
Research Article
Int J Nutr Sci. 2021; 6(2): 1052.
Reappraisal of Factors Disturbing the Relationship between Body Water Volumes and Total Body Electrical Resistance in Patients on Hemodialysis
Schotman JM1*, van Borren MMGJ2, Wetzels JFM3, Kloke HJ3, Reichert LJM1, Doorenbos CJ4 and de Boer H1
1Department of Internal Medicine, Rijnstate Hospital, Arnhem, The Netherlands
2Department of Clinical Chemistry, Rijnstate Hospital, Arnhem, The Netherlands
3Department of Nephrology, Radboud University Medical Center, Radboud Institute for Health Sciences, Nijmegen, The Netherlands
4Department of Internal Medicine, Deventer Hospital, Deventer, The Netherlands
*Corresponding author: Schotman JM, Department of Internal Medicine, Rijnstate Hospital, Wagnerlaan 55, 6800 TA Arnhem, The Netherlands
Received: April 27, 2021; Accepted: May 24, 2021; Published: May 31, 2021
Abstract
Background: Measurements of Total Body Electrical Resistance (TBER) are used to improve fluid balance management in patients on Hemodialysis (HD). This approach is based on the inverse relation that exists between TBER and body water volumes. Interpretation errors may occur if TBER measurements are affected by factors that are not related to changes in body water. Aim of this paper was to provide an overview of the methodological artifacts commonly encountered in a clinical setting, and to strengthen current evidence of their disturbing effects by performing additional experiments.
Methods: This study includes an analysis of available literature data, supplemented with additional experiments in healthy adults and patients. A cutoff of 2.7% was used to classify changes in TBER as significant within individual subjects.
Results: Electrode position, electrode interference, differences of measurements performed at the right or left side of the body, presence of orthopedic prosthesis located in the limbs, fluid redistribution induced by longterm changes in body position, and electrolyte abnormalities were the main disturbing factors that can induce a significant change in TBER. Other factors either had no significant disturbing effect or could be easily avoided.
Conclusion: TBER measurements require a high degree of standardization to minimize interpretation errors.
Keywords: Bioimpedance; Hemodialysis; Single-frequency; Measurement errors; Standardization
Introduction
Whole-body Bioelectrical Impedance Analysis (BIA) can be used for fluid balance monitoring in patients on Hemodialysis (HD) and has been shown to have significant clinical benefits, such as improvements in fluid status and blood pressure, reduction of antihypertensive medication, and a reduced number of intradialytic hemodynamic events [1-5]. Nevertheless, clinicians remain reluctant to use the BIA technique in clinical practice, probably because of concerns about the accuracy of volume estimations in individual patients. In the present paper, we will discuss the potential sources of these inaccuracies, based on findings described in the literature as well as data from additional experiments.
BIA is an umbrella term used to encompass a number of different technologies, of which Single-Frequency BIA (SF-BIA), Multifrequency BIA (MF-BIA), and Bioimpedance Spectroscopy (BIS) are the most commonly used methodologies. It is important to note that they are all based on a two-step procedure. The first step is measurement of Total Body Electrical Resistance (TBER) by skin electrodes positioned on the hand and foot or a predefined body segment. The second step includes translation of the measured TBER into body water volumes, either based on empirically derived algorithms or more complex models [6-10]. Therefore, inaccuracies may either be related to factors that only affect the measurement of TBER itself, or to computation errors caused by invalid assumptions in the algorithms translating TBER into body water volumes.
The electrical measurement of TBER itself is very precise, with an analytical coefficient of variation ranging from 0.07 to 0.30%, and therefore this cannot account for the observed inaccuracies in patients [11-14]. In a well-controlled setting, changes in TBER observed during HD are tightly correlated with the changes in body water volume induced by Ultrafiltration (UF) [14-16], indicating that TBER measurements are useful to monitor hydration and to guide UF. However, this does not exclude the possibility that methodical errors or specific clinical conditions can induce changes in TBER that have no relation with changes in body water volume. If these disturbing factors are not recognized, a non-volume related decrease or increase in TBER will be incorrectly translated into an increase or decrease in calculated body water, respectively. This will lead to either over- or underestimation of the actual hydration status, and patients may receive the wrong treatment.
Knowledge of all factors affecting TBER in any specific situation is required to avoid interpretation errors. This applies to all BIA approaches using algorithms to calculate body water, as well as all BIA methods that are based on raw data analysis. Several studies already provided an overview of sources of error affecting TBER and recommended ways to minimize or avoid them [11,17-21]. Examples of factors that were addressed are electrode placement, body position during measurement, and the impact of food and beverage. However, other issues commonly encountered during HD, like blood pressure measurements coinciding with TBER measurements, HD related changes in body temperature, and the impact of posture dependent fluid redistribution have received limited attention. Moreover, the safety of TBER measurements in patients with implanted electronic cardiac devices also remains subject of debate.
Most data about TBER disturbances have been derived from studies in healthy subjects. It is not well known to what extent these results are applicable in patients on HD. The aim of this study was to extend the currently available information on methodological factors and conditions that disturb the relationship between TBER and body water volume in patients on HD, in order to minimize errors in hydration status assessment.
Methods
Subjects
This study was performed in various subgroups of healthy adults and patients visiting either Rijnstate Hospital (Arnhem, the Netherlands) or Deventer Hospital (Deventer, the Netherlands). The studies were approved by the local ethics committee of both institutions, and all subjects gave their informed consent prior to participation.
TBER measurements
Literature results and additional experiments were based on TBER measured at a frequency of 50kHz, unless stated otherwise. This choice was made because 50kHz BIA was the earliest proposed method for the estimation of body water, and because the majority of research on interfering factors has been performed at this particular current frequency. The BIA101 Anniversary (Akern bioresearch srl, Pontassieve, Italy) was used to measure whole-body TBER in additional experiments, using skin-gel electrodes. In fact, TBER is not measured directly, but calculated from measured total body impedance and phase. Under normal conditions, TBER was measured at the right body side in controls and at the non-shunt side in patients on HD, with subjects in semi-recumbent position and with limbs slightly abducted from the body. Current injection electrodes were placed below the phalangeal-metacarpal joints of the index and middle finger, and below the phalangeal-metatarsal joints of the second and third toe. Detection electrodes were placed in the middle of the posterior aspect of the wrist proximal from the imaginary line at level of the styloid process of the radius, and at the ventral side of the ankle joint distal from the imaginary line at level of the lateral malleolus.
Critical difference
The concept of critical difference of TBER measurements was used as an objective criterium to quantify the impact of the investigated error for individual patients. It is defined as the smallest difference needed to consider a change in TBER significant within a single subject, with a confidence interval of 95% [22]. The critical difference of a TBER measurement in adults is 2.7%, and is based on analytical precision of the TBER instrument and biological variation within subjects [7,14,23]. For example, in patients with an ECW volume of 18L, a critical difference of 2.7% implies that the volume change has to be at least 0.49L in order to become detectable within a single subject. In this study the critical difference is used as a cutoff to describe the impact magnitude for each disturbing factor separately. However, in a clinical setting more than one disturbing factor may occur. In such cases the errors need to be added up. To avoid a significant disturbance, it should be realized that the sum of the all errors factors should not exceed 2.7%.
