecal Microbiota. Nutrients. 2019; 11: 337.
- Gloria Dominguez-Bello M, Godoy-Vitorino F, Knight R, Blaser MJ. Role of the microbiome in human development. Gut. 2019; 68: 1108-1114.
- Warner BB. The contribution of the gut microbiome to neurodevelopment and neuropsychiatric disorders. Pediatric Research. 2019; 85: 216-224.
- Kho ZY, Lal SK. The Human Gut Microbiome - A Potential Controller of Wellness and Disease. Frontiers in Microbiology. 2018; 9: 1835.
- Berding K, Donovan SM. Microbiome and nutrition in autism spectrum disorder: current knowledge and research needs. Nutrition Reviews. 2016; 74: 723-736.
- Baio J, Wiggins L, Christensen DL, Maenner MJ, Daniels J, Warren Z, et al. Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years - Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2014. MMWR. Surveillance Summaries. 2018; 67: 1-23.
- Carmo C, Naia L, Lopes C, Rego AC. Mitochondrial Dysfunction in Huntington’s Disease. Advances in experimental medicine and biology. 2018; 1049: 59-83.
- Srikantha P, Mohajeri MH. Microbiota-Gut-Brain-Axis and Autism Spectrum Disorders. 2018.
- Wasilewska J, Klukowski M. Gastrointestinal symptoms and autism spectrum disorder: links and risks - a possible new overlap syndrome. Pediatric health, medicine and therapeutics. 2015; 6: 153-166.
- Jones L, Kumar J, Mistry A, Sankar Chittoor Mana T, Perry G, Reddy VP, et al. The Transformative Possibilities of the Microbiota and Mycobiota for Health, Disease, Aging, and Technological Innovation. Biomedicines. 2019; 7: 24.
- Mukhtar K, Nawaz H, Abid S. Functional gastrointestinal disorders and gutbrain axis: What does the future hold? World Journal of Gastroenterology. 2019; 25: 552-566.
- Grossi E, Melli S, Dunca D, Terruzzi V. Unexpected improvement in core autism spectrum disorder symptoms after long-term treatment with probiotics. SAGE open medical case reports. 2016; 4: 2050313X16666231.
- Tomova A, Husarova V, Lakatosova S, Bakos J, Vlkova B, Babinska K, et al. Gastrointestinal microbiota in children with autism in Slovakia. Physiology & Behavior. 2015; 138: 179-187.
- Wang M, Zhou J, He F, Cai C, Wang H, Wang Y, et al. Alteration of gut microbiota-associated epitopes in children with autism spectrum disorders. Brain, Behavior, and Immunity. 2019; 75: 192-199.
- Rinninella E, Raoul P, Cintoni M, Franceschi F, Miggiano G, Gasbarrini A, et al. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms. 2019; 7: 14.
- Gill SR, Pop M, DeBoy RT, Eckburg PB, Turnbaugh PJ, Samuel BS, et al. Metagenomic Analysis of the Human Distal Gut Microbiome. Science. 2006; 312: 1355-1359.
- Rowland I, Gibson G, Heinken A, Scott K, Swann J, Thiele I, et al. Gut microbiota functions: metabolism of nutrients and other food components. European Journal of Nutrition. 2018; 57: 1-24.
- Tian X, Hellman J, Horswill AR, Crosby HA, Francis KP, Prakash A. Elevated Gut Microbiome-Derived Propionate Levels Are Associated With Reduced Sterile Lung Inflammation and Bacterial Immunity in Mice. Frontiers in Microbiology. 2019; 10: 159.
- Cani PD. Human gut microbiome: hopes, threats and promises. Gut. 2018; 67: 1716-1725.
- Mueller NT, Bakacs E, Combellick J, Grigoryan Z, Dominguez-Bello MG. The infant microbiome development: mom matters. Trends in molecular medicine. 2015; 21: 109-117.
- Zhu B, Wang X, Li L. Human gut microbiome: the second genome of human body. Protein & Cel1. 1: 718-725.
- Ma N, Guo P, Zhang J, He T, Kim SW, Guolong Zhang, et al. Nutrients Mediate Intestinal Bacteria–Mucosal Immune Crosstalk. Frontiers in Immunology. 2018; 9: 5.
- Flint HJ, Scott KP, Duncan SH, Louis P, Forano E. Microbial degradation of complex carbohydrates in the gut. Gut Microbes. 2012; 3: 289-306.
- Janusz G, Pawlik A, Sulej J, Swiderska-Burek U, Jarosz-Wilkolazka A, Paszczynski A. Lignin degradation: microorganisms, enzymes involved, genomes analysis and evolution. FEMS Microbiology Reviews. 2017; 41: 941-962.
