
Research Article
Int J Nutr Sci. 2022; 7(1): 1063.
Atmospheric Water Generator (AWG): New Innovative Technology to Overcome Global Water Scarcity
Vibha Bhardwaj*
Director Environment Laboratories, Ras Al KhaimahMunicipality, United Arab Emirates
*Corresponding author: Dr. Vibha Bhardwaj, Director Environment Laboratories, Ras Al Khaimah Municipality, United Arab Emirates
Received: June 10, 2022; Accepted: June 24, 2022; Published: July 01, 2022
Abstract
Freshwater scarcity is an ever-growing concern for human society. The atmosphere contains water in the form of water vapor; moisture etc., within that amount almost 35% of the water is wasted. Alternative new technologies are urgently needed to overcome the rapidly increasing global water scarcity. Atmospheric water is a potential source of potable water, as the earth’s atmosphere contains tons of fresh water (98% in a vapor state). The atmospheric water generator (AWG) converts water vapor into liquid water and is a capable solution for water scarcity. The major aim of this research work is to provide safe and clean drinking water to those areas which are facing water scarcity problems. In this research work we provide the comprehensive analysis of the chemical and biological profiles of water, produced for several months by three different AWG. Physical parameters, heavy metals and microbiological parameters were analyzed in the water. The main elements found were aluminum, calcium, magnesium, and potassium. The sampling site, likely affected the chemical composition of the produced atmospheric water. Nevertheless, the produced water nearly always met the WHO drinking water standards. In this research work, scientific evidence has been presented that supports, AWG can be an alternative potential source of water to cope up with water scarcity problem and provide safe drinking water throughout the year.
Keywords: Atmospheric Water Generator; Water Quality; Drinking Water; Alternative Water Resource; Atmospheric Water
Abbreviations
AWG: Atmospheric Water Generator; SD: Standard Deviation; EMB: Eosin Methylene Blue; EPA: Environmental Protection Agency; WHO: World Health Organization.
Introduction
Water paucity is an ever-growing concern for human society (Mekonnen et al, 2016). Currently, over 2 billion people across the globe are experience high water scarcity, a number that is expected to rise with population growth and the intensity of climate change (UNESCO, 2019). Perseverance for water scarcity is based on various water-saving strategies, retrieving used water, and water production. Seawater desalination by reverse osmosis is the most common and competent water-production method (Semiat, 2018). However, it needs a large saline or brackish water source and is therefore not applicable in regions with no access to brackish or sea water. Moreover, desalination plants require large capital investments. Distillation is therefore not relevant to poor and non-coastal regions, many of which suffer from chronic severe water scarcity. Production of atmospheric water is another potential source of potable water. The earth’s atmosphere is a huge and renewable water resource, containing approximately 12,900 billion tons of fresh water (Li et al, 2018)98% vapor and the rest in a condensed state (clouds and fog).Atmospheric water consists of the water droplets formed when atmospheric water vapor condenses on surfaces with temperatures below the dew point temperature. The main advantage of using atmospheric water as a drinking water source is that there is no need to build a water-transport infrastructure; harvesting apparatuses can be placed almost anywhere (away from the coastline).
There is a disruptive technology emerging in the marketplace that may provide a better choice: Atmospheric Water Generators (AWG) (Figure 1) which produces drinkable water from surrounding air. This provides the potential to enlarge water availability during shortages, contamination events, and other issues that can interrupt drinking water services. Natural disasters, such as hurricanes, and public water infrastructure failures, such as pipe corrosion resulting in contamination issues, have increased the interest in AWG technology as both emergency and long-term supply solutions. Water production rates are highly dependent upon the air temperature and the amount of water vapour (i.e., humidity) in the air. The most commonly used AWG systems employ condenser and cooling coil technology to pull moisture from the air in the same way a household dehumidifier does.
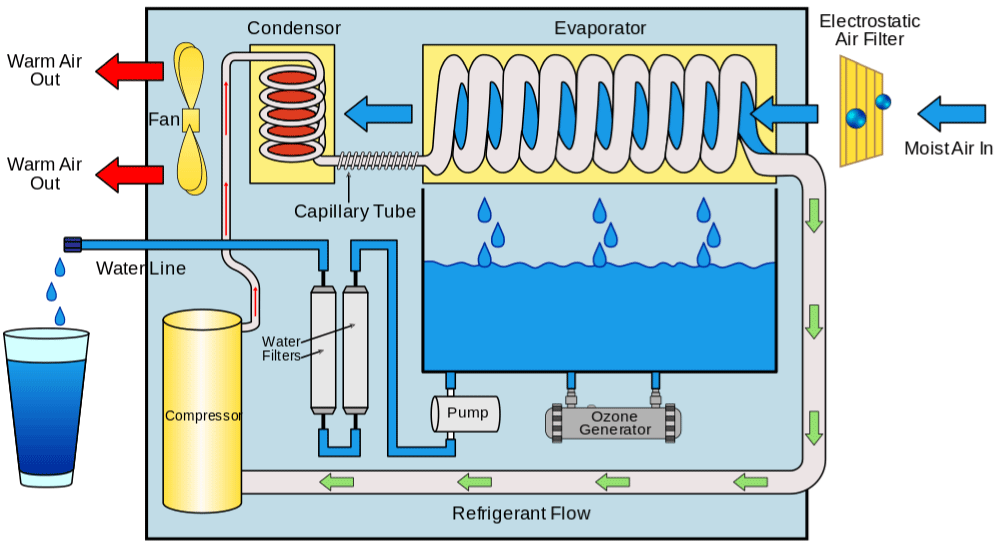
Figure 1: Air Water Generator system.
