
Research Article
Int J Nutr Sci. 2022; 7(1): 1062.
Relationship of Body Mass Index and Clinical Outcomes in Patients with Acute Kidney Injury: Systematic Review and Meta-analysis
Nsengimana B, Guo Y, Jin Y, Wei W* and Ji S*
Department of Biochemistry and Molecular Biology, School of Basic Medical sciences, Henan University, Henan, China
*Corresponding author: Wenqiang Wei, School of Basic Medical Sciences, Henan University, Kaifeng, China
Shaoping Ji, School of Basic Medical Sciences, Henan University, Kaifeng, China
Received: March 14, 2022; Accepted: April 08, 2022; Published: April 15, 2022
Abstract
Background: A higher body mass index (BMI) is considered as risk factor of developing chronic kidney diseases. However, its impact on acute kidney injury (AKI) remains debatable. This meta-analysis aimed to scrutinize the research evidence regarding the association of BMI and AKI development.
Methods: Eligible studies published until August, 2021 were searched by using electronic databases. Review Manager (RevMan) was used to evaluate the association of BMI and AKI by considering the odd ratio (OR) with 95% confidence interval (CI). Sensitivity analysis and publication bias were assessed.
Results: A total of 69,190 participants were obtained from 15 included studies. The pooled results show that the overall AKI incidence was 24.9%. OR of AKI in obese, overweight, and underweight were 1.22, 95% CI: 0.98 to 1.52, 1.2, 95% CI: 1.01 to 1.42, and 0.9, 95% CI: 0.78 to 1.02 respectively. AKI mortality was associated with underweight group with OR of 1.45, 95% CI: 1.04 to 2.01. AKI stages were statistically insignificant.
Conclusion: High incidence of AKI and high AKI mortality rate are associated with elevated BMI and low BMI respectively, hence awareness and control measures on BMI should be taken into account to prevent AKI burden. Further studies are recommended.
Keywords: AKI; BMI; Clinical outcome
Abbreviations
AKI: Acute Kidney Disease; APACHE: Acute Physiology and Chronic Health Evaluation; BMI: Body Mass Index; BUN: Blood Urea Nitrogen; CI: Confidence Interval; eGFR: estimated Glomerular Filtration Rate; LOS: Length of Stay; ICU: Intensive Care Unit; OR: Odd Ratio; PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses; PROSPERO: International Prospective Register of Systematic; SD: Standard deviation
Background
Despite BMI’s consideration as a tool for evaluating the nutritional status, its increment remains associated with different health comorbidities such as cardiovascular diseases, type 2 diabetes, and chronic kidney diseases [1-5].
The impact of overweight and obesity as a global epidemic is intense. BMI average is raising over 0.4 to 0.5 kg/ m2 in each decade worldwide [6]. It has been stated that 39% of adults were overweight in 2016. In 2020, 39 million of under 5 years old were overweight or obese, and the trend estimates that 2.7 billion adults will be overweight in 2050 globally [7-9]. In USA, the severe obesity folded over 9.2% from 2000 to 2018 [10]. In similar vein, a study carried out in England reports that overweight rate is increasing up to 40% in men [11]. Based on the aforementioned studies, a growing rate of BMI in global and regional is alarming. A rationale for researchers to explore the association of BMI and other diseases.
In the past decades, obesity-related nephropathy has been recognized due to several factors including type 2 diabetes, hypertension, intraglomerular pressure, and glomerulomegaly resulting in chronic kidney diseases [12]. Currently, findings show that AKI-obesity is associated a high number of patients in intensive care unit (ICU) [13]. 25% of ICU patients are obese with OR of 1.89 compared to general population [14]. So far, the confounding results have been found. Some studies established that more BMI is correlated with high prevalence of AKI and ICU- mortality compared to normal BMI, whereas, others proved that high mortality rate exists in underweight compared to overweight [15,16]. Therefore, the current meta-analysis aimed to scrutinize the research evidence regarding the association of BMI and AKI as the outcome of critically ill patients which remains inconsistent.
Methodology
Protocol and registration
This meta-analysis was conducted in accordance with Preferred Reporting Items for Systematic Reviews and Meta- Analyses (PRISMA) guidelines [17]. The protocol was registered in International Prospective Register of Systematic (PROSPERO) database (Registration number: CRD42021272156).
Searching strategies
An electronic search was conducted in Pubmed, Embase, Medline, Google Scholar, and Scopus databases for retrieving the articles published until August, 2021. The search term with Boalean Operators used were: “BMI” OR “body mass index” OR “overweight” OR “obese” OR “normal weight” OR “underweight” AND “acute kidney disease” OR “AKI” OR “kidney injury” OR “kidney failure”. The language applied was English.
Inclusion and exclusion criteria
The study included the original articles that evaluated the association of BMI and AKI. The first criterion was if the participants were classified into underweight, normal weight, overweight, and obese. The second criterion was the analyzed outcomes which were included but not limited to, AKI development, AKI stage, intensive care unit stay, time used to stay in hospital, comorbidities (hypertension and diabetes mellitus). The excluded studies in metaanalysis were reviews, case reports, newspapers, conference papers, comments, and other studies that were not published in English and those conducted on the participants who are under 18 age old.
Study selection
Based on eligibility criteria, two independent reviewers screened the selected studies. They firstly removed the duplicates and other studies based on exclusion criteria by screening the titles and abstracts. The full-text of remaining studies were further revised for checking their eligibilities. Any discrepancies between the two investigators were solved by a third reviewer in mutual consensus.
Data extraction and quality assessment
The data were extracted by two independent authors based on a standardized form which is recommended by Cochrane. The extracted information was year of publication, design of study, country, participants’ demographic features (age, height, weight, and gender), and outcomes: glomerulus filtration rate features, AKI mortality, AKI stage, LOS in ICU, LOS in hospital, acute physiology and chronic health evaluation (APACHE II). Participants group was classified as underweight, normal, overweight, and obese based on BMI <18.5kg/m², BMI ≥18.5 <25kg/m2, BMI ≥25 <30kg/m², BMI ≥30kg/ m² respectively. Newcastle-Ottawa quality assessment tool was used to assess the quality of the cohorts and the risk of bias [18], more than six stars were considered as high quality to meet the eligibility criteria in meta-analysis. A funnel plot was used to evaluate the publication bias (more or equal to six included studies were considered).
Statistical analysis
Statistical analysis was executed by RevMan 5.0.25 (Nordic Cochrane Centre, Cochrane Collaboration, UK). Mann-Whitney U test was used to evaluate the hypothesis and P <0.05 was considered as statistical significance. For continuous and dichotomous data, mean difference and OR in 95% CI were calculated respectively. A random effect model was used to assess the pooled OR and 95% CI. I2 was used to assess the heterogeneity, where 0% to 40%, 30% to 60%, 50% to 90%, and 90% to 100% was considered as minimal, moderate, substantial, considerable heterogeneity respectively, and P <0.1 designated the significance. Sensitivity analysis was used to assess the consistence of results.
Results
Study flow and characteristics
A total of 284,212 articles were retrieved through online searching the different databases including PubMed (169,107), Embase (57,726), Medline (3,042), Google Scholar (45,900), and Scopus (8,437). A total of 115,051 duplicates were removed, resulting in 169,161 articles which screened for the title and abstract. Subsequently, 229 articles were identified after removing 50,783 narrative reviews and 118,149 irrelevant articles. Among 55 full articles which checked for eligibility, 39 articles were excluded due to the lack of the related report of BMI and AKI outcomes. 17 articles were included in systematic review and 15 articles were considered in meta-analysis (Figure 1).
A total of 69,190 participants were included in these studies which carried out in 7 countries namely China (n=4), Denmark (n=1) Korea (n=4), Portugal (n=1), Turkey (n=1), Singapore (n=1), and USA (n=3). Study design in all studies was retrospective, except one which was a prospective study (Table 1).