Statistical analysis
Results of additional experiments were presented as mean ± standard deviation (SD). Differences between groups were tested by a student’s t-test and intra-individual changes in TBER by paired twotailed t-test. Linear regression was performed to explore the relation between TBER and UF volume (temperature study). A P-value of <0.05 was considered statistically significant.
Experiments and Results
Impact of alcohol cleaning
TBER is measured by four adhesive electrodes applied to the skin. To achieve a good signal quality, the skin has to be proper and dry. In case of dirty or oily skin, impeding proper attachment of the electrodes, the skin can be cleaned with alcohol prior to attachment of the electrodes. However, it has been suggested that alcohol might dehydrate the skin and, thereby, increase the electrodeskin impedance [24]. Evans et al. investigated the impact of alcohol cleaning on the TBER in only two patients. TBER measured before and directly after alcohol cleaning, showed an increase in TBER of 1.3% (7.0Ω) and 1.5 % (7.2Ω), respectively [11]. A similar study in 46 adults found a small but significant increase of 0.4% [25]. Because of the limited data available we decided to study this aspect in a group of 18 healthy subjects (9 men and 9 women). In this study, alcohol cleaning was associated with a very small, non-significant decrease in TBER of 0.5 ± 2.1 Ω or 0.1 ± 0.4% (P=0.29). Changes within subjects ranged from -6.0 to 3.4 Ω (-1.0 to 0.6%) and never exceeded the critical difference. Note that this topic is only relevant for skingel electrode type devices. It does not apply for stand-on devices or devices using tactile electrodes.
In conclusion: Cleaning of the skin with alcohol does not affect TBER.
Shielding of the cables
When performing TBER measurements it is important to know the degree of shielding of the electrode cables. If shielding is incomplete, electrical interference may occur between the cables or between the cables and metallic bed frames, which will disturb the measurement of TBER. A study in 46 adults showed a small error of 0.2% related to the use of a metallic hospital bed [21]. To extent current knowledge, we studied further this aspect with BIA 101 cables in 20 healthy controls (9 men and 11 women). Cables were attached to the skin electrodes placed at the standard anatomical positions on the hand and foot. Initially, TBER was measured with the cables clearly separated, and then while holding the cables in parallel contact to each other. Interference was found to be negligible with the cables in parallel contact, with a mean decrease in TBER of 1.0 ± 0.7 Ω or 0.2 ± 0.1% (P <0.001). Impact at individual level was far below the critical difference of 2.7%, with values ranging from -3.1 to -0.3 Ω (-0.4 to -0.1%).
In conclusion: The cables of the BIA101 device are well shielded. When measurements are performed with other devices and at other frequencies, shielding check is recommended.
Impact of detection electrode position
TBER measurements are often used for long-term monitoring. Since TBER is directly related to length of the segment that is measured [8], electrode position should be the same at each occasion to obtain comparable data. In a study of two subjects, Evans et al. reported that a decrease of the hand-to-foot detection electrode distance was associated with a decline in TBER of about 1.7% per cm proximalization of the hand electrode pair and of 1.2 % per cm proximalization of the foot electrode pair [11]. Gartner et al. studied 8 subjects and confirmed that proximalization of the detection electrode was associated with a linear decrease in TBER [26].
To extend current evidence, we evaluated the impact of electrode displacement in 20 healthy subjects (9 men and 11 women). TBER measurements were first performed with electrode pairs applied to standard anatomical positions, and then after shifting the detection electrode in steps of 1cm, up to either 3cm distally or 3cm proximally, while maintaining the baseline position of the injection electrodes.
TBER proved very sensitive to detection electrode displacement (Figure 1). It decreased by 11.1 ± 2.3 Ω/cm (2.1 ± 0.3 %/cm) for proximal shifts of the hand detection electrode and by 8.3 ± 2.0 Ω/cm (1.5 ± 0.2 %/cm) for proximal shifts of the foot detection electrode. At individual level, the impacts ranged from 7.5 to 14.4 Ω/cm (1.6 to 2.6 %/cm) for shifts of the hand electrode and from 4.7 to 11.8 Ω/cm (1.0 to 1.9 %/cm) for shifts of the foot electrode. In 7 of 20 subjects (35%), one centimeter distalization of the hand detection electrode caused an increase in TBER that exceeded the critical difference.
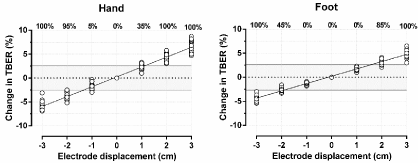
Figure 1: Impact of detection electrode displacements of the hand and foot on Total Body Electrical Resistance (TBER) measured in individual patients. Electrode
proximalization is indicated by negative distances and distalization by positive distances. The percentages at the top of the figure represent the percentage of
patients with a change in TBER exceeding the critical difference of 2.7% (gray area).
In conclusion: Rigorous standardization of detection electrode position is very important to obtain reproducible results.
Impact of inter-electrode distance
A minimal distance of 5cm between the detection and injection electrode is advised to prevent electrode interference [27,28]. However, available literature supporting this recommendation is limited. Gartner et al. measured TBER while decreasing the interelectrode distance between the detection and injection electrode at the hand and foot by a proximalization of the injector electrode in 8 subjects. Some interference was observed when the inter-electrode distance became <3cm [26]. Russo et al. also observed interference at an inter-electrode distance of 3cm or less, with a mean overestimation of TBER of 1.0% in 104 healthy adults [18].
To evaluate the results of previous studies, we performed a similar study in 20 healthy subjects (10 men and 10 women). Initially, electrodes were applied to the standard anatomical positions. Baseline TBER was measured at an inter-electrode distance of 6 cm and, subsequently, TBER was measured while gradually shifting the hand and foot injection electrodes separately from 6 (baseline) to 0 cm distance to the detection electrodes, in shifts of 1cm, and while maintaining fixed positions of the detection electrodes.
As demonstrated in Figure 2, a decrease in inter-electrode distance to 5cm already caused a detectable interference with a small increase in TBER of 0.4 ± 0.6 Ω or 0.1 ± 0.1 % for the hand (P <0.05) and 1.5 ± 0.8 Ω or 0.3 ± 0.2 % for the foot (P<0.001). TBER overestimation gradually increased with each cm decrease in inter-electrode distance. At 0cm distance it had increased by 8.4 ± 2.6 Ω or 1.7 ± 0.5 % for the hand (P <0.001) and by 15.1 ± 4.3 Ω or 2.9 ± 0.7 % for the foot (P<0.001), where it exceeded its critical difference in 12 of 20 (60%) patients. Overestimation of TBER that exceeds the critical difference can occur for inter-electrode distances <2cm.
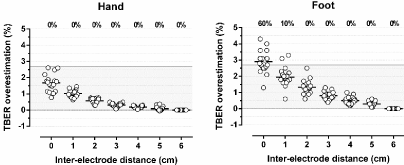
Figure 2: Impact of changes in inter-electrode distance of the hand and foot on Total Body Electrical Resistance (TBER). Data are presented as mean and
individual values. TBER measured with the pair of electrodes at 6 cm distance represents baseline TBER. The percentages at the top of the figure represent the
percentage of patients with an overestimation of TBER exceeding the critical difference of 2.7% (gray area).