Source: https://en.wikipedia.org/wiki/Atmospheric_water_generator
Atmospheric water generators potentially can produce at the point-of-need fresh and environment friendly water without the plastic waste or carbon footprint of bottled water. For those relying on municipal water supplies with probable drinking water contaminants, especially contaminants due to aging infrastructure like lead pipes, AWG offers a means to disconnect from traditional water supplies. Of course, drawing water from air does not guarantee the water is free from contaminants, especially from particulates and lead. This means that AWGs must be designed to prevent airborne contaminants from being present in the produced drinking water. System designs should include multiple obstructions to prevent airborne contaminants, including point-of-use drinking water treatment technology and disinfection methods like ultraviolet light to ensure water safety. Further, the materials used to construct AWG systems must be safe and must not leach contaminants into the product water.
Relative humidity of the air and temperature are the primary determinants governing the proficiency of water generation by AWGs. Generally, the greater the atmospheric humidity and the warmer the air temperature the more efficient the water production. AWG technologies are energy intensive, with many deploying solar energy to power the equipment or drive the water harvesting process.
The AWG is considered a promising option as an alternative or supplemental source of innocuous drinking water, the quality of which, as already noted, is dependent on air and meteorological parameters. As far as we know, all studies analyzing the profiles of harvested dew water have looked at water from passive dew condensers. In this research work we provide the comprehensive analysis of the chemical and biological profiles of water, produced for several months by three different AWG. Physical parameters, heavy metals, inorganic ions, and microbiological parameters were analyzed in the water. Our aim was to determine whether the atmospheric water produced by an AWG can meet the WHO/EPA drinking water standards and is safe for drinking and also can combat water scarcity.
Material and Methods
Collection of Samples
Water samples were taken directly from three different AWG (Thirty samples) (at different interval), at the highest possible level of sterility and caution, to avoid external contamination. The water from the AWG container was collected into clean 1 L glass bottles. The bottles were closed and kept. Water was then immediately portioned into the various test tubes as needed. The test tubes were stored in a 4°C refrigerator until analyses. Each water sampling was accompanied by blank that were subjected to all of the same processes. The blank contained 200mL distilled water in a 1 L glass bottle that was closed with a stopper. Its purpose was to check that there is no contamination in the process (test tubes, vials, or refrigerator) and that there is no contamination in the instrument used to perform the analysis.
Analytical Methods
Physical and Chemical Analysis: Metal composition was analyzed by inductively coupled plasma-optical emission spectrometry (ICP-OES; Analytik JenaPQ9000, Germany), which was calibrated to detect and quantify a variety of metal elements in the range of several micrograms per liter to hundreds of milligrams per liter. The list of analyzed metals is given in Table 1. Only drinking water can be analyzed directly without any dilution or rest of other samples will undergo acid microwave digestion. Standard solutions for linearity calibration were prepared at concentration of 0.25ppm, 0.5ppm, 1.0ppm, 2.0ppm. The prepared standard and samples were kept properly. Checked the availability of Argon gas (grade 5) supply with proper pressure (4-6 bar), switch on cooler, auto sampler and then instruments after that switch on software. The plasma chamber should be free from moisture or dry. The method as per our analysis requirement was prepared. The sequence was prepared for analysis. The plasma icon and the gas flow was maintained as per standard: Nebulizer gas-0.5 L/min, Plasma gas-0.5 L/Min, Auxiliary gas-15 L/ min. Purge sprayed two times and the ignite plasma, waited for 5 min for plasma saturation then started analysis. For higher concentration used radial view (Na,K,Ca, Mg). pH was determined using a calibrated multimeter (WTW 3430, Germany).