Author, Year, Reference
Study design
Country
Population n
Number with BMI/total patients (%)
Aim of the study
Comorbidities
Outcomes
Vasquez 2020 [19]
Prospective cohort study
USA
463
463/553 (84%)
BMI and AKI after severe trauma
Hypertension, Diabetes mellitus, chronic kidney disease, and congestive heart failure
-
Zou 2017 [20]
Retrospective study
China
8,455
8,455/13083 (65%)
BMI and AKI after cardiac surgery
Hypertension and Diabetes mellitus,
AKI mortality, duration of mechanical ventilation, LOS in ICU, and LOS in hospital
Ju 2018 [21]
Retrospective study
Korea
468
468
BMI as AKI predictor in critically ill patients
diabetes mellitus, hypertension; cardiovascular disease; liver cirrhosis; chronic kidney disease; and acute respiratory distress syndrome
APACHE II score, SOFA score, ICU admission, MV duration, ICU LOS, hospital LOS, ICU death, and hospital death
Argalious 2017 [22]
Retrospective
USA
8,543
8,543/121,745 (7%)
BMI and AKI after laparoscopic surgery
Diabetes mellitus, hypertension; coronary artery disease, and chronic obstructive pulmonary disease
AKI and hospital mortality
Park 2017 [23]
Retrospective study
Korea
203
203/334 (61%)
BMI and AKI in liver transplantation recipients
hepatitis B virus; hepatitis C virus, Primary biliary cirrhosis, Autoimmune hepatitis, hypertension, and diabetes mellitus
AKI incidence, ICU stay, hospital stay, and hospital mortality
Kim 2018 [24]
Observational study
Korea
1,144
1144/2391(48%)
BMI and AKI in renal replacement therapy
Cancer, diabetes mellitus, hypertension, myocardiac infarction, congestive heart failure, cerebrovascular attack, peripheral vascular disease, and chronic obstructive pulmonary disease
AKI , APACHE II, and SOFA
Wang 2019 [25]
Retrospective cohort study
China
1120
1120/1271(88.1%)
BMI and AKI in renal replacement therapy
Myocardial infarction, congestive heart failure, cerebrovascular disease, diabetes mellitus, and hypertension
-
Kim 2017 [26]
Observational study
Korea
212
212/573(36.9)
BMI and AKI in renal replacement therapy
Diabetes mellitus, hypertension, congestive heart failure, cerebrovascular attack, and cancer
Mortality, hospital LOS, and ICU LOS
Liu 2021 [27]
Retrospective cohort study
China
115
115/137 (83.9)
BMI and AKI after aortic arch surgery
Cerebrovascular disease, diabetes mellitus, hypertension, and kidney malperfusion
postoperative AKI, Length of ICU, length of in hospital, and hospital mortality
Liu 2018 [28]
Retrospective cohort study
12,555
12555/35474(35.3%)
BMI and AKI
Hypertension, hypertension, and cardiovascular disease
AKI, mortality within 90 days of admission, and length of stay
MacLaughlin 2021 [29]
Prospective multisite cohort study
USA
1477
1477/1603(92.1%)
BMI and Chronic kidney disease after AKI
Diabetes, chronic heart failure, and cardiovascular disease
AKI stages, ICU, and hospital mortality
Gameiro 2018 [30]
Retrospective cohort study
Portugal
456
456/722(63.1%)
Obesity and AKI in patients with sepsis
Hypertension, diabetes, and infection
AKI, LOS in hospital, LOS in ICU, ICU mortality, and hospital mortality
Sabaz 2021 [13]
Retrospective cohort study
Turkey
4,459
4459/7227(61.6%)
BMI on AKI and ICU mortality
Hypertension, Diabetes, Cerebrovascular disease, Malignancy, Hepatic disease, Psychiatric disorder, Dementia, chronic obstructive pulmonary disease, chronic renal failure, coronary artery disease, and gastrointestinal bleeding
AKI, mechanic ventilation, APACHE 2, SOFA, and LOS in ICU
Zhou 2020 [31]
Retrospective cohort study
China
244
244/341(71.5%)
Overweight and AKI after liver transplantation
Hypertension, diabetes mellitus, chronic kidney disease, encephalopathy, ascites, and liver disease
AKI and hospital mortality
Wang 2021 [32]
Retrospective cohort study
China
15174
-
BMI and AKI in critically ill patients
Congestive heart failure, cardiac arrhythmias, valvular disease, hypertension, renal disease, Liver disease, uncomplicated and complicated diabetes, metastatic cancer and coagulopathy,
AKI stage, SOFA, ICU LOS, and mortality
Moon 2018 [33]
Retrospective cohort study
Korea
3018
3018/3089(97.7%)
Obesity and AKI after coronary artery bypass grafting
Hypertension, and diabetes mellitus,
AKI
Pedersen 2016 [35]
Regional cohort study
Denmark
13529
11411/16111 (70.8)
BMI and AKI after hip fracture surgery
Chronic kidney disease, diabetes, and Charlson comorbidity
AKI, mortality and hospital stay
Table 1: Details of the included studies.
The male participants who included in the studies were 33,478/69,190 (48.3%), and the range of mean age was 47.8-87.05. More about patients’ baseline features including albumin, uric acid, estimated glomerular filtration rate (eGFR), serum creatinine, and blood urea nitrogen (BUN) baseline, height, weight, and comorbidities like hypertension, and diabetes mellitus were summarized in Table 2.
Author year, Ref
BMI (kg/m2) categories
Age, years, median (SD)
Hypertension (%)
Albumin baseline, g/L
UA baseline, μmol/L
eGFR baseline, mL/min
SCr baseline, μmol/L
BUN baseline, mmol/L
Height, cm
Body weight, kg
Male, n (%)
Diabetes mellitus, n (%)
Pedersen 2016 [35]
Underweight
84 (78-89)
-
-
-
-
-
-
-
-
168 (13.2%)
70 (5.5%)
Normal
84 (78-89)
-
-
-
-
-
-
-
-
1817 (27.6%)
651 (9.9%)
Overweight
82 (76-87)
-
-
-
-
-
-
-
-
938 (33.9%)
480 (17.3%)
Obese
80 (74-86)
-
-
-
-
-
-
-
-
201 (25.7%)
206 (26.3%)
Zou 2017 [20]
Normal
52.6 ± 14.1
1,042 (23.6)
40.2 ± 3.6
354.7 ± 117.4
91.9 ± 25.1
77.4 ± 25.3
6.6 ± 2.9
163.4 ± 7.2
58.0 ± 6.6
2,251 (51.0)
292 (6.6)
Overweight
55.3 ± 12.2
59.4 (16.7)
40.3 ± 3.3
377.8 ± 141.8
89.3 ± 23.1
80.9 ± 24.0
6.3 ± 2.2
166.4 ± 7.0
71.2 ± 6.8
1,644 (65.1)
307 (12.2)
Obese
55.0 ± 11.9
344 (48.9)
40.4 ± 3.1
396.0 ± 109.5
87.3 ± 23.1
83.0 ± 24.4
6.3 ± 2.0
166.1 ± 8.0
83.0 ± 8.2
471 (66.9)
111 (15.8)
Underweight
47.8 ± 16.6
97 (12.0)
39.8 ± 4.1
342.0 ± 114.6
98.2 ± 28.6
73.4 ± 25.0
6.6 ± 3.0
163.6 ± 7.3
46.0 ± 5.2
340 (42.2)
38 (4.7)
Ju 2018 [21]
Normal
68.6 ± 14.1
-
-
-
-
-
-
-
-
197 (41)
94 (33.0)
Overweight
64.9 ± 13.8
44 (55.0)
-
-
-
-
-
-
-
47 (7)
34 (42.5)
Obese
57.0 ± 15.9
-
-
-
-
-
-
-
-
116 (63.4%)
-
Underweight
71.3 ± 12.7
34 (33.3)
-
-
-
-
-
-
-
62 (13)
27 (26.5)
Argalious 2017 [22]
Normal
54 ± 17
461 (31)
-
-
-
-
-
-
-
391 (26)
107 (7)
Overweight
57 ± 15
7354 (44)
-
-
-
-
-
-
-
644 (39)
243 (15)
Obese
56 ± 14
700 (56)
-
-
-
-
-
-
-
394 (31)
253 (20)
Morbidly obese
49.3 ± 13.1
-
-
-
-
-
-
-
-
23 (31.1%)
26 (35.1%)
Underweight
49 ± 17
21 (21)
-
-
-
-
-
-
-
20 (20)
2 (2)
Park 2017 [23]
Normal
54.38 ± 7.33
14 (18.9)
-
-
-
-
-
-
-
58 (78.4)
21 (28.4)
Underweight
53.68 ± 8.91
9 (24.3)
-
-
-
-
-
-
-
26 (70.3)
14 (17.0%)
Kim 2018 [24]
Normal
65.0 ± 13.6
217 (53.8)
2.6 ± 0.6
35 (8.7)
33.1 ± 22.3
2.5 ± 1.3
35 (8.7)
-
-
241 (59.8)
140 (34.7)
Overweight
63.9 ± 14.0
110 (50.0)
2.6 ± 0.6
21 (9.5)
32.7 ± 24.4
2.9 ± 1.9
21 (9.5)
-
-
155 (70.4)
79 (35.9)
Obese
61.3 ± 14.5
233 (55.3)
2.6 ± 0.6
46 (10.9)
29.0 ± 18.8
2.9 ± 1.7
46 (10.9)
-
-
248 (58.7)
153 (36.3)
Underweight
62.3 ± 17.2
40 (40.4)
2.5 ± 0.5
13 (13.1)
33.2 ± 18.0
2.6 ± 1.5
13 (13.1)
-
-
61 (61.6)
27 (27.6)
Table 2: Details on patients’ baseline features.