- Rastelli M, Knauf C, Cani PD. Gut Microbes and Health: A Focus on the Mechanisms Linking Microbes, Obesity, and Related Disorders. Obesity. 2018; 26: 792-800.
- Lerner A, Neidhöfer S, Matthias T. The Gut Microbiome Feelings of the Brain: A Perspective for Non-Microbiologists. Microorganisms. 2017; 5: 66.
- Cristiano C, Lama A, Lembo F, Mollica MP, Calignano A, Mattace Raso G, et al. Interplay Between Peripheral and Central Inflammation in Autism Spectrum Disorders: Possible Nutritional and Therapeutic Strategies. Frontiers in Physiology. 2018; 9: 184.
- Kang DW, Park JG, Ilhan ZE, Wallstrom G, LaBaer J, Adams JB, et al. Reduced Incidence of Prevotella and Other Fermenters in Intestinal Microflora of Autistic Children. PLoS ONE. 2013; 8: e68322.
- Macfabe DF. Autism: metabolism, mitochondria, and the microbiome. Glob Adv Health Med. 2013; 2: 52-66.
- Pulikkan J, Maji A, Dhakan DB, Saxena R, Mohan B, Anto MM, et al. Gut Microbial Dysbiosis in Indian Children with Autism Spectrum Disorders. Microbial Ecology. 2018; 76: 1102-1114.
- Sanctuary MR, Kain JN, Chen SY, Kalanetra K, Lemay DG, Rose DR, et al. Pilot study of probiotic/colostrum supplementation on gut function in children with autism and gastrointestinal symptoms. PLOS ONE. 2019; 14: e0210064.
- Cowan CSM, Hoban AE, Ventura-Silva AP, Dinan TG, Clarke G, Cryan JF. Gutsy Moves: The Amygdala as a Critical Node in Microbiota to Brain Signaling. BioEssays. 2018; 40.
- Israelyan N, Margolis KG. Serotonin as a link between the gut-brainmicrobiome axis in autism spectrum disorders. Pharmacol Res. 2018; 132: 1-6.
- Israelyan N, Margolis KG. Reprint of: Serotonin as a link between the gutbrain- microbiome axis in autism spectrum disorders. Pharmacol Res. 2019; 140: 115-120.
- Vuong HE, Hsiao EY. Emerging Roles for the Gut Microbiome in Autism Spectrum Disorder. Biol Psychiatry. 2017; 81: 411-423.
- Yang Y, Tian J, Yang B. Targeting gut microbiome: A novel and potential therapy for autism. Front Microbiol. 2018; 194: 111-119.
- Salem I, Ramser A, Isham N, Ghannoum MA. The Gut Microbiome as a Major Regulator of the Gut-Skin Axis. Front Microbiol. 2018; 9: 1459.
- Silva DD, Rapior S, Hyde KD, Bahkali AH. Medicinal mushrooms in prevention and control of diabetes mellitus. Fungal Diversity. 2012; 56: 1-29.
- Akgul H, Sevindik M, Coban C, Alli H, Selamoglu Z. New Approaches in Traditional and Complementary Alternative Medicine Practices: Auricularia auricula and Trametes versicolor. Journal of Traditional Medicine & Clinical Naturopathy. 2017; 6.
- Abd-alwahab WIA, Al-dulaimi FKY, Abdulqader AT. Effect of mushroom cooked in olive oil on some physiological and biochemical parameters of human. EurAsian Journal of BioSciences. 2018; 12: 393-397.
- Kapahi M, Sachdeva S. Mycoremediation potential of Pleurotus species for heavy metals: a review. Bioresour Bioprocess. 4: 32.
- Soetan KO, Olaiya CO, Oyewole OE. The importance of mineral elements for humans, domestic animals and plants: A review. African Journal of Food Science. 2010; 4: 200-222.
- Pellow J, Solomon EM, Bernard CM. Complementary and Alternative Medical Therapies for Children with Attention-Deficit/Hyperactivity Disorder (ADHD). Alternative medicine review. 2011; 16: 323-337.
- Myers SM, Johnson CP. American Academy of Pediatrics, Council on Children with Disabilities. Management of children with autism spectrum disorders. Pediatrics. 2007; 120: 1162-1182
- Perrin JM, Coury DL, Hyman SL, Cole L, Reynolds AM, Clemons T. Complementary and Alternative Medicine Use in a Large Pediatric Autism Sample. Pediatrics. 2012; 130: S77-S82.
- Mannion A, Leader G. Gastrointestinal Symptoms in Autism Spectrum Disorder: A Literature Review. Review Journal of Autism and Developmental Disorders. 2014; 1: 11-17.
- LeClerc S, Easley D. Pharmacological therapies for autism spectrum disorder: a review. P & T : a peer-reviewed journal for formulary management. P T. 40: 389-397.