PARAMETER
AWG 1
AWG2
AWG3
Standard Reference
Physical appearance
Clear and color less
Clear and color less
Clear and color less
Clear and color less
pH
7.62
7.89
7.89
6.5-8.5
Heavy Metal (mg/L)
Silver
Not Detected
Not Detected
0.003
Not more than 0.1 mg/L (EPA)
Aluminum
0.15
0.012
0.017
Not more than 0.2 mg/L (WHO)
Boron
0.18
0.029
Not Detected
Not more than 2.0 mg/L(EPA)
Barium
0.002
0.028
Not Detected
Not more than 2.0 mg/L(EPA)
Bismuth
Not Detected
Not Detected
Not Detected
-
Calcium
8.507
11.01
3.667
1-135 mg/L(EPA)
Cadmium
Not Detected
Not Detected
Not Detected
Not more than 0.005 mg/L(EPA)
Cobalt
Not Detected
Not Detected
Not Detected
Not more than 2.0 mg/L(EPA)
Chromium
0.004
Not Detected
Not Detected
Not more than 0.1 mg/L(EPA)
Copper
Not Detected
Not Detected
Not Detected
Not more than 1.3 mg/L(EPA)
Iron
Not Detected
Not Detected
0.000
Not more than 0.2 mg/L(EPA)
Gallium
Not Detected
Not Detected
Not Detected
-
Indium
Not Detected
Not Detected
Not Detected
-
Potassium
0.653
5.673
10.38
-
Magnesium
2.35
2.667
0.698
25-50 mg/L(EPA)
Manganese
Not Detected
Not Detected
Not Detected
Not more than 0.05 mg/L(EPA)
Sodium
0.479
2.483
11.29
Not more than 20 mg/L(EPA)
Nickel
Not Detected
Not Detected
Not Detected
Not more than 0.1 mg/L(EPA)
Lead
Not Detected
Not Detected
Not Detected
Not more than 0.015 mg/L(EPA)
Strontium
0.034
0.040
0.005
Not more than 4.0 mg/L(EPA)
Titanium
0.003
Not Detected
0.002
-
Zinc
0.000
0.006
0.000
Not more than 0.02 mg/L(EPA)
1) MCL-Maximum Contaminant Level for drinking water from a public water supply system. From “Current Drinking Water Standards”, E.P.A. Office of Water. https://www.freedrinkingwater.com/water_quality/chemical/water
2) http://www.who.int/water_sanitation_health/dwq/chemicals/
Table 1: Results of Physical and Chemical parameters of water from three different Atmospheric Water Generator (AWG) with standard specification from EPA¹ and WHO².
Microbiological Analysis: Microbiological analysis of water samples was started as soon as possible after collection of samples to avoid unpredictable changes in the microbial population. Gram staining was performed for morphological characterization. Nutrient agar (NA) as a basal medium MacConkey and EMB agar as a differential medium and VRB agar as a special medium were used to determine enteric bacteria. Inoculation in Nutrient broth for characterization in liquid media. Enteric bacteria isolated on respective selective or differential media were identified on the basis of their colonial, morphological and Biochemical properties (Table 2) following Bergey’s Manual of Determinative Bacteriology, 1994. Coliform counts were performed using standard Membrane filtration technique on VRB Agar.
Parameters
AWG 1
AWG 2
AWG 3
- Citrate Utilization
+
+
+
- Lysine
+
+
+
- Ornithine
+
+
+
- Urease
+ / -
-
-
- TDA
-
-
-
- Nitrate reduction
+
+
+
- H2S production
-
-
-
- Glucose
+
+
+
- Adonitol
+
+
+
- Lactose
+
+
+
- Arabinose
+
+
+
- Sorbitol
+
+
+
Table 2: Results of Biochemical analysis of water from three different Atmospheric Water Generator.
Chemicals
The chemicals used in the present investigation were of analytical grade and of high purity from Merck. Standard used for analysis were purchased from Germany and USA.
Statistical Analysis
The tests were performed in triplicates. Data are expressed as mean. Pair wise comparisons were performed. Experimental error was determined for triplicate and expressed as standard deviation (SD).
Results and Discussion
In the present probe, we analyzed various parameters (physical, chemicals, microbiological) in the dew water. The results were compared to the drinking water guidelines of the WHO/EPA standards. A total of 30 water samples were collected from three different AWG at different interval of time. None of the measured chemicals exceeded drinking water standards, although the standard deviation (SD) between samples was significant due to the varied climatic conditions. We had some concerns about the active dew collector inside the AWG apparatus becoming contaminated. However, examination showed this not to be the case, as the values on subsequent days were not affected by each other.
Metals
Metals are indispensable to human health but excess amounts can lead to severe health effects, and their quantity in the dew water must be monitored. A total of 22 metals were studied in this research from three different AWG (Table 1). The concentrations of the common metals zinc, aluminum, and copper were in order of magnitude within the limit range of drinking water standards. However, previous studies have also shown the impact of several long-range transport processes on the characteristics of aerosol (Heo et al, 2017). Thus, during the winter, zinc originated mostly from sources (vehicles).