Wang 2019 [25]
Normal
64.85 ± 13.73
216 (53.87)
-
-
-
32.44 ± 22.51
55.83 ± 28.07
-
-
242 (60.3)
138 (34.41)
Overweight
63.94 ± 13.61
113 (50.45)
-
-
-
31.75 ± 22.63
56.79 ± 31.36
-
-
154 (68.7)
83 (37.05)
Obese
61.24 ± 14.59
224 (55.31)
-
-
-
28.86 ± 18.52
54.57 ± 29.82
-
-
238 (58.7)
146 (36.05)
Underweight
63.10 ± 17.48
38 (42.22)
-
-
-
35.22 ± 22.51
60.70 ± 35.26
-
-
53 (58.8)
22 (24.72)
Liu 2018 [27]
Normal
63 ± 19.5
1579 (36.4)
-
-
-
-
-
-
-
2329 (53.7)
1039 (23.9)
Overweight
62 ± 17.3
1519 (38.9)
-
-
-
-
-
-
-
2264 (58.0)
1072 (27.5)
Obese
57 ± 15.9
1144 (44.2)
-
-
-
-
-
-
-
1306 (50.5)
837 (32.4)
MacLaughlin 2021 [29]
Underweight
70 ± 19.4
653 (37.8)
-
-
-
-
-
-
-
856 (49.6)
341 (19.7)
Normal
64.3 (16.0)
-
-
-
-
70 (31)
-
-
-
88 (65.6)
43 (32)
Overweight
65.9 (12.7)
-
-
-
-
64 (25)
-
-
-
174 (78)
87 (39)
Obese
62.3 (11.2)
-
-
-
-
65 (26)
-
-
-
248 (62.6)
253 (64)
Underweight
61.7 (15.1)
-
-
-
-
74 (36)
-
-
-
9 (56.2)
4 (25)
Gameiro 2018 [30]
Normal
63.9 ± 16.5
141 (43)
1.9 ± 0.6
-
-
-
-
-
-
203 (61.3)
63 (19)
Obese
64.4 ± 14.8
71 (56.8)
1.9 ± 0.5
-
-
-
-
-
61 (48.8)
40 (32)
Sabaz 2021 [13]
Normal
57.88 ± 21.53
495 (27.5)
-
-
-
-
-
-
-
1153 (64)
270 (15.0)
Overweight
61.16 ± 18.0
662 (37.4)
-
-
-
-
-
-
-
1088 (61.5)
397 (22.4)
Obese
64.69 ± 15.53
481 (54.2)
-
-
-
-
-
-
-
258 (29.1)
326 (36.7)
Zhou 2020 [31]
All participants
54.8 (9.6)
69 (28.04)
-
-
-
-
-
-
-
244 (99)
86 (34.9)
Wang 2021 [32]
Normal
81.42 ± 61.16
749 (15.99)
-
-
-
-
26.39 ± 21.27
-
-
2683 (57.29)
1087 (23.2)
Overweight
74.72 ± 47.22
815 (16.03)
-
-
-
-
26.03 ± 20.38
-
-
3412 (67.11)
1481 (29.1)
Obese
66.80 ± 31.95
824 (16.38)
-
-
-
-
28.21 ± 21.77
-
-
2973 (59.12)
2125 (42.2)
Underweight
87.05 ± 71.09
55 (14.55)
-
-
-
-
26.59 ± 21.97
-
-
153 (40.48)
65 (17.1)
Moon 2018 [33]
Normal
66.7 ± 9.88
53.4
3.7 ± 0.46
69.7 ± 20.74
-
-
-
836 (74.0)
43.2
Overweight
64.7 ± 9.48
60.4
3.8 ± 0.65
70.2 ± 19.63
-
-
-
-
493 (77.4)
45
Obese
60.0 ± 11.60
72.7
3.9 ± 0.60
73.4 ± 20.06
-
-
-
-
829 (70.2)
49.6
Underweight
70.8 ± 9.95
53.5
3.7 ± 0.60
66.0 ± 23.59
-
-
-
-
44 (62)
28.2
Vasquez 2020 [19]
All participants
42 (28-60)
127 (27.4)
-
-
-
-
-
-
-
350 (75.5)
31 (6.6)
Liu 2021 [27]
All participants
48.7 ± 10.4
92 (80)
-
-
-
-
-
-
-
86 (74.7)
7 (6.08)
Kim 2017 [26]
All participants
61.8 ± 13.2
100 (47.1)
-
-
-
-
-
-
-
138 (65.09)
58 (27.3)
Table 2 off 1:
Quality assessment
The Newcastle-Ottawa tool was used to determine the quality of each eligible study. The maximum star designed for each study was nine: four stars for selection, two stars for comparability, three stars for the outcome. A study with greater or equal to seven stars was considered as high quality. Among seventeen studies, seven studies [13,23,28-30,32,35] scored eight points, eight studies [19,21,22,24- 26,29,31] scored seven points and two studies [27,33] scored six points (Supplementary Table 1). Meta-analysis included fourteen study based on the quality scale. There was no obvious risk of publication bias which was assessed based on funnel plot (Supplementary 1 Figure 1).
Overall analysis
Based on BMI, the current systematic review assessed the different patient’s outcomes in the included studies including AKI incidence, AKI mortality, length of stay in intensive care (LOS in ICU), and APACHE II score. Among these studies, the highest incidence of AKI, AKI mortality, ICU mortality, Hospital mortality, highest APACHE II score, long stay in ICU, and long stay in hospital were found in obese population (92.8 %), underweight population (9.5%), overweight population (43.8%), underweight population (70.7%), overweight population (27.5 ± 9.1), underweight population (44 (20, 95)), underweight population (35 (14–222)) respectively, as they are summarized in Table 3. Table 4 summarizes the percentage of comorbidities (hypertension and diabetes mellitus) in different groups. The overall percentage of hypertension and diabetes mellitus was 34.4% and 20.03% respectively. The highest percentage of hypertension (46.2%) and diabetes (34.3%) was in obese group. The percentage of hypertension in underweight, normal-weight, and overweight was 19.9%, 26.9%, and 31.4% respectively. The percentage of diabetes mellitus in underweight, normal-weight, and overweight was 5.9%, 13.4%, and 19.9% respectively.
Author year
BMI (kg/m2) categories
AKI-RRT Incidence (%)
AKI mortality (%)
MV-free days
ICU mortality (%)
LOS in ICU
hospital length of stay
Hospital mortality
AKI-RRT mortality
AKI (%)
AKI STAGE 1 (%)
AKI STAGE 2 (%)
AKI STAGE 3 (%)
AKI stage 2-3 (%)
Renal replacement therapy
APACHE II score
Pedersen, 2016
Underweight (n=1272)
-
-
-
-
-
9 (5-13)
24 (23.1%)
-
128 (10)
96 (7.5%)
22 (1.7%)
10 (0.8%)
-
-
-
Normal (n=6588)
-
-
-
-
-
10 (5-14) 6.6
97 (14.1%)
-
782 (11.9)
572 (8.7%)
158 (2.4%)
52 (0.8%)
-
-
-
Overweight (n=2769)
-
-
-
-
-
10 (6-14)
35 (10.7%)
-
345 (12.4)
249 (9.0%)
69 (2.5%)
27 (1.0%)
-
-
-
Obese (n=782)
-
-
-
-
-
11 (7-16)
20 (15.2%)
-
140 (17.9)
92 (11.8%)
33 (4.2%)
15 (1.9%)
-
-
-
Zou 2017
Normal
74/1,368 (5.4)
82/1,368 (6.0)
1 (1, 2)
-
40 (20, 88)
13 (10, 18)
-
45/74 (60.8)
1,368 (31.0)
1,010 (73.8)
205 (15.0)
153 (11.2)
358 (26.2)
-
-
Overweight
44/922 (4.8)
35/922 (3.8)
1 (1, 2)
-
39 (20, 86)
14 (11, 18)
-
16/44 (36.4)
922 (36.5)
667 (72.3)
154 (16.7)
101 (11.0)
255 (27.7)
-
-
Obese
17/324 (5.2)
14/324(4.3)
1 (1, 2)
-
40 (19, 93)
14 (11, 18)
-
10/17 (58.8)
324 (46.0)
223 (68.8)
67 (20.7)
34 (10.5)
101 (31.2)
-
-
Underweight
13/241 (5.4)
23/241 (9.5)
1 (1, 2)
-
44 (20, 95)
14 (10, 18)
-
9/13 (69.2)
241 (29.9)
161 (66.8)
51 (21.2)
29 (12.0)
80 (33.2)
-
-
Ju 2018
Normal
121 (42.3)
9.8 ± 19.8
10.3 ± 21.0
-
-
-
-
-
-
-
-
-
-
66 (23.1)
18.8 ± 8.8
Overweight
-
-
7.4 ± 10.5
35 (43.8)
6.9 ± 9.8
40 (50.0)
29 (36.3)
-
-
-
-
28 (35)
21.4 ± 10.0
Obese
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
Underweight
-
-
10.7 ± 11.8
41 (40.2)
11.7 ± 13.4
-
48 (47.1)
-
10 (9.8)
-
-
-
-
19 (18.6)
16.7 ± 7.5
Argalious 2017
Normal
-
-
-
-
-
-
9 (0.6)
-
34 (2.3)
27 (1.8)
5 (0.3)
2 (0.1)
-
-
-
Overweight
-
-
-
-
-
-
9 (0.5)
-
53 (3.2)
42 (2.5)
8 (0.5)
3 (0.2)
-
-
-
Obese
-
-
-
-
-
-
5 (0.4)
-
37 (2.9)
28 (2.2)
5 (0.4)
4 (0.3)
-
-
-
Morbidly obese
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
Underweight
-
-
-
-
-
-
1 (1)
-
4 (4)
3 (3)
1 (1)
0
-
-
-
Park 2017
Normal
-
-
-
-
10.0 ± 8.8
26 (15-110)
2 (2.7)
-
30 (40.5)
23 (31.1)
7 (9.5)
0
-
-
-
Underweight
-
-
-
-
12.41 ± 10.96
35 (14-222)
2 (5.4)
-
13 (35.1)
10 (27.0)
2 (5.4)
1 (2.7)
-
-
-
Kim 2018
Normal
-
-
-
-
9 (3-20)
20 (7-46)
256 (63.5)
-
-
-
-
-
-
-
27.4 ± 8.0
Overweight
-
-
-
-
6 (3-15)
23 (6.5-45.5)
136 (61.8)
-
-
-
-
-
-
-
27.5 ± 9.1
Obese
-
-
-
-
8 (3-16)
21 (8-41)
239 (56.8)
-
-
-
-
-
-
-
26.7 ± 8.4
Underweight
-
-
-
-
5 (2-14)
8 (2-30)
70 (70.7)
-
-
-
-
-
-
-
26.4 ± 8.3
Wang 2019
Normal
-
-
319 (79.55%)
-
-
-
-
-
-
-
122 (30.42%)
279 (69.58%)
-
-
27.86 ± 7.47
Overweight
-
-
176 (78.57%)
-
-
-
-
-
-
-
67 (29.91%)
157 (70.09%)
-
-
27.64 ± 8.57
Obese
-
-
313 (77.28%)
-
-
-
-
-
-
-
90 (22.22%)
315 (77.78%)
-
-
26.77 ± 8.13
Underweight
-
-
70 (77.78%)
-
-
-
-
-
-
-
14 (15.56%)
76 (84.44%)
-
-
25.81 ± 7.55
Table 3: Incidence of AKI and outcomes.