In conclusion: Inter-electrode distance <2cm will induce electrode interference that exceeds the allowable error of 2.7%.
Left-right differences
The majority of published TBER normal values have been obtained by measurements in healthy adults, performed at the right side of the body. To avoid any bias caused by left-right differences in body composition, measurements in patients should therefore preferably be performed at the right side. However, in some conditions this is not feasible, such as in patients with right-sided limb amputation or in patients on HD with an arteriovenous shunt in the right arm. In such cases, the validity of left-sided TBER measurements based on right-sided normal values may be questioned. A switch from right to left-sided measurements is only valid if the population normal range is similar for both sides, and if TBER differences between left and right sided measurements within a patient does not exceed the critical difference.
Previous studies investigating TBER left-right differences have produced conflicting results. In a study of 46 healthy men and 46 healthy women, left-sided TBER was 8.0 ± 16 Ω higher (1.6%) than right-sided TBER (P <0.01) [29]. In contrast, Di Iorio et al. found that left-sided TBER was 1.4 ± 2.3% lower than right-sided TBER in 20 healthy right-handed subjects (10 men and 10 women) [19]. These discrepant finding may be partially related to differences in right/left side dominance [21,30]. We investigated left-right differences in 91 healthy subjects (44 men and 47 women). Right-hand dominance was present in 81% of men and in 82% of women. Figure 3 shows left-sided and right-sided TBER values in men and women. Although leftsided TBER tended to be higher in men, left-right differences were not significant, with a mean difference of 2.5 ± 15.3 Ω or 0.6 ± 3.2% (P=0.29, Table 1). In women, left-sided TBER was significantly higher than right-sided TBER, with a small difference of 5.7 ± 15.9 Ω or 1.0 ± 2.9% (P=0.02). Left-right differences did not significantly differ between men and women (P=0.32). At individual level, left-right differences may be large, ranging from -34.6 to 28.8 Ω (-6.5 to 5.9%) in men and from -16.0 to 48.1 Ω (-2.6 to 8.5%) in women. As shown in Figure 3, side-switching may introduce an error that exceeds the critical difference in 17 of 44 men (39%) and in 11 of 47 women (23%). As TBER is for 90 to 95% explained by the resistance of the limbs [31], and limb conduction is mainly determined by the limb lean body mass [32,33], left-right differences in TBER may be related to differences in limb muscle mass. This relationship was however not investigated in this study.
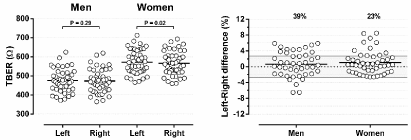
Figure 3: Left-sided and right-sided Total Body Electrical Resistance (TBER), and left-right differences in TBER in healthy subjects. Data are presented as mean
and individual values. The percentages at the top of the figure represent the percentage of patients with a TBER difference exceeding the critical difference of
2.7% (gray area).
Left sided TBER (O)
Right sided TBER (O)
Difference (%)
Healthy controls
Men (N = 44)
476 ± 59
473 ± 62
0.6 ± 3.2
Women (N = 47)
572 ± 62
566 ± 61
1.0 ± 2.9#
TBER P-side (O)
TBER NP-side (O)
Difference (%)
THP patients
Men (N = 25)
436 ± 40
441 ± 36
-1.2 ± 4.1
Women (N = 25)
515 ± 48
522 ± 55
-1.0 ± 3.9
TKP patients
Men (N = 25)
439 ± 53
449 ± 51
-2.3 ± 3.4#
Women (N = 25)
512 ± 71
526 ± 74
-2.7 ± 3.0*
Values are presented as mean ± SD.
Abbreviations: TBER: Total Body Electrical Resistance; P: Prothesis; NP: Non-Prosthesis; THP: Total Hip Prosthesis; TKP: Total Knee Prosthesis.
Level of significance: #P<0.05; *P<0.001.
Table 1: Overview of absolute values and differences in TBER measured at left and right side in controls and at Prosthesis (P) and Non-Prosthesis (NP) side in patients with Total Hip Prosthesis (THP) and patients with Total Knee Prosthesis (TKP).
In conclusion: Because left-right differences at a group level were small, the right-sided TBER normal range may be used for left-sided measurements, if necessary. However, in view of the large differences within individuals, it is strongly recommended to perform all repeated TBER measurements on the same side.
Impact of orthopedic prosthesis
BIA manuals advise to exclude patients with orthopedic prosthesis at the measuring side to prevent metal artifacts. To our knowledge, there is little evidence to support this advice. We found only one study. The impact of Total Hip Prosthesis (THP) on TBER was investigated in 203 patients, 3 months after surgery [34]. In contrast to expectations, THP did not significantly affect TBER measurements, with a mean TBER of 553 ± 98 Ω at the Prosthesis (P) side and 550 ± 92 Ω at Non-Prosthesis (NP) side (P=0.40). To examine this unexpected finding we repeated the THP study, and added a study about the impact of Total Knee Prosthesis (TKP). Measurements were performed in the outpatient clinic, at least 6 months after surgery to avoid errors induced by postoperative edema.
The impact of THP on TBER as well as that of TKP was studied in 100 orthopedic patients (50 men and 50 women). In patients with a THP (25 men and 25 women), TBER tended to be slightly lower at the P-side, by -5.2 ± 18.1 Ω or -1.2 ± 4.1% in 25 men (P=0.16) and by -6.3 ± 20.1 Ω or -1.0 ± 3.9% in 25 women (P=0.13), but statistical significance was not reached (Table 1). In contrast, TKP significantly lowered TBER, with a mean P-NP difference of -10.4 ± 14.8 Ω or -2.3 ± 3.4% in 25 men (P<0.05) and -14.5 ± 16.2 Ω or -2.7 ± 3.0 in 25 women (P <0.001). The impact of THP and TKP on TBER did not significantly differ between men and women (P=0.85 and P=0.35, respectively).
Although mean P-NP difference was not significant for THP, individual differences may be large, ranging from -32.8 to 41.4 Ω (-8.7 to 9.1%) in men and from -47.0 to 33.4 Ω (-8.9 to 8.4%) in women. In 14 of 25 men (56%) and in 11 of 25 women (44%), P-NP difference exceeded the critical difference (Figure 4). Individual P-NP differences in patients with TKP ranged from -48.2 to 13.2 Ω (-11.0 to 3.0%) in men and from -51.8 to 22.1 Ω (-8.7 to 4.5%) in women. P-NP differences exceeded the critical difference in 11 of 25 men (44%) and 13 of 25 women (52%). 10 of 25 men (40%) and 12 of 25 women (48%) showed a significant decrease in TBER at P-side. The small impact of the THP can be explained by its location in the electrical field of the trunk with its low contribution to TBER because of its large cross-sectional area [31]. Metallic prosthesis located in the limbs, such as TKP, will increase limb conduction and thus decrease TBER. Data on the impact of metallic prosthesis in the arm and shoulder are currently not available.