- Kraneveld AD, Szklany K, de Theije CGM, Garssen J. Gut-to-Brain Axis in Autism Spectrum Disorders. Int Rev Neurobiol. 2016; 131: 263-287.
- Piwowarczyk A, Horvath A, Lukasik J, Pisula E, Szajewska H. Gluten- and casein-free diet and autism spectrum disorders in children: a systematic review. Eur J Nutr. 2018; 57: 433-440.
- Trovato A, Pennisi M, Crupi R, Di Paola R, Alario A, Modafferi S, et al. Neuroinflammation and Mitochondrial Dysfunction in the Pathogenesis of Alzheimer’s Disease: Modulation by Coriolus Versicolor (Yun-Zhi) Nutritional Mushroom. J Neurol Neuromedicine. 2017; 2.
- Dai X, Stanilka JM, Rowe CA, Esteves EA, Nieves C, Spaiser SJ, et al. Consuming Lentinula edodes (Shiitake) Mushrooms Daily Improves Human Immunity: A Randomized Dietary Intervention in Healthy Young Adults. J Am Coll Nutr. 2015; 34: 478-487.
- Thinschmidt, JS, King MA, Korah M, Perez PD, Febo M, Miyan J, et al. Central neural activation following contact sensitivity peripheral immune challenge: evidence of brain-immune regulation through C fibres. Immunology. 2015; 146: 206-216.
- Ayeka PA. Potential of Mushroom Compounds as Immunomodulators in Cancer Immunotherapy: A Review. Evidence-Based Complementary and Alternative Medicine. 2018; 1-9.
- Patel S, Goyal A. Recent developments in mushrooms as anti-cancer therapeutics: a review. 3 Biotech. 2012; 2: 1-15.
- Kennedy DO. B Vitamins and the Brain: Mechanisms, Dose and Efficacy - A Review. Nutrients. 2016; 8: 68.
- Lindequist U, Niedermeyer THJ, Jülich WD. The pharmacological potential of mushrooms. Evid Based Complement Alternat Med. 2005; 2: 285-299.
- Jeong MH, Seo MJ, Park JU, Kang BW, Kim KS, Lee JY, et al. Effect of cordycepin purified from Cordyceps militaris on Th1 and Th2 cytokines in mouse splenocytes. J Microbiol Biotechnol. 2012; 22: 1161-1164.
- Kominsky DJ, Campbell EL, Colgan SP. Metabolic Shifts in Immunity and Inflammation. J Immunol. 2010; 184: 4062-4068.
- Mizuno M, Nishitani Y. Immunomodulating compounds in Basidiomycetes. J Clin Biochem Nutr. 2013; 52: 202-207.
- Chaste P, Leboyer M. Autism risk factors: genes, environment, and geneenvironment interactions. Dialogues Clin Neurosci. 2012; 14: 281-292.
- Bölte S, Girdler S, Marschik PB. The contribution of environmental exposure to the etiology of autism spectrum disorder. Cell Mol Life Sci. 2019; 76: 1275- 1297.
- Karimi P, Kamali E, Mousavi S, Karahmadi M. Environmental factors influencing the risk of autism. J Res Med Sci. 2017; 22: 27.
- Kishore K, Singh M. Effect of bacosides, alcoholic extract of Bacopa monniera Linn. (brahmi), on experimental amnesia in mice. Indian J Exp Biol. 2005; 43: 640-645.
- Chaudhari KS, Tiwari NR, Tiwari RR, Sharma RS. Neurocognitive Effect of Nootropic Drug Brahmi (Bacopa monnieri) in Alzheimer’s Disease. Ann Neurosci. 2017; 24: 111-122.
- Mori K, Inatomi S, Ouchi K, Azumi Y, Tuchida T. Improving effects of the mushroom Yamabushitake (Hericium erinaceus) on mild cognitive impairment: a double-blind placebo-controlled clinical trial. Phytother Res. 2009; 23: 367-372.
- Li IC, Lee LY, Tzeng TT, Chen WP, Chen YP, Shiao YJ, et al. Neurohealth Properties of Hericium erinaceus Mycelia Enriched with Erinacines. Behav Neurol. 2018; 5802634.
- Sabaratnam V, Kah-Hui W, Naidu M, David PR. Neuronal Health – Can Culinary and Medicinal Mushrooms Help J Tradit Complement Med. 2013; 3: 62-68.
- Tuli HS, Sandhu SS, Sharma AK. Pharmacological and therapeutic potential of Cordyceps with special reference to Cordycepin. 3 Biotech. 2014; 4: 1-12.
- Li B, Hou Y, Zhu M, Bao H, Nie J, Zhang GY, et al. 3’-Deoxyadenosine (Cordycepin) Produces a Rapid and Robust Antidepressant Effect via Enhancing Prefrontal AMPA Receptor Signaling Pathway. Int J Neuropsychopharmacol. 2016; 19: pyv112.