Liu 2018
Normal
-
-
-
414 (9.5)
5 (3-10)
-
564 (35.1)
-
-
-
-
-
-
Overweight
-
-
-
239 (6.1)
4 (3-8)
-
-
-
488 (30.4)
-
-
-
-
-
-
Obese
-
-
-
88 (3.4)
4 (2-7)
-
-
-
279 (17.4)
-
-
-
-
-
-
Underweight
-
-
-
256 (14.8)
7 (4-14)
-
-
-
275 (17.1)
-
-
-
-
-
-
MacLaughlin 2021
Normal
-
-
-
-
-
-
-
-
-
100 (75%)
18 (13%)
16 (12%)
-
-
-
Overweight
-
-
-
-
-
-
-
-
-
174 (78%)
25 (11%)
24 (11%)
-
-
-
Obese
-
-
-
-
-
-
-
-
-
264 (67%)
75 (19%)
57 (14%)
-
-
-
Underweight
-
-
-
-
-
-
-
-
-
15 (94%)
0
1 (6%)
-
-
-
Gameiro 2018
Normal
-
-
256 (77.3)
-
81 (24.5)
38.8 ± 39.3
113 (34.1)
-
283 (85.5)
-
-
-
-
-
-
Overweight
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
Obese
-
-
94 (75.2)
27 (21.6)
32.6 ± 39.3
40 (32)
-
116 (92.8)
-
-
-
-
-
-
Underweight
-
-
-
-
-
-
-
-
-
-
-
-
-
-
Sabaz 2021
Normal
-
-
4.81 (2.74-11.14)
548 (30.4)
5.45 (3-12.72)
-
-
-
1172 (65.1)
98 (5.4)
156 (8.7)
918 (51.0)
-
-
24 (17-29)
Overweight
-
-
5.73 (2.51-12.16)
556 (31.4)
6.54 (2.84-13.58)
-
-
-
1149 (64.9)
118 (6.7)
185 (10.4)
846 (47.8)
-
-
25 (19-30)
Obese
-
-
5.89 (2.75-12.47)
307 (34.6)
6.81 (3.32-13.88)
-
-
-
620 (69.8)
57 (6.4)
101 (11.4)
462 (52.0)
-
-
26 (19-31)
Underweight
-
-
-
-
-
-
-
-
-
-
-
-
-
-
Zhou 2020
Normal
-
-
-
-
-
-
-
-
28.7
-
-
-
-
-
-
Overweight
-
-
-
-
-
-
-
-
47.7
-
-
-
-
-
-
Obese
-
-
-
-
-
-
-
-
50.50%
-
-
-
-
-
-
Underweight
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
Wang 2021
Normal
-
-
-
-
-
-
812 (17.34)
-
-
1079 (23.04)
786 (16.78)
2818 (60.18)
-
-
-
Overweight
-
-
-
-
-
-
687 (13.51)
-
-
1119 (22.01)
749 (14.73)
3216 (63.26)
-
-
-
Obese
-
-
-
-
-
-
667 (13.26)
-
-
920 (18.29)
811 (16.13)
3298 (65.58)
-
-
-
Underweight
-
-
-
-
-
-
71 (18.78)
-
-
98 (25.93)
81 (21.43)
199 (52.65)
-
-
-
Moon 2018
Normal
-
-
-
-
-
-
-
-
25.6
-
-
-
-
-
-
Overweight
-
-
-
-
-
-
-
-
26.7
-
-
-
-
-
-
Obese
-
-
-
-
-
-
-
-
35.5
-
-
-
-
-
-
Underweight
-
-
-
-
-
-
-
-
29.6
-
-
-
-
-
-
Table 3 off 1:
Comorbidities
Sub-group
Event
Total
Percentage
Hypertension
Underweight
335
1683
19.90%
Normal-weight
4010
14885
26.90%
Overweight
3996
12705
31.40%
Obese
6771
14650
46.20%
Total
15112
43923
34.40%
Diabetes mellitus
Underweight
154
2577
5.90%
Normal-weight
2246
16786
13.30%
Overweight
2071
10390
19.90%
Obese
3576
10403
34.30%
Total
8047
40156
20.03%
Table 4: Percentage of Comorbidities and BMI.
BMI and AKI
The incidence of AKI among the included studies in metaanalysis was 24.9%. The subgroups analysis shows that the highest incidence was 30.1% in overweight population, and the smallest was 18% in underweight group (Table 5).
Sub-group
Event (AKI)
Total
Percentage
Underweight
429
2404
18%
Normal-weight
4184
16441
25.40%
Overweight
3064
10157
30.10%
Obese
2393
11412
21%
Overall total
10070
40414
24.90%
Table 5: Incidence of AKI in sub-groups.
The risk of developing AKI in the overweight group was more likely than normal-weight group, OR was 1.2, 95% CI: 1.01 to 1.42, P=0.03), there was substantial heterogeneity among overweight studies with I²=78%, P=0.0001. The association of AKI in obese group was more likely higher than in normal group, even it is not statistically significant, OR was 1.22, 95% CI: 0.98 to 1.52, P=0.08, there was substantial heterogeneity with I2=86%, P=0.00001. The results in underweight group show that 10% were less likely to develop AKI compared to normal-weight group, even it was not statistically significant, OR was 0.9, 95% CI: 0.78 to 1.02, P=0.11, with a minimal heterogeneity, I²=7%, P=0.38 as shown in Figure 2a-2c. A sensitivity analysis were conducted after removing the outlier in underweight and overweight group, results remain consistent to the primary findings. However, in the overweight group, the sensitivity analysis shows the statistical significant results with OR of 1.32, 95% CI: 1.16 to 1.5, I²=36%, P=0.0001 (Supplementary 2 Figure 1).