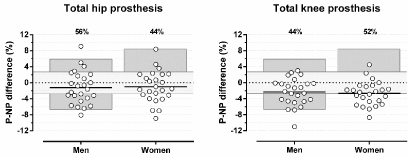
Figure 4: Differences in Total Body Electrical Resistance (TBER) between Prothesis (P) and Non-Prothesis (NP) side in patients with total hip or total knee
prosthesis. Data are presented as mean and individual values. The percentages at the top of the figure represent the percentage of patients with a TBER difference
exceeding the critical difference of 2.7% (light gray area). The dark grey areas represent the total range of left-right differences in healthy subjects.
The P-NP data were compared to the observed left-right differences in healthy controls. At group level, mean P-NP differences in TBER exceeded mean left-right differences observed in healthy controls, i.e. P=0.06 in men and P=0.03 in women with THP and P<0.001 for men and women with TKP. Figure 4 shows individual P-NP differences in relation to total range of normally existing left-right differences in healthy controls. THP as well as TKP induced a decrease in TBER at P-side with a magnitude that exceeded that of left-right differences in controls, i.e. in 4% of men and 32% of women with THP and in 12% of men and 48% of women with TKP. This indicates that both types of prosthesis have a disturbing effect on TBER.
In conclusion: Our study confirmed that THP did not significantly affect TBER at group level. However, its impact in individual patients can be large and may exceed the normally existing left-right differences in healthy subjects. TKP systematically lowered TBER at the prosthesis side, at group level as well as in individuals. We therefore recommend excluding measurements performed at the THP and TKP side.
Impact of electronic cardiac devices
Safety of performing TBER measurements in patients with Cardiac Implantable Electronic Devices (CIED) is subject of debate. Electrical interference induced by the BIA device might result in under-or oversensing of the lead, inappropriate shocks, inhibition of pacing or other malfunction of the CIED [35]. Theoretically, it is not likely that a 50kHz electrical current will induce electrical interference, as this frequency is far away from cardiac frequencies and because these high frequency currents are attenuated by band-pass filters. However, current evidence demonstrating lack of interference is still limited. Three studies examined the safety of TBER measurements at frequencies of 5 to 500 kHz in a group of respectively 20, 63 and 43 patients with implanted defibrillators [35-37]. In these studies, shock therapy was temporarily disabled to prevent inappropriate shocks. They all reported that TBER can be safely measured in patients without cardiac monitoring, as interference with the CIED was not observed in any of the patients. Safety was also established by the studies of Pinto et al. and Chabin et al., in which TBER was measured at frequencies between 5 and 100 kHz in 62 and 200 patients with implanted pacemakers or defibrillators, and with on-set of therapy [38,39]. We extended current evidence by measurement of TBER to a 50kHz current during a regular CIED check in 55 patients with pacemakers or defibrillators with on-set of therapy. Interference or malfunction of the CIED due to BIA was not observed. Of note, BIA devices performing measurements at lower frequencies require a similar validation procedure to assess instrument specific safety.
In conclusion: Despite the manufacturers’ recommendation to avoid BIA in patients with CIED, there is no evidence of interference when measurements are performed at frequencies ranging between 5 and 500 kHz.
Impact of blood pressure measurement
Blood Pressure (BP) measurements are performed every 30 minutes during HD to monitor hemodynamic stability. In theory, brief cuff inflations may drive extracellular water from the arm segment and thereby induce an increase TBER. As studies on this phenomenon were lacking, we investigated the magnitude and duration of the BP effect on TBER in 14 healthy subjects (6 men, 8 women). The BP cuff was attached to the right upper arm. The cuff pressure was rapidly raised to 200mmHg and then gradually deflated in 30 seconds. TBER measurements were performed before the BP measurement and then every 5 seconds from the start of cuff deflation until the TBER value had returned to its baseline. BP measurements induced a significant increase in TBER with a maximum value of 4.8 ± 0.6 Ω or 0.8 ± 0.1 % (P<0.001). Individual maximum values range from 1.9 to 8.4 Ω (0.4 - 1.6 %) and all were below the critical difference. The duration of the impact of BP measurement on TBER was 39.4 ± 4.6 seconds, ranging from 8 to 60 seconds.
In conclusion: The impact of brief cuff inflations for BP measurements is small. Moreover, it can be easily avoided by performing all TBER measurements before, or at least one minute after BP measurement.
Impact of HD related changes in body temperature
Fever with a body core temperature >38.5°C is associated with a 9% decrease of TBER [19]. It is therefore recommended to exclude patients with large changes in body core temperature from TBER measurement. It has also been shown that changes in ambient temperature can affect TBER by its effect on skin temperature and skin blood flow [40-42]. Liang et al. found that TBER increased by 1.43% for every °C decrease in skin temperature; whereas an increase in skin temperature induced by a rise in ambient temperature was associated with a 0.88%/°C decrease in TBER [41]. Kushner et al. reported an increase in TBER of 1.7%/°C decrease in skin temperature and a decrease in TBER of 1%/°C increase in skin temperature, induced by cooling and heating blankets [17].
HD treatment may be associated with changes in body core and skin temperature when cool dialysates with a temperature of 35-36°C is used to prevent body heating during HD [43-45]. In addition, high Ultrafiltration (UF) volumes that lead to hypovolemia may induce a decrease in skin temperature as result of compensatory vasoconstriction. Van der Sande et al. found that an UF volume of 3.1 ± 1.0 L was associated with a decrease in arm skin temperature of -0.9 ± 0.9 °C [46].
As the impact of HD related changes in body temperature on TBER has never been investigated we performed such a study in 30 patients on HD (19 men and 11 women). Dialysate temperature was 35.3 ± 0.3 °C. Measurements of TBER, core temperature, and skin temperature of the hand and foot were performed every 30 minutes during a single HD session. Skin temperature measurements were performed close to the position of the detection electrodes. Core temperature was measured by the tympanic method (Genius 2, Medtronic, Minneapolis, USA) and skin temperature of the hand and foot by an infrared device (Fora IR10, ForaCare Suisse AG, Gallen, Switzerland). The results of Liang et al. and Kushner et al. indicate that a 2°C decrease in skin temperature may induce an increase in TBER exceeding the critical difference of 2.7% [17,41]. This change in skin temperature was therefore used as a cutoff to compare groups.
As presented in Figure 5, core temperature remained stable during HD with a baseline temperature of 36.4 ± 0.5 °C (P=0.32). Hand skin temperature decreased from 35.3 ± 0.4 °C to 34.8 ± 0.8 °C (P <0.05) and foot skin temperature from 34.8 ± 1.5 °C to 33.2 ± 3.2 °C (P <0.05). Changes in core temperature ranged from -0.9 to 0.8 °C. One of 30 patients (3%) had a clinically relevant decrease in hand skin temperature of -2.1°C and 7 of 30 patients (23%) had a decrease in foot skin temperature >2°C, ranging from -2.4 to -8.3°C. Decreases in skin temperature occurred mainly during the second half of HD. To evaluate whether the observed decrease in skin temperature was associated with a significant rise in TBER we examined the TBER-UF volume relationship during HD for temperature effects. The mean UF volume of 2.2 ± 0.7 L induced an increase in TBER of 63.9 ± 19.6 Ω or 12.1 ± 3.5%. Individual changes in TBER during HD were tightly related to corresponding UF volumes, with R² ranging from 0.85 to 0.99. We hypothesized that TBER rises associated with skin temperature drops would be detectable as a steeper TBER-UF volume slope during the course of HD. To verify this, TBER/volume slopes (expressed in ohm per liter UF) were determined for the first part (0 to 120 min) and second part of HD (120 to 240 min). Then, the change in slope (Δslope) during HD of patients with foot temperature changes >2°C was compared to the Δslope of patients without clinically relevant changes in skin temperature. As shown in Figure 5, Δslope values were not significantly different between both groups (P=0.69), indicating that HD related changes in skin temperature did not affect TBER measured during HD. This may be explained by the fact that changes in skin temperature were limited to the distal extremities, which are largely outside the TBER measuring area that extends from the wrist to the ankle joint.