- Clements CC, Castro VM, Blumenthal SR, Rosenfield HR, Murphy SN, Fava M, et al. Prenatal antidepressant exposure is associated with risk for attention-deficit hyperactivity disorder but not autism spectrum disorder in a large health system. Mol Psychiatry. 2015; 20: 727-734.
- Hirsch KR, Smith-Ryan AE, Roelofs EJ, Trexler ET, Mock MG. Cordyceps militaris Improves Tolerance to High-Intensity Exercise After Acute and Chronic Supplementation. J Diet Suppl. 2016; 1-13.
- Tang W, Gao Y, Chen G, Gao H, Dai X, Ye J, et al. A Randomized, Double- Blind and Placebo-Controlled Study of a Ganoderma lucidum Polysaccharide Extract in Neurasthenia. J Med Food. 2005; 8: 53-58.
- Gill SK, Rieder MJ. Toxicity of a traditional Chinese medicine, Ganoderma lucidum, in children with cancer. Can J Clin Pharmacol. 2008; 15: e275-285.
- Jin X, Ruiz Beguerie J, Sze DM, Chan GC. Ganoderma lucidum (Reishi mushroom) for cancer treatment. Cochrane Database Syst Rev. 2016; 4: CD007731.
- Guzmán G. Hallucinogenic Mushrooms in Mexico: An Overview. Economic Botany. 2008; 62: 404-412.
- Byock I. Taking Psychedelics Seriously. J Palliat Med. 2018; 21: 417-421.
- Gilbert JA, Krajmalnik-Brown R, Porazinska DL, Weiss SJ, Knight R. Toward Effective Probiotics for Autism and Other Neurodevelopmental Disorders. Cell. 2013; 155: 1446-1448.
- Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, et al. Microbiota Modulate Behavioral and Physiological Abnormalities Associated with Neurodevelopmental Disorders. Cell. 2013; 155: 1451-1463.
- Howland MA, Sandman CA, Glynn LM. Developmental origins of the human hypothalamic-pituitary-adrenal axis. Expert Rev Endocrinol Metab. 2017; 12: 321-339.
- Curtin P, Austin C, Curtin A, Gennings C, Arora M. Dynamical features in fetal and postnatal zinc-copper metabolic cycles predict the emergence of autism spectrum disorder. Science Advances. 2018; 4: eaat1293.
- Miller NZ. Aluminum in Childhood Vaccines Is Unsafe. Journal of American Physicians and Surgeons. 2016; 21: 109-117.
- Fattorusso A, Di Genova L, Dell’Isola G, Mencaroni E, Esposito S. Autism Spectrum Disorders and the Gut Microbiota. Nutrients. 2019; 11: 521.
- Singh RK, Chang HW, Yan D, Lee KM, Ucmak D, Wong K, et al. Influence of diet on the gut microbiome and implications for human health. J Transl Med. 2017; 15: 73.
- Zhang H, Yu L, Yi M, Li K. Quantitative studies on normal flora of seborrhoeic dermatitis. Chin J Dermatol. 1999; 32: 399-340.
- Bakare MO, Munir KM, Kinney DK. Association of hypomelanotic skin disorders with autism: links to possible etiologic role of vitamin-D levels in autism? Hypothesis (Tor). 2011; 9: e2.
- Kawicka A, Regulska-Ilow B. How nutritional status, diet and dietary supplements can affect autism. A review. Rocz Panstw Zakl Hig. 2013; 64: 1-12.
- Goodrich AJ, Volk HE, Tancredi DJ, McConnell R, Lurmann FW, Hansen RL, et al. Joint effects of prenatal air pollutant exposure and maternal folic acid supplementation on risk of autism spectrum disorder. Autism Res. 2018; 11: 69-80.
- Wagner CL, Hollis BW. The Implications of Vitamin D Status During Pregnancy on Mother and her Developing Child. Front Endocrinol (Lausanne). 2018; 9.
- Perreault M, Moore CJ, Fusch G, Teo KK, Atkinson SA. Factors Associated with Serum 25-Hydroxyvitamin D Concentration in Two Cohorts of Pregnant Women in Southern Ontario, Canada. Nutrients. 2019; 11: 123.
- Balasubramanian B, Bhatt CV, Goyel NA. Genetic studies in children with intellectual disability and autistic spectrum of disorders. Indian J Hum Genet. 2009; 15: 103-107.
- Pulendran B, Artis D. New Paradigms in Type 2 Immunity. Science. 2012; 337: 431-435.
- Tagalakis V, Meng L, Fombonne E, Strulovitch J, Tse J. Social Skills Training for Adolescents with Asperger Syndrome and High-Functioning Autism. J Autism Dev Disord. 2007; 37: 1960-1968.