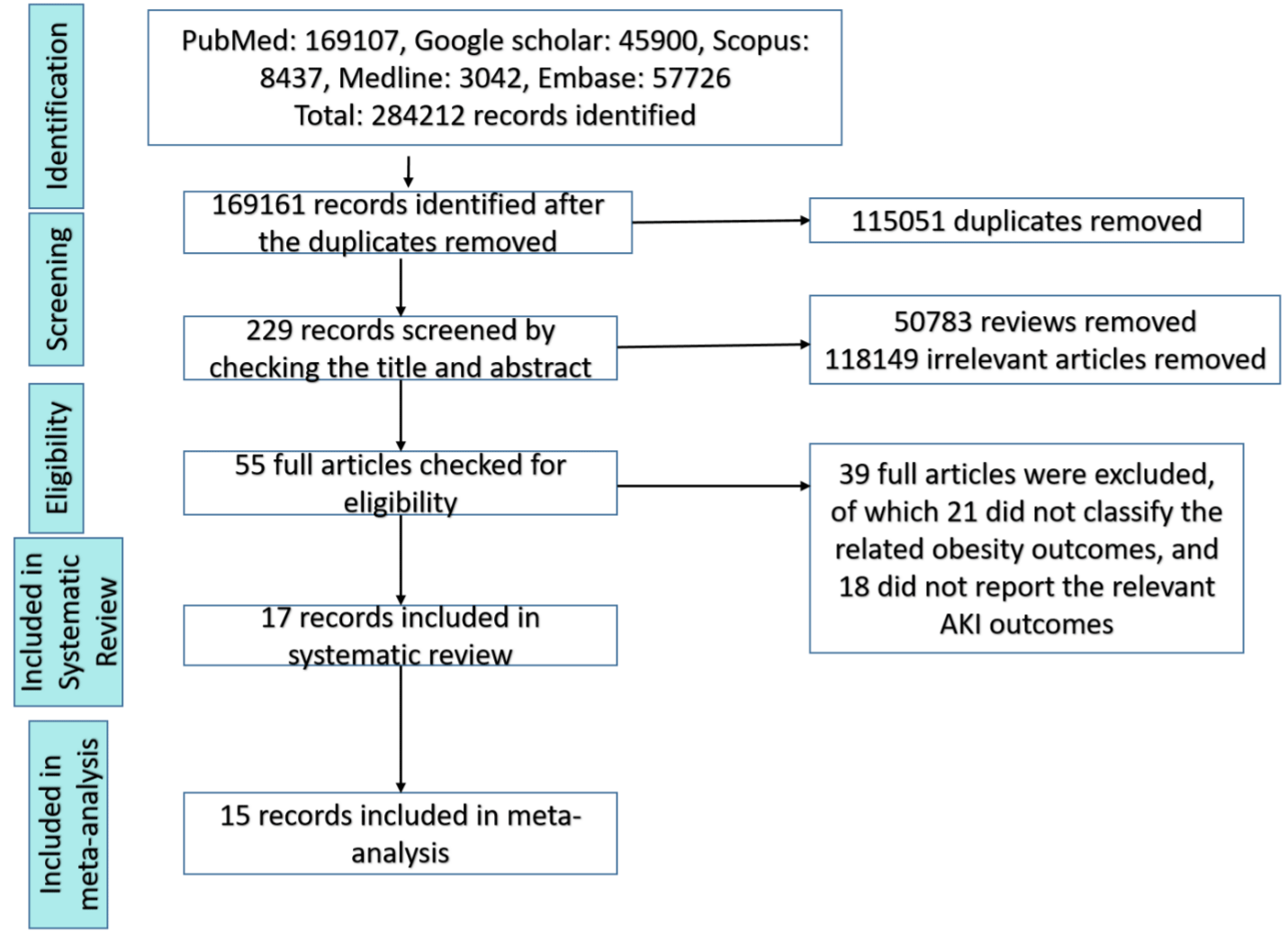
Figure 1: Workflow Chart.
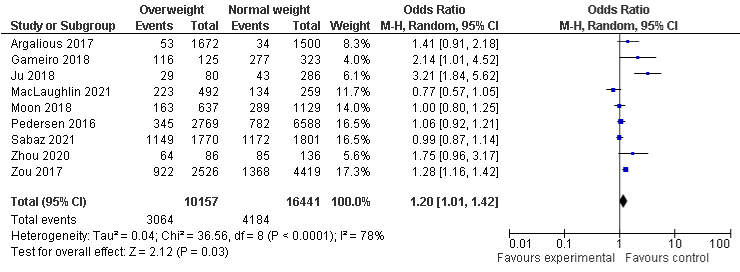
Figure 2a: AKI and Overweight.
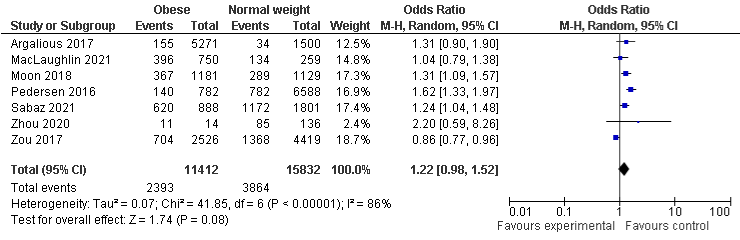
Figure 2b: AKI and Obese.
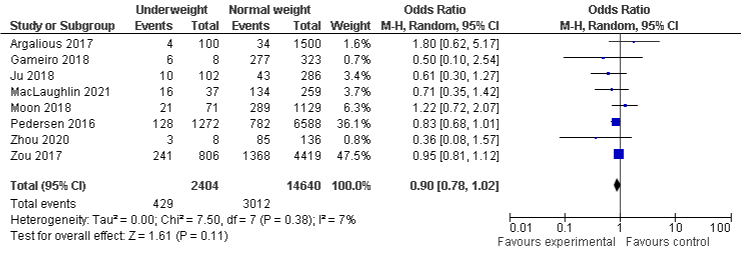
Figure 2c: AKI and Underweight.
BMI and AKI stage 1: The overall analysis of BMI and AKI stage 1 in six and seven included studies shows that 4% underweight and 5% obese patients were less likely to experience AKI stage 1 compared with normal population, with OR of 0.96, 95% CI: 0.74 to 1.6, P=0.77 and 0.95, 95% CI: 0.74 to 1.22, P=0.69, but, both findings were not statistically significant. The results reveal that overweight patients were slightly more likely to experience AKI stage 1 compared to normal-weight, even if it was not statically significant, OD was 1.01, 95% CI: 0.91 to 1.11, p=0.90. There was a moderate heterogeneity in underweight group with I²=53%, P=0.06, a minimal heterogeneity in overweight group with I²=26%, P=0.23, and a substantial heterogeneity in obese group with I²=81%, P=0.0001 (Figure 3a-3c).
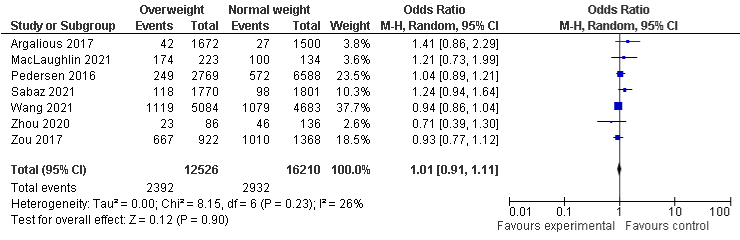
Figure 3a: AKI stage 1 and Overweight.
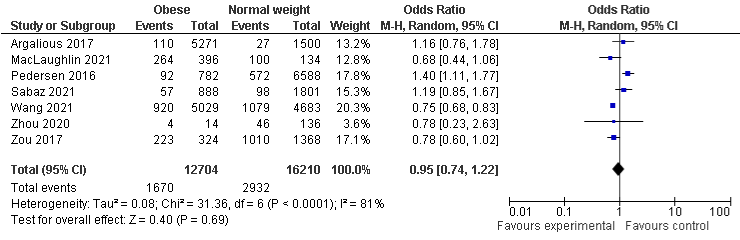
Figure 3b: AKI stage 1 and Obese.
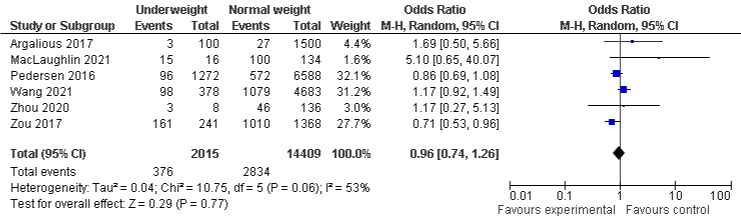
Figure 3c: AKI stage 1 and Underweight.
BMI and AKI stage 2: The overall meta-analysis of BMI and AKI stage 2 demonstrates that 8% in underweight (seven studies) and overweight (eight studies) sub-groups are less likely to develop AKI stage 2, but not statistically significant, the OR in underweight and overweight group were 0.92, 95% CI: 0.57 to 1.46, P=0.71 and 0.92, 95% CI: 0.47 to 1.77, P=0.79, respectively. Conversely, obese group (eight studies) was statistically insignificant more likely to develop AKI stage 2, OR was 1.24, 95% CI: 0.96 to 1.61, P=0.1. A substantial, considerable, and substantial heterogeneity with I²=73%, P=0.001, I²=98%, P=0.00001, and I²=78%, P=0.0001 were found in underweight, overweight, and obese group respectively (Figure 4a- 4c).