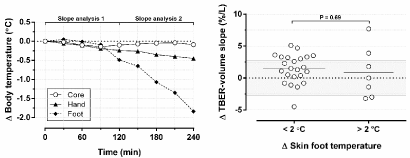
Figure 5: Left: overview of mean changes (Δ) in core, hand, and foot temperature during hemodialysis, plotted against time. Right: the impact of individual changes
in skin foot temperature on slope of the linear relation between total body electrical resistance and ultrafiltration volume. For patients with and without changes in
skin foot temperature >2°C, slopes of the first part of HD were compared to slopes of the second part of HD.
In conclusion: Changes in core or skin temperature can inversely affect TBER. Uncomplicated HD does not affect core temperature, but can be associated with a significant decline in foot skin temperature. However, this had no detectable effect on TBER. We recommend that in case of fever or hypothermia TBER measurements should be postponed until normalization of core temperature has occurred.
Body position during TBER measurement
Brief changes in body position are common during a HD session, for example to reach for a book or to get something to eat. Some studies reported that these short-term changes in body position affect TBER [47,48]. In 69 healthy controls, Allinson et al. demonstrated that transition from supine to semi-supine and sitting position induces a decrease in TBER of -1.4% and -8.6% respectively, which may be related to a reduced length of conduction path between the electrodes [47]. Fenech et al. investigated the transition from supine to sitting position in 8 patients on HD and found that TBER decreased by 2.3%, which was completely reversible within a few minutes when patients returned to their initial position [48].
To extend these data, we performed additional studies on brief position changes in 20 patients on HD (13 men and 7 women). TBER was measured before HD in semi-recumbent baseline position and then in sitting position, with the legs permanently straight forward in the first experiment and with legs permanently down in the second experiment (Figure 6). Transition from semi-supine to sitting position with the legs straight forward induced a decrease in TBER of -8.1 ± 3.8 Ω or -1.7 ± 0.8 % (P <0.001). Individual changes ranged from -0.8 to -18.8 Ω (-0.1 to -3.9%). The change in TBER exceeded the critical difference in 2 of 20 patients (10%) after 1 minute and in 1 of 20 patients (5%) after 5 minutes in sitting position (Figure 6). The impact of transition from baseline to sitting position with the legs down was somewhat higher. TBER decreased by -9.5 ± 4.2 Ω or -2.0 ± 0.9 % (P <0.001). Individual results ranged from -0.6 to -15.5 Ω (-0.1 to -3.6%), and the impact exceeded the critical difference in 5 of 20 patients (25%) after 1 as well as after 5 minutes in sitting position. When the subjects returned to semi-supine position, the effect was completely reversible within one minute, which is in line with the results of Fenech et al.
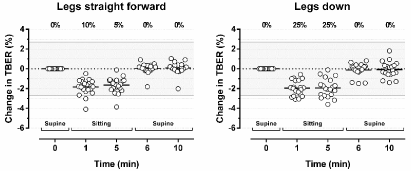
Figure 6: The impact of a change in body position from semi-supine position to sitting position with the legs straight forward and sitting position with the legs down
on Total Body Electrical Resistance (TBER). Data are presented as mean and individual values. The percentages at the top of the figure represent the percentage
of patients with a change in TBER exceeding the critical difference of 2.7% (gray area).
In conclusion: As TBER normal ranges have been assessed in supine position, it is advised to measure all subjects accordingly. Standardization of body position is important. TBER measurements have to be postponed for at least one minute after brief position changes.
Posture dependent fluid redistribution
TBER measurements are generally performed in supine position, after an equilibration period of 5 to 15 minutes. Such an equilibration period is commonly recommended because a prolonged change from standing to supine position is known to be associated with an increase in TBER due to fluid redistribution from the legs to the trunk [17,49,50]. The change from upright to supine position reduces the effect of gravity, lowers leg intravascular pressure and promotes the reabsorption of interstitial fluid from the leg into the circulation. Information about the time course and impact of this reabsorption process is limited and has only been studied in small groups of healthy adults [17,50-52]. Roos et al. demonstrated a 3.3% increase in TBER after 60 minutes of recumbency in 10 healthy subjects [51]. Kushner et al. measured TBER during a 4-hour period in 9 healthy controls and demonstrated a 3% rise in TBER after 1 hour, which increased to 5% after 2 hours and to 6% after 4 hours [17]. These results suggest that an equilibration time of 5 to 15 minutes is too short to create a steady state situation in healthy controls.
As TBER responses to prolonged posture changes may be quantitatively different in dialysis patients, in particular those with fluid excess, we studied the individual responses in 23 patients on HD (19 men and 4 women) [53]. On the day after HD, and also after an equilibration time of 15 minutes in semi-recumbent position, TBER was measured every 30 minutes for 2 hours while maintaining the semi-recumbent position. Baseline TBER increased by 11.6 ± 8.3 Ω or 2.4 ± 1.7 % after 60 minutes (P <0.001) and by a total of 18.9 ± 11.2 Ω or 3.8 ± 2.3 % after 120 minutes (P<0.001). As shown in Figure 7, variation at individual level was large, with ΔTBER ranging from -10.6 to 36.4 Ω (-2.0 to 6.8%) after 120 minutes. At that moment, the change in TBER exceeded its critical difference in 17 of 23 patients (74%). The exponential fit in Figure 7 or Equation 1 indicates that a steady state is still not reached after 120 minutes in semi-supine position. The time constant of 72 minutes indicates that the redistribution effect continues for at least 240 minutes before reaching steady state. After two hours 70% of plateau level is reached, and this increased to 91% after four hours. At that moment, the extrapolated total posture dependent rise in TBER was 4.9%, which is in line with the observations of Kushner et al.
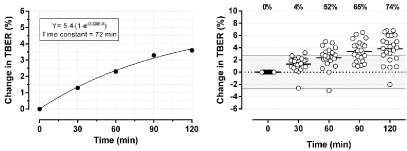
Figure 7: Impact of posture dependent redistribution of fluid on Total Body Electrical Resistance (TBER), presented as mean and individual changes in TBER
during a period of 120 minutes after transition from standing to semi-supine position. The exponential curve fit can be used to calculate the time needed to reach
steady state. The percentages at the top of the figure represent the percentage of patients with a change in TBER exceeding the critical difference of 2.7% (gray
area).
ΔTBER after posture change (%) = 5.4 • (1-e-0.0096•Time) (Eq. 1)
Since BIA equations to calculate body fluid volumes are validated on TBER measurements performed after a brief equilibration period, the TBER increase induced by posture dependent fluid redistribution will continue and will be incorrectly translated as a decrease in body fluid volume when measurements are performed after a longer period in supine position, such as in patients measured at the end of a 3 to 4 hour HD session. In these cases, Equation 1 may be used to estimate the mean impact of posture dependent fluid redistribution on TBER. However, due to the large variation between subjects and conditions, it cannot be used to correct data at individual level.
In conclusion: Posture dependent redistribution induces an increase in TBER that mainly occurs in the first 2 hours, but may continue to up to 4 hours. This phenomenon will introduce errors in the hydration assessment when TBER measurements are performed after a longer period in supine position.
Impact of electrolyte abnormalities
Algorithms translating TBER into body water volumes assume a constant value for the specific electrical resistance of body water (resistivity, ρ). The value for ρ is empirically determined in bioimpedance spectroscopy, or indirectly incorporated in the regression equations used in single-frequency BIA [54]. However, resistivity is not a constant in patients on HD or in other patients with electrolyte abnormalities. In vitro studies demonstrated that plasma electrolyte levels are major determinants of ρ in ECW (ρecw), in particular sodium and chloride levels [55,56]. This implies that clinical conditions with electrolyte abnormalities will be associated with abnormal values for ρecw, such as may occur in patients on HD. As these changes in ρecw are not related to changes in body water, they may disturb the relation between TBER and body water volumes [57,58]. Scharfetter et al. demonstrated that changes in ρecw during conventional HD induced by electrolyte abnormalities caused ECW volume estimation errors of up to 15% [58]. The relative impact of electrolyte shifts on TBER has been investigated in only a few studies. Roos et al. demonstrated that the change in plasma sodium is inversely related to the change in TBER measured at 50kHz (TBER50) in 10 healthy subjects, with R² = 0.90 (P <0.001). Based on their results, it can be calculated that TBER50 will change by 1% for each 3.3mmol/L change in plasma sodium [51]. We investigated the impact of electrolyte shifts during HD on extracellular TBER (TBERe) in 23 patients on HD, with near normal electrolyte levels at start of HD and with only small plasma-dialysate gradients. We found that 8% of the increase in TBERe during a conventional 4-hour HD is explained by electrolyte diffusion in well-controlled HD patients [53]. The results of these studies indicate that the impact of electrolyte abnormalities on TBER should not be underestimated. It may exceed the critical difference in patients with major electrolyte abnormalities. We therefore recommend performing TBER measurements at the time when ECW composition is likely to be as close as possible to that of healthy controls. In patients on HD, this occurs at the end of HD [53,59].
In conclusion: Major electrolyte abnormalities may induce a non-volume related change in TBER which is incorrectly translated as a change in body water. We advise to measure TBER at the end of HD, when electrolyte levels will have normalized and TBER will be most closely related to ECW.
Discussion
This study provides an overview of methodological artifacts that may occur when TBER measurements are performed in human subjects. It is a summary of the findings that have been uncovered in the past 30 years by researchers active in this field. The additional experiments we performed to extend current knowledge confirmed or strengthened most of the findings previously reported in the literature. Awareness of these confounding factors and the importance of highly standardized measurements can’t be overemphasized. Although the mean impact may seem small for most of these factors, the large SD’s suggested large errors in individual subjects. If not accounted for, these artifacts will be incorrectly translated into body water volumes, and thus lead to either over- or underestimation of a subject’s actual hydration status.
A summary of methodological factors and conditions affecting TBER is provided in Table 2. Measurements should be performed under highly standardized conditions to minimize errors in the body volume prediction. The concept of critical difference was used as a cutoff to define the significance of changes in TBER induced by each of the confounding factors. It should be noticed that the critical difference represents the total error budget for a single measurement. This implies that the sum of the impact of all disturbing factors should not exceed 2.7%. The disturbing effect of each confounding factors should therefore be reduced to a minimum. To achieve that, we advise to:
Artifact
Impact on TBER
Advice
Alcohol cleaning
No impact
To achieve good signal quality, the skin can be cleaned with alcohol if necessary
Shielding of the cables
No impact with BIA101 device
Examine the quality of shielding for other devices
Detection electrode position
Changes in TBER ranging from 1.0 to 2.6 %/cm
Standardize detection electrode position
Electrode interference
Inter-electrode distance < 2cm induces an increase in TBER that may exceed the CD
An inter-electrode distance of at least 2cm
Left-right differences
Side-switching introduced differences in TBER ranging from -6.5 to 8.5%
Perform all repeated TBER measurements on the same body side
Orthopedic prosthesis
THP and TKP may induce a significant decrease in TBER at P-side in individual patients
Perform TBER measurements at NP-side and exclude measurements performed at P-side
Electronic cardiac devices
Safe for TBER measurements at frequencies of 5 to 500kHz
Investigate safety when measuring TBER at other frequencies
Blood pressure measurements
Minor impact of BP measurement simultaneously with TBER, rapidly disappearing
Perform all TBER measurements before or > one minute after BP measurement
HD induced changes in body temperature
Changes in hand and foot skin temperature did not affect TBER; Changes in core temperature were not observed
As core temperature may affect TBER we advise to monitor body temperature in case of suspected fever or hypothermia
Short-term changes in body position
TBER changes of up to 3.9%, but the effect was completely reversible within one minute
Perform repeated measurements always in the same body position
Posture dependent fluid redistribution
Long-term changes in body position induce a significant increase in TBER that continues for more than 4 hours
Correct the TBER for the impact of fluid redistribution when measurements are performed after a longer period in supine position
Electrolyte abnormalities
Major electrolyte abnormalities may induce a significant change in TBER
TBER measurements at the time ECW composition is as close as possible to that of healthy controls (at the end of HD)
Abbreviations: TBER: Total Body Electrical Resistance; CD: Critical Difference; THP: Total Hip Prosthesis; TKP: Total Knee Prosthesis; P-side: Prosthesis-side; NP-side: Non-Prosthesis side; BP: Blood Pressure; HD: Hemodialysis; ECW: Extracellular Water.
Table 2: Summary of methodological factors and conditions affecting TBER.
• Standardize electrode positioning, by an anatomically fixed position of the detection and injection electrodes.
• Perform repeated measurements always at the same body side and in the same body position.
• Not measure TBER at the prosthesis side.
• Exclude patients with abnormal core temperature.
• Consider TBER correction for posture dependent redistribution of fluid when measurements are performed after a longer period in supine position. Correction can be performed based on Equation 1. However, this equation can only estimate mean impact of fluid redistribution and does not account for large interindividual variability.
• Perform measurements at the time when ECW composition is likely to be as close as possible to that of healthy subjects. Generally, TBER measurements are performed at the start of HD. However, at the end of HD, electrolyte levels are most comparable to normal. Interpretation errors may therefore be reduced by a switch to end-of- HD measurements.