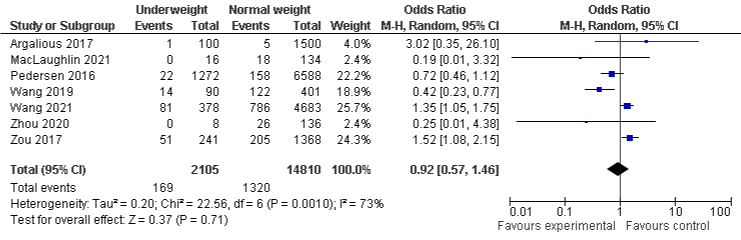
Figure 4a: AKI stage 2 and Underweight.
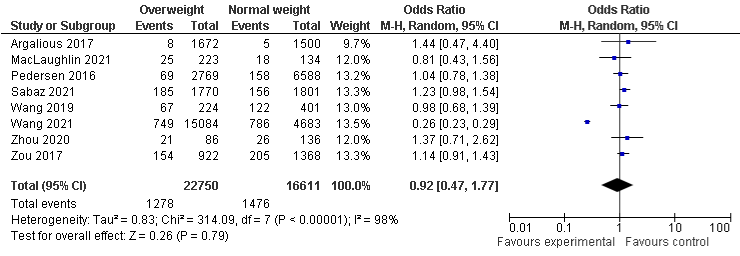
Figure 4b: AKI stage 2 and Overweight.
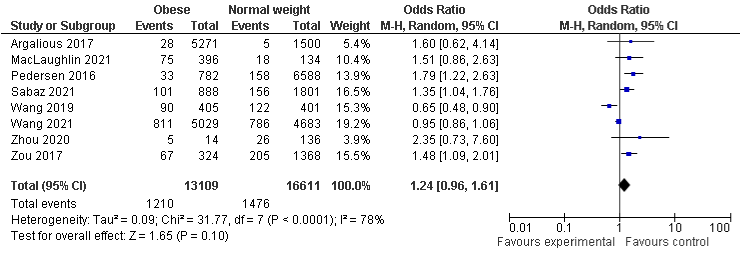
Figure 4c: AKI stage 2 and Obese.
BMI and AKI stage 3: The pooled results of BMI and AKI stage 3 of seven studies show that obese population were more likely to develop AKI stage 3 with OR 1.27, 95% CI: 1.06 to 1.51, P=0.008. The other subgroups are also more likely to develop AKI stage 3, but not statistically significant, OR in underweight (seven included studies) and overweight (eight included studies) group were 1.08, 95% CI: 0.71 to 1.65, P=0.72 and 1.06, 95% CI: 0.90, 1.25, P=0.47, respectively. The studies are associated with moderate, moderate to substantial, and substantial heterogeneity with I²=60%, P=0.02, I²=60%, P=0.02, and I²=62%, P=0.01 in obese, underweight, and overweight group respectively (Figure 5a-5c).
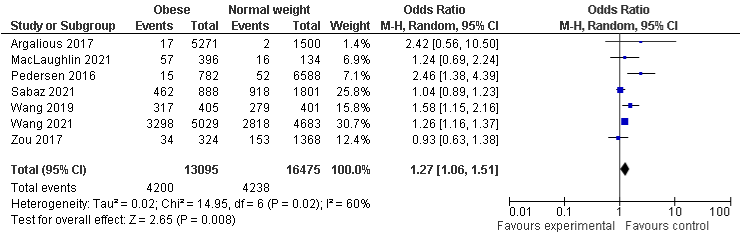
Figure 5a: AKI stage 3 and Obese.
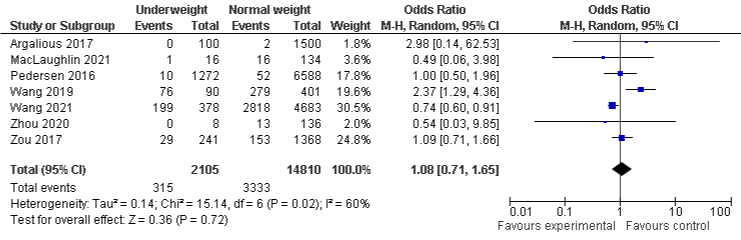
Figure 5b: AKI stage 3 and Underweight.
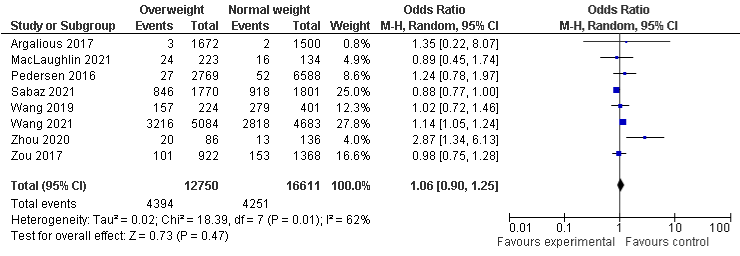
Figure 5c: AKI stage 3 and Overweight.
BMI and AKI stage 2-3: There was only one study which analyzed the association of BMI and AKI stage 2-3 development. The study showed that underweight population were more likely to develop AKI stage 2-3, OR was 1.4, 95% CI: 1.04 to 1.88, P=0.02. Moreover, the overweight and obese population were more likely to develop AKI stage 2-3, but not statistically significant, ORs were 1.08, 95% CI: 0.89 to 1.30, p=0.43 and 1.28, 95% CI: 0.98, 1.66, P=0.07 (Figure 6a-6c).

Figure 6a: AKI stage 2-3 and Underweight.

Figure 6b: AKI stage 2-3 and Overweight.

Figure 6c: AKI stage 2-3 and Obese.
BMI and AKI patients with clinical outcomes
BMI and AKI mortality: The study showed that underweight population were more likely to experience AKI mortality, OR was 1.44, 95% CI: 1.04 to 2.00, P=0.03. However, overweight and obese population which were less likely to be associated with AKI mortality, even it was statistically insignificant, ORs were 0.73, 95% CI: 0.53 to 1.01, P=0.05 and 0.71, 95% CI: 0.40 to 1.27, P=0.24 respectively. A minimal, and substantial heterogeneity with I²=0%, P=0.46, I²=22%, P=0.26, and I²=82%, P=0.02 were found in underweight, overweight, and obese group respectively (Figure 7a-7c).
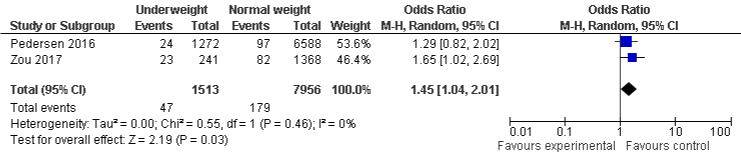
Figure 7a: AKI mortality and Underweight.
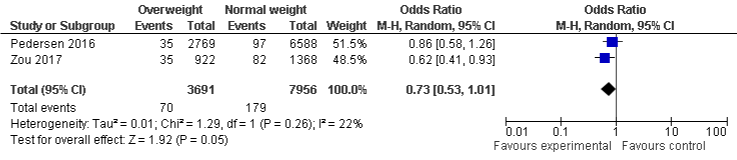
Figure 7b: AKI mortality and Overweight.
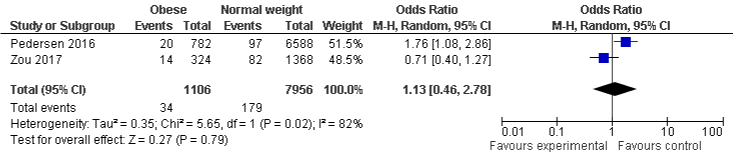
Figure 7c: AKI mortality and Obese.
BMI and LOS in ICU: The pooled results of two included studies show that 31% of obese patients stay in ICU for a short period of time compared to normal-weight group, OR 0.69, 95% CI: 0.37 to 1.01, p=0.0001. Similarly, underweight (four studies) and overweight (three) group were also more likely to stay in ICU for short time compared to normal-weight, but it was no statistically significant, OR: 0.25, 95% CI: -0.86 to 1.36, p=0.66 and -1.0, 95% CI: -3.23 to 1.23, p=0.38, respectively. There was A minimal, and moderate heterogeneity with I²=0%, P=0.84, I²=9%, P=0.35, and I²=58%, P=0.09 were found in obese underweight, and overweight group respectively (Figure 8a-8c).
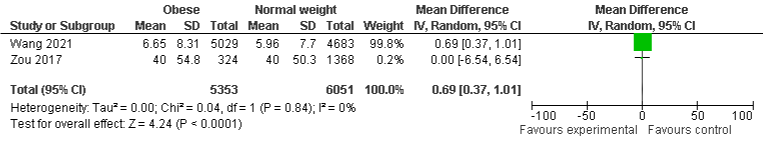
Figure 8a: Overall survival, autologous stem cell transplant (ASCT) versus no ASCT (p=0.12).