A limitation of this review is that most of the literature results and all additional experiments were mainly based on TBER obtained at a current frequency of 50kHz (TBER50). At this frequency electrical currents will mainly flow through the ECW space but also partially though the intracellular compartment [8,14,60,61]. The BIS-derived extracellular resistance (TBERe), obtained by a mathematical extrapolation of the frequency dependent resistance-reactance plane, is generally considered as the most accurate reflection of true extracellular resistance and ECW volume [61]. The size of the compartment that is being measured by TBERe is smaller than that by TBER50, and therefore changes in ECW compartment will have a larger impact on TBERe than on TBER50 [14]. This implies that disturbing factors are likely to have a larger impact on TBERe than on TBER50.
Conclusion
TBER measurements should be performed under highly standardized conditions to avoid or minimize interpretation errors induced by non-volume related changes in TBER.
Acknowledgment
The authors want to thank the staff and nurses of the dialysis department in Rijnstate Hospital and Deventer Hospital for enabling BIA measurements, and Leny Blonk and Moniek Vroemen for their help with including patients with orthopedic prosthesis in Rijnstate Hospital. This study was financed by the Radboud-Rijnstate PhD funding.
References
- Hur E, Usta M, Toz H, Asci G, Wabel P, Kahvecioglu S, et al. Effect of fluid management guided by bioimpedance spectroscopy on cardiovascular parameters in hemodialysis patients: a randomized controlled trial. Am J Kidney Dis. 2013; 61: 957-965.
- Machek P, Jirka T, Moissl U, Chamney P, Wabel P. Guided optimization of fluid status in haemodialysis patients. Nephrol Dial Transplant. 2010; 25: 538- 544.
- Moissl U, Arias-Guillén M, Wabel P, Fontseré N, Carrera M, Campistol JM, et al. Bioimpedance-guided fluid management in hemodialysis patients. Clin J Am Soc Nephrol. 2013; 8: 1575-1582.
- Onofriescu M, Hogas S, Voroneanu L, Apetrii M, Nistor I, Kanbay M, et al. Bioimpedance-guided fluid management in maintenance hemodialysis: a pilot randomized controlled trial. Am J Kidney Dis. 2014; 64: 111-118.
- Ponce P, Fham J, Gligoric-Fuerer O, Kreuzberg U. Fluid management in haemodialysis: Conventional versus Body Composition Monitoring (BCM) supported management of overhydrated patients. Port J Nephrol Hypert. 2014; 28: 239-248.
- Piccoli A, Pillon L, Dumler F. Impedance vector distribution by sex, race, body mass index, and age in the United States: standard reference intervals as bivariate Z scores. Nutrition. 2002; 18: 153-167.
- Schotman JM, van Borren MMGJ, Kooistra MP, Doorenbos CJ, de Boer H. Towards personalized hydration assessment in patients, based on measurement of total body electrical resistance: Back to basics. Clin Nutr ESPEN. 2020; 35: 116-122.
- Kyle UG, Bosaeus I, De Lorenzo AD, Deurenberg P, Elia M, Gómez JM, et al. Bioelectrical impedance analysis-part I: review of principles and methods. Clin Nutr. 2004; 23: 1226-1243.
- Ellis KJ. Human body composition: in vivo methods. Physiol Rev. 2000; 80: 649-680.
- Khalil SF, Mohktar MS, Ibrahim F. The theory and fundamentals of bioimpedance analysis in clinical status monitoring and diagnosis of diseases. Sensors. 2014; 14: 10895-10928.
- Evans W, McClagish H, Trudgett C. Factors affecting the in vivo precision of bioelectrical impedance analysis. Appl Radiat Isot. 1998; 49: 485-487.
- Roche AF, Chumlea WC, and Guo S. Identification and validation of new anthropometric techniques for quantifying body composition. DTIC Document. Division of human biology, department of pediatric, Wright state University School of Medicine, Yellow Springs, Ohio. 1986.
- Wabel P, Chamney P, Moissl U, Schultheiss B, Rode C, Wieskotten S, et al. Reproducibility of Bioimpedance Spectroscopy (BIS) in health and disease. Nephrol Dial Transplant. 2007; 22: 137.
- Schotman JM, Hazeleger LR, van Borren MMGJ, Wetzels JFM, Kloke HJ, Reichert LJM, et al. Optimal Current Frequency for the Detection of Changes in Extracellular Water in Patients on Hemodialysis by Measurement of Total Body Electrical Resistance. Clin Nutr ESPEN (accepted for publication). 2021; 43: 302-307.
- Jebb S, Elia M. Assessment of changes in total body water in patients undergoing renal dialysis using bioelectrical impedance analysis. Clin Nutr. 1991; 10: 81-84.
- Dou Y, Cheng X, Liu L, Bai X, Wu L, Guo W, et al. Development and validation of a new dry weight estimation method using single frequency bioimpedance in hemodialysis patients. Blood Purif. 2011; 32: 278-285.
- Kushner RF, Gudivaka R, Schoeller DA. Clinical characteristics influencing bioelectrical impedance analysis measurements. Am J Clin Nutr. 1996; 64: 423S-427S.
- Gualdi-Russo E, Toselli S. Influence of various factors on the measurement of multifrequency bioimpedance. Homo. 2002; 53: 1-16.
- Di Iorio BR, Terracciano V, Bellizzi V. Bioelectrical impedance measurement: errors and artifacts. J Ren Nutr. 1999; 9: 192-197.
- Brantlov S, Ward LC, Jødal L, Rittig S, Lange A. Critical factors and their impact on bioelectrical impedance analysis in children: a review. J Med Eng Technol. 2017; 41: 22-35.
- González-Correa C, Caicedo-Eraso J, editors. Bioelectrical Impedance Analysis (BIA): a proposal for standardization of the classical method in adults. J. Phys. Conf. Ser. 2012; 407: 012018
- Fraser CG. Biological variation: from principles to practice. Amer. Assoc. for Clinical Chemistry. 2001.
- Schotman J, van Borren M, Kooistra M, Doorenbos C, de Boer H. Corrigendum to ‘Towards personalized hydration assessment in patients, based on measurement of total body electrical resistance: Back to basics’. Clin Nutr ESPEN. 2020; 36: 172-173.
- Oster CD. Proper Skin Prep Helps Ensure ECG Trace Quality. 3M Medical.
- Gonzalez C, Evans J, Smye S, Holland P. Variables affecting BIA measurement of body water. Med Biol Eng Comput. 1999; 37: 106-107.
- Gartner A Fau - Maire B, Maire B Fau - Delpeuch F, Delpeuch F Fau - Sarda P, Sarda P Fau - Dupuy RP, Dupuy Rp Fau - Rieu D, Rieu D. Importance of electrode position in bioelectrical impedance analysis. Am J Clin Nutr. 1992; 56: 1067-1068.
- Gartner A, Sarda P, Dupuy R, Maire B, Delpeuch F, Rieu D. Bioelectrical impedance analysis in small-and appropriate-for-gestational-age newborn infants. Eur J Clin Nutr. 1994; 48: 425-432.
- Kyle UG, Bosaeus I, De Lorenzo AD, Deurenberg P, Elia M, Gómez JM, et al. Bioelectrical impedance analysis-part II: utilization in clinical practice. Clin Nutr. 2004; 23: 1430-1453.