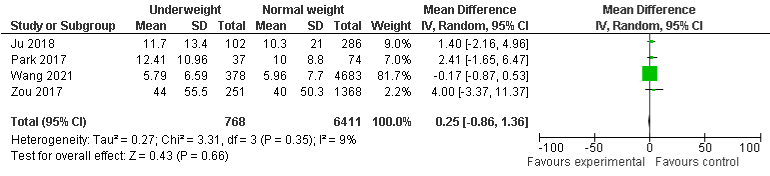
Figure 8b: LOS in ICU and Underweight.
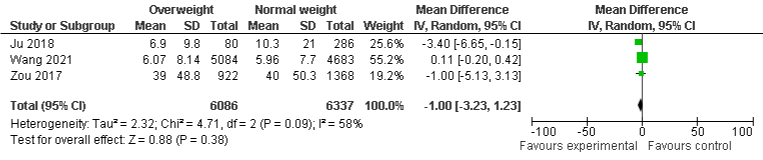
Figure 8c: LOS in ICU and Overweight.
BMI and LOS in hospital: Compared with normal weight group, findings of four included studies (statistically insignificant) reveal that 96% of underweight are not associated with LOS in hospital with OR of 0.04, 95% CI: -1.79 to 1.87, P=0.96. Moreover, results of three included studies show that 73% of overweight patients were less likely to stay in hospital for long time compared with normal-weight group, OR was 0.27, 95% CI: -0.83 to 1.38, P=0.63. However, two included studies show that there was no difference of LOS in hospital for obese group compared with normal-weight group, OR was 1.0, 95% CI: 0.62 to 1.38, P=0.0001. There was substantial, and minimal heterogeneity with I²=85%, P=0.0002, I²=89%, P=0.0001 and I²=0%, P=1 were found in underweight, and overweight group respectively (Figure 9a-9c).
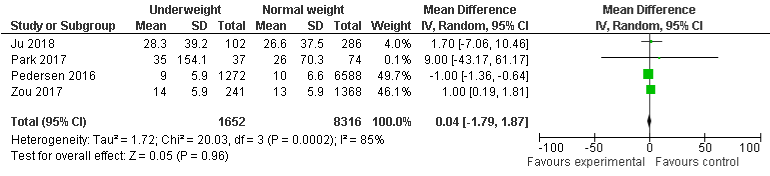
Figure 9a: LOS in Hospital and Underweight.
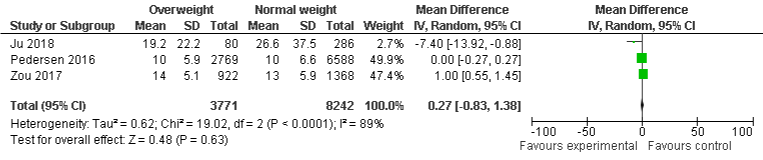
Figure 9b: LOS in Hospital and Overweight.
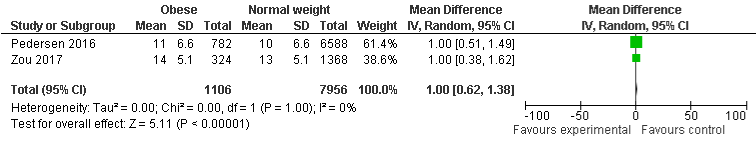
Figure 9c: LOS in Hospital and Obese.
BMI and hospital mortality: Results from three included studies in underweight group and one study in obese group show that there were less associated with hospital mortality compared to normal weight group, OR was -23.33, 95% CI: -34.87 to -11.79, p=0.0001 and -4, 95% CI: -4.04 to -3.96, P=0.00001 respectively. There was considerable heterogeneity among these included cohorts in underweight group with I²=99%, P=0.0001. The findings from two included cohorts show that overweight was also less associated with hospital mortality, but not statistically significant, OR: -40.08, 95% CI: -119.45 to 39.30, P=0.32. The heterogeneity was considerable with I²=99%, P=0.00001. There one study included in this meta-analysis showed that the number of hospital mortality in obese population was lesser compared normal weight group, OR: -4.0, 95% CI of -4.04 to -3.96, p=0.00001, heterogeneity is not applicable (Figure 10a-10c).
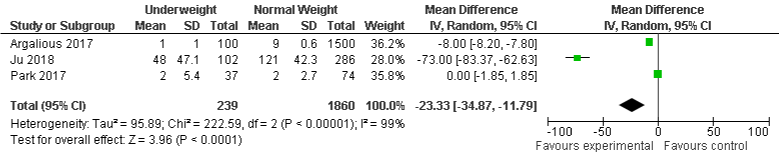
Figure 10a: Hospital mortality and Underweight.
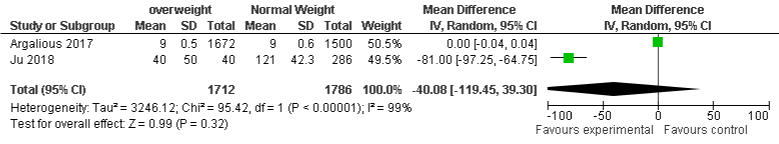
Figure 10b: Hospital mortality and Overweight.

Figure 10c: Hospital mortality and Obese.
Discussion
Current review introduces the possibility of obesity paradox in AKI, where higher BMI is associated with AKI morbidity, while underweight group is associated with AKI mortality.
Based on the meta-analysis results, AKI incidence was 24.9% and higher AKI incidence in subgroup was 30.1% in overweight, while based systematic analysis, findings demonstrate that the highest incidence of AKI was 92.8% in obese group. These findings show that AKI incidence trend is associated with high BMI. AKI stages were not statistically associated with BMI, except AKI stage 2-3 was more likely to develop in underweight with OR of 1.4. Besides, AKI mortality rate was high in underweight group compared to normalweight group, while high incidence was associated with elevated BMI. The current findings are in the line with a retrospective study with 11,736 participants conducted in Australia, where morbid obese and overweight patients were 2.9 1.4 times more likely to develop renal failure and morbidity respectively. The findings of this study found that AKI mortality was not associated with high BMI [34]. Moreover, a cohort study carried out in Denmark from 2005-2011 with 13,529 participants shows that 17.9% were obese patients while 11.9% were normal-weight, nevertheless, AKI mortality was 23.1%, 14.1%, 10.7%, 15.2% in underweight, normal-weight, overweight, and obese patients respectively [35].
Although the higher BMI is accompanied with low rate of AKI mortality, much caution could be taken as the current systematic review reveals that high BMI is associated with different comorbidities (hypertension and diabetes mellitus), where percentage of hypertension in overweight and obese was 31.1% and 46.2% respectively, which are high compared to underweight (19.9%). Moreover, percentage of diabetes mellitus in overweight and obese was 19.9% and 34.3% respectively, which is also high compared with underweight (5.9%). Based on these comorbidities, recent studies have shown that hypertension and diabetes mellitus are associated with AKI in overweight and obese patients. For instance, a retrospective study carried out in Poland with 215 patients shows that among 70% of patients with hypertension were associated with 85%, 75%, and 30% of post-renal AKI, renal AKI, and pre-renal AKI respectively [36]. Besides, Worldwide Acute Kidney Injury Epidemiology in Neonates (AWAKEN) database was used to collect the data in the study enrolled 2162 neonates, where the overall AKI was 29.9% with the association of hypertension over 41.2% compared to 26.2% of control group [37]. Nevertheless, it has been shown that hemoglobin A1c more than 9% is associated with AKI with OR of 1.29, 95% CI: 1.18-1.41 up to 1.33, 95% CI: 1.13-1.57 compared with baseline A1c of 6-6.9% [38]. Moreover, a cohort study of 16,700 participants shows that 48.6% of diabetes patients versus 17.2% controls are more likely to develop AKI [39]. Based on these findings, there might be a great impact of diabetes and hypertension on AKI development. Therefore, more studies are welcomed to reveal the association between these comorbidities and AKI, and related cellular mechanism behind. Moreover, particularly to the current results, it will be interesting to understand why and how low BMI induces AKI mortality in future.
Altogether, the link of low BMI and mortality for patients with AKI shows a more clinically useful observation, therefore clinicians should be especially vigilant when managing AKI risk.
The study was challenged with different limitations. For instance, BMI can be affected by different factors like ethnicity which can increase the heterogeneity [40]. Besides, different sample sizes contribute to the different power of the study which affects the pooled result and result in the study heterogeneity.
Conclusion
Although high BMI is known to enhance the chronic kidney diseases, having a higher BMI might be associated with AKI morbidity, while low BMI might be associated with AKI mortality. More studies are recommended.
Declaration
Funding: This work was supported by the National Natural Science Foundation of China (No.31371386 SP.J).
Authors’ contribution: BN collected the data, analyzed and prepared the manuscript. BN, YG, and YJ organized the manuscript. WW and SJ supervised and revised the manuscript. All authors read and approve the final manuscript.
References
- Bull CJ, Bell JA, Murphy N, Sanderson E, Davey Smith G, Timpson NJ, et al. Adiposity, metabolites, and colorectal cancer risk: Mendelian randomization study. BMC Med. 2020; 18: 396.