- Graves JE, Pollock ML, Colvin AB, Van Loan M, Lohman TG. Comparison of different bioelectrical impedance analyzers in the prediction of body composition. Am J Human Biol. 1989; 1: 603-611.
- Ward LC, Isenring E, Dyer JM, Kagawa M, Essex T. Resistivity coefficients for body composition analysis using bioimpedance spectroscopy: Effects of body dominance and mixture theory algorithm. Physiol Meas. 2015; 36: 1529- 1549.
- Organ LW, Bradham GB, Gore DT, Lozier SL. Segmental bioelectrical impedance analysis: theory and application of a new technique. J Appl Physiol. 1994; 77: 98-112.
- Faes T, Van der Meij H, De Munck J, Heethaar R. The electric resistivity of human tissues (100Hz-10MHz): a meta-analysis of review studies. Physiol Meas. 1999; 20: R1-10.
- Geddes LA, Baker LE. The specific resistance of biological material-a compendium of data for the biomedical engineer and physiologist. Med Biol Eng. 1967; 5: 271-293.
- Steihaug OM, Bogen BE, Kristoffersen MH, Ranhoff AH. Bones, blood and steel: How bioelectrical impedance analysis is affected by hip fracture and surgical implants. J Electr Bioimpedance. 2017; 8: 54-59.
- Buch E, Bradfield J, Larson T, Horwich T. Effect of bioimpedance body composition analysis on function of implanted cardiac devices. Pacing Clin Electrophysiol. 2012; 35: 681-684.
- Meyer P, Makhlouf A-M, Mondouagne Engkolo LP, Trentaz F, Thibault R, Pichard C, et al. Safety of bioelectrical impedance analysis in patients equipped with implantable cardioverter defibrillators. J Parenter Enter Nutr. 2017; 41: 981-985.
- Garlini LM, Alves FD, Kochi A, Zuchinali P, Zimerman L, Pimentel M, et al. Safety and Results of Bioelectrical Impedance Analysis in Patients with Cardiac Implantable Electronic Devices. Braz J Cardiovasc Surg. 2020; 35: 169-174.
- Pinto LW, Gandra SV, Alves MC, Gomes I, Sternick EB. Bioelectrical impedance analysis of body composition: influence of a newly implanted cardiac device. J Electr Bioimpedance. 2017; 8: 60-65.
- Chabin X, Taghli-Lamallem O, Mulliez A, Bordachar P, Jean F, Futier E, et al. Bioimpedance analysis is safe in patients with implanted cardiac electronic devices. Clin Nutr. 2019; 38: 806-811.
- Buono MJ, Burke S, Endemann S, Graham H, Gressard C, Griswold L, et al. The effect of ambient air temperature on whole-body bioelectrical impedance. Physiol Meas. 2004; 25: 119-123.
- Liang M, Su H-F, Lee N-Y. Skin temperature and skin blood flow affect bioelectric impedance study of female fat-free mass. Med Sci Sports Exerc. 2000; 32: 221-227.
- Medrano G, Bausch R, Ismail A, Cordes A, Pikkemaat R, Leonhardt S, editors. Influence of ambient temperature on whole body and segmental bioimpedance spectroscopy measurements. J Phys: Conf Ser. 2010; 224: 012128.
- Fine A, Penner B. The protective effect of cool dialysate is dependent on patients’ predialysis temperature. Am J Kidney Dis. 1996; 28: 262-265.
- Rosales LM, Schneditz D, Morris AT, Rahmati S, Levin NW. Isothermic hemodialysis and ultrafiltration. Am J Kidney Dis. 2000; 36: 353-361.
- Pérgola PE, Habiba NM, Johnson JM. Body temperature regulation during hemodialysis in long-term patients: is it time to change dialysate temperature prescription? Am J Kidney Dis. 2004; 44: 155-165.
- van der Sande FM, Rosales LM, Brener Z, Kooman JP, Kuhlmann M, Handelman G, et al. Effect of ultrafiltration on thermal variables, skin temperature, skin blood flow, and energy expenditure during ultrapure hemodialysis. J Am Soc Nephrol. 2005; 16: 1824-1831.
- Allison G, Singer K, Marshall R. The effect of body position on bioelectrical resistance in individuals with spinal cord injury. Disabil Rehabil. 1995; 17: 424-429.
- Fenech M, Jaffrin M, Malmen U. Reversibility of artifacts of fluid volume measurements by bioimpedance caused by position changes during dialysis. Int J Artif Organs. 2002; 25: 217-222.
- Maw G, Mackenzie I, Taylor N. Redistribution of body fluids during postural manipulations. Acta Physiol Scand. 1995; 155: 157-163.
- Rush EC, Crowley J, Freitas IF, Luke A. Validity of hand-to-foot measurement of bioimpedance: standing compared with lying position. Obesity. 2006; 14: 252-257.
- Roos A, Westendorp R, Frölich M, Meinders A. Tetrapolar body impedance is influenced by body posture and plasma sodium concentration. Eur J Clin Nutr. 1992; 46: 53-60.
- Zhu F, Schneditz D, Wang E, Levin NW. Dynamics of segmental extracellular volumes during changes in body position by bioimpedance analysis. J Appl Physiol. 1998; 85: 497-504.
- Schotman JM, van Borren MMGJ, Wetzels JFM, Kloke HJ, Reichert LJM, Doorenbos CJ, et al. Impact of diffusion, ultrafiltration, and posture on total body electrical resistance in patients on hemodialysis. J Appl Physiol. 2021; 130: 318-324.
- Ward LC. Bioelectrical impedance analysis for body composition assessment: reflections on accuracy, clinical utility, and standardisation. Eur J Clin Nutr. 2019; 73: 194-199.
- Schotman J, van Borren M, Wetzels J, Kloke H, Reichert L, de Boer H. Assessment of Plasma Resistivity as a Surrogate for Extracellular Fluid Resistivity: Analytical Performance and Impact of Fluid Composition. Ann Biomed Eng. 2019; 47: 1463-1469.
- Fuller H. The electrical impedance of plasma: a laboratory simulation of the effect of changes in chemistry. Ann Biomed Eng. 1991; 19: 123-129.
- Rees A, Ward L, Cornish B, Thomas B. Sensitivity of multiple frequency bioelectrical impedance analysis to changes in ion status. Physiol Meas. 1999; 20: 349-362.
- Scharfetter H, Wirnsberger G, Holzer H, Hutten H. Influence of ionic shifts during dialysis on volume estimations with multifrequency impedance analysis. Med Biol Eng Comput. 1997; 35: 96-102.
- El-Kateb S, Davenport A. Changes in intracellular water following hemodialysis treatment lead to changes in estimates of lean tissue using bioimpedance spectroscopy. Nutr Clin Pract. 2016; 31: 375-377.
- Buchholz AC, Bartok C, Schoeller DA. The validity of bioelectrical impedance models in clinical populations. Nutr Clin Pract. 2004; 19: 433-446.
- Matthie J, Zarowitz B, De Lorenzo A, Andreoli A, Katzarski K, Pan G, et al. Analytic assessment of the various bioimpedance methods used to estimate body water. J Appl Physiol. 1998; 84: 1801-1816.