- Han C, Liu C, Geng J, Tang Y, Li Y, Wang Y, et al. Black and Green Tea Supplements Ameliorate Male Infertility in a Murine Model of Obesity. J Med Food. 2020; 23: 1303-1311.
- Teleka S, Jochems SHJ, Häggström C, Wood AM, Järvholm B, Orho- Melander M, et al. Association between blood pressure and BMI with bladder cancer risk and mortality in 340,000 men in three Swedish cohorts. Cancer Med. 2021; 10: 1431-1438.
- Qiao W, Zhang X, Kan B, Vuong AM, Xue S, Zhang Y, et al. Hypertension, BMI, and cardiovascular and cerebrovascular diseases. Open Med (Wars). 2021; 16: 149-155.
- Martin WP, Bauer J, Coleman J, Dellatorre-Teixeira L, Reeve JLV, Twomey PJ, et al. Obesity is common in chronic kidney disease and associates with greater antihypertensive usage and proteinuria: evidence from a crosssectional study in a tertiary nephrology centre. Clin Obes. 2020; 10: e12402.
- Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, et al; Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Body Mass Index). National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet. 2011; 377: 557-567.
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet. 2017; 390: 2627-2642.
- Prevalence of Obesity. 2021.
- Obesity and Overweight. 2021.
- Kompaniyets L, Goodman AB, Belay B, Freedman DS, Sucosky MS, Lange SJ, et al. Body Mass Index and Risk for COVID-19-Related Hospitalization, Intensive Care Unit Admission, Invasive Mechanical Ventilation, and Death - United States, March-December 2020. MMWR Morb Mortal Wkly Rep. 2021; 70: 355-361.
- Muttarak R. Normalization of Plus Size and the Danger of Unseen Overweight and Obesity in England. Obesity (Silver Spring). 2018; 26: 1125-1129.
- Tsuboi N, Okabayashi Y, Shimizu A, Yokoo T. The Renal Pathology of Obesity. Kidney Int Rep. 2017; 2: 251-260.
- Sabaz MS, Asar S, Sertçakacilar G, Sabaz N, Çukurova Z, Hergünsel GO. The effect of body mass index on the development of acute kidney injury and mortality in intensive care unit: Is obesity paradox valid? Ren Fail. 2021; 43: 543-555.
- Caussy C, Pattou F, Wallet F, Simon C, Chalopin S, Telliam C, et al; COVID Outcomes HCL Consortium and Lille COVID-Obesity Study Group. Prevalence of obesity among adult inpatients with COVID-19 in France. Lancet Diabetes Endocrinol. 2020; 8: 562-564.
- Guo M, Gao Y, Wang L, Zhang H, Liu X, Zhang H. Early Acute Kidney Injury Associated with Liver Transplantation: A Retrospective Case-Control Study. Med Sci Monit. 2020; 26: e923864.
- Kim H, Kim H, Lee M, Cha MU, Nam KH, An SY, et al. The impact of disease severity on paradoxical association between body mass index and mortality in patients with acute kidney injury undergoing continuous renal replacement therapy. BMC Nephrol. 2018; 19: 32.
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009; 62: 1006-1012.
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010; 25: 603-605.
- Vasquez CR, DiSanto T, Reilly JP, Forker CM, Holena DN, Wu Q, et al. Relationship of body mass index, serum creatine kinase, and acute kidney injury after severe trauma. J Trauma Acute Care Surg. 2020; 89: 179-185.
- Zou Z, Zhuang Y, Liu L, Shen B, Xu J, Luo Z, et al. Role of Body Mass Index in Acute Kidney Injury Patients after Cardiac Surgery. Cardiorenal Med. 2017; 8: 9-17.
- Ju S, Lee TW, Yoo JW, Lee SJ, Cho YJ, Jeong YY, et al. Body Mass Index as a Predictor of Acute Kidney Injury in Critically Ill Patients: A Retrospective Single-Center Study. Tuberc Respir Dis (Seoul). 2018; 81: 311-318.
- Argalious MY, Makarova N, Leone A, Cywinski J, Farag E. Association of Body Mass Index and Postoperative Acute Kidney Injury in Patients Undergoing Laparoscopic Surgery. Ochsner J. 2017; 17: 224-232.
- Park JY, Park JH, Lee SS, Ri HS, Kim HJ, Choi YM, et al. The Association of Preoperative Body Mass Index with Acute Kidney Injury in Liver Transplantation Recipients: A Retrospective Study. Korean J Crit Care Med. 2017; 32: 265-274.
- Kim H, Kim H, Lee M, Cha MU, Nam KH, An SY, et al. The impact of disease severity on paradoxical association between body mass index and mortality in patients with acute kidney injury undergoing continuous renal replacement therapy. BMC Nephrol. 2018; 19: 32.
- Wang H, Shi Y, Bai ZH, Lv JH, Sun JL, Pei HH, et al. Higher body mass index is not a protective risk factor for 28-days mortality in critically ill patients with acute kidney injury undergoing continuous renal replacement therapy. Ren Fail. 2019; 41: 726-732.
- Kim H, Kim J, Seo C, Lee M, Cha MU, Jung SY, et al. Body mass index is inversely associated with mortality in patients with acute kidney injury undergoing continuous renal replacement therapy. Kidney Res Clin Pract. 2017; 36: 39-47.
- Liu T, Fu Y, Liu J, Liu Y, Zhu J, Sun L, et al. Body mass index is an independent predictor of acute kidney injury after urgent aortic arch surgery for acute DeBakey Type I aortic dissection. J Cardiothorac Surg. 2021; 16: 145.
- Liu AYL, Wang J, Nikam M, Lai BC, Yeoh LY. Low, rather than High, Body Mass Index Is a Risk Factor for Acute Kidney Injury in Multiethnic Asian Patients: A Retrospective Observational Study. Int J Nephrol. 2018; 2018: 3284612.
- MacLaughlin HL, Pike M, Selby NM, Siew E, Chinchilli VM, Guide A, et al; ASSESS-AKI Study Investigators. Body mass index and chronic kidney disease outcomes after acute kidney injury: a prospective matched cohort study. BMC Nephrol. 2021; 22: 200.
- Gameiro J, Gonçalves M, Pereira M, Rodrigues N, Godinho I, Neves M, et al. Obesity, acute kidney injury and mortality in patients with sepsis: a cohort analysis. Ren Fail. 2018; 40: 120-126.
- Zhou J, Lyu L, Zhu L, Liang Y, Dong H, Chu H. Association of overweight with postoperative acute kidney injury among patients receiving orthotopic liver transplantation: an observational cohort study. BMC Nephrol. 2020; 21: 223.
- Wang B, Li D, Gong Y, Ying B, Cheng B, Sun L. Body Mass Index is Associated with the Severity and All-Cause Mortality of Acute Kidney Injury in Critically Ill Patients: An Analysis of a Large Critical Care Database. Biomed Res Int. 2021; 2021: 6616120.
- Moon H, Lee Y, Kim S, Kim DK, Chin HJ, Joo KW, et al. Differential Signature of Obesity in the Relationship with Acute Kidney Injury and Mortality after Coronary Artery Bypass Grafting. J Korean Med Sci. 2018; 33: e312.
- Yap CH, Mohajeri M, Yii M. Obesity and early complications after cardiac surgery. Med J Aust. 2007; 186: 350-354.
- Pedersen AB, Gammelager H, Kahlert J, Sørensen HT, Christiansen CF. Impact of body mass index on risk of acute kidney injury and mortality in elderly patients undergoing hip fracture surgery. Osteoporos Int. 2017; 28: 1087-1097.
- Dylewska M, Chomicka I, Malyszko J. Hypertension in patients with acute kidney injury. Wiad Lek. 2019; 72: 2199-2201.
- Kraut EJ, Boohaker LJ, Askenazi DJ, Fletcher J, Kent AL; Neonatal Kidney Collaborative (NKC). Incidence of neonatal hypertension from a large multicenter study (Assessment of Worldwide Acute Kidney Injury Epidemiology in Neonates-AWAKEN). Pediatr Res. 2018; 84: 279-289.
- Xu Y, Surapaneni A, Alkas J, Evans M, Shin JI, Selvin E, et al. Glycemic Control and the Risk of Acute Kidney Injury in Patients With Type 2 Diabetes and Chronic Kidney Disease: Parallel Population-Based Cohort Studies in U.S. and Swedish Routine Care. Diabetes Care. 2020; 43: 2975-2982.
- Hapca S, Siddiqui MK, Kwan RSY, Lim M, Matthew S, Doney ASF, et al; BEAt-DKD Consortium. The Relationship between AKI and CKD in Patients with Type 2 Diabetes: An Observational Cohort Study. J Am Soc Nephrol. 2021; 32: 138-150.
- Krueger PM, Coleman-Minahan K, Rooks RN. Race/ethnicity, nativity and trends in BMI among U.S. adults. Obesity (Silver Spring). 2014; 22: 1739- 1746.