We detected significant concentrations of calcium (Ca2+), magnesium (Mg2+), sodium (Na+), and potassium (K+) ions, which are considered to be major ions in water geochemistry (Table 1). Potassiumion do not have either WHO or IL drinking water thresholds but other metal have standards from EPA. It should be noted that Mg2+ deficiency and Ca2+ deficiency in drinking water can cause various health problems, such as tooth loss, rickets, and cardiac infarction (Rosborg et al, 2019). Therefore, the dew water should be supplemented with these essential minerals e.g. calcium to a concentration of 1-135 mg/L (EPA) and 25–50 mg/L magnesium (EPA) (WHO, 2009), as recommended for drinking water.
Other metals were found in negligible quantities and did not exceed the drinking water standards. There is no minimum required concentration for these substances in drinking water (Table 1). However, it is important to keep track of them as they can warn of potential contamination, possibly from the AWG (e.g., iron). The metals in the water originate from both local emissions (transportation, industry, and marine aerosols) and long-range atmospheric transport. Nevertheless, the overall levels of the metals in the produced dew water were much lower than the EPA and WHO and IL drinking water standards.
pH
The pH values of the water samples from AWG ranged from 7.62 to 7.89; with a median value of 7.8.While the WHO does not have a pH standard, the IL standard ranges between 6.5 and 9.5. pH values of dew water vary significantly across sites, ranging between 4.0 and 7.9, with wide daily variations due to the variable sources of ions. However, in most studies, the mean pH values usually remain fairly close to neutral (Beysens et al, 2018).
Microbiological Analysis
Microbiological analysis of water samples was started as soon as possible after collection of samples to avoid unpredictable changes in the microbial population. Gram staining was performed for morphological characterization. In AWG1 Gram + Ve and Gram – Ve bacteria were observed under microscopic examination, some slides shows mixed culture of bacteria i.e. Cocci, short bacilli found. In AWG 2, Gram – Ve bacteria were observed under microscopic examination, some slides show mixed culture of bacteria i.e. Cocci, but it was observed G-ve short bacilli predominantly. In AWG 3, mixed culture of bacteria i.e. Cocci, short bacilli were observed which were both Gram + Ve and Gram – Ve bacteria under microscopic examination.MacConkey and EMB agar as a differential medium and VRB agar as a special medium were used to determine enteric bacteria. Isolation of Microorganism by spread plate method on EMB Agar in all samples showed numerous purple black colour colonies observed. Outer ring of purple colony was creamy white in initial incubation. Colonies are isolated and in groups, circular in shape, sticky, glistening. Nutrient agar (NA) as a basal medium. On Nutrient Agarminute/small colonies of microorganism were observed, which were buff colour and yellow colour colonies after incubation at 37°C for 24 to 48 hours. On MacConkey Agar, which was used as differential medium, numerous colonies were observed, colour of the medium was changed from pink to yellow and colonies observed were purplish pink in colour. Inoculation in nutrient broth for characterization in liquid media. Growth observed in nutrient broth, due to that turbidity increased. There was also pellicle formation in all samples. Faecal and total coliform counts were performed using the standard membrane filtration technique. The 100 ml water sample was filtered using 0.45 mm pore size, 47 mm diameter filter membrane as described by APHA (1998). On inoculation on VRB medium, colour of media changes from pink to yellow, numerous colonies observed were reddish colour on membrane filter of 0.45μ, mucoid, round. Enteric bacteria isolated on respective selective or differential media were identified on the basis of their colonial, morphological and Biochemical properties (Table 2) following Bergey’s Manual of Determinative Bacteriology, 1994.
According to biochemical characterization in all the water samples from three different AWG, Citrate utilization, lysine, ornithine, urease, nitrate reduction, H2S production, glucose, Adonitol, lactose, Arabinose, Sorbitol were found to be positive. According to the present investigation, the bacterial species identified were members of the Enterobactericae family (Table 2). One of the reasons may be due to less human intervention. But it is important to note that the limited presence may be due to that coliform bacteria which are widely found in nature and do not necessarily indicate faecal pollution (Binnie et al., 2002; Griffith et al., 2003).
Conclusion
Our comprehensive research investigation suggests that the production of dew water by an AWGin a large urban area can provide safe drinking water, throughout the year. There was high variability in the concentrations of most substances between the water samples. Special attention and monitoring efforts should be paid to the only compounds that exceeded the drinking water standards. In conclusion, we demonstrate that the atmospheric water produced by AWGs can be a potential source of potable water, which may assist in dealing with the severe water scarcity existing across the globe, and specifically in remote and inland regions. Therefore, future research efforts should examine the influence of anthropogenic air pollution, meteorological conditions, and atmospheric processes on the chemical characteristics of the produced dew water.
Conflict of Interest
We declare that we have no conflict of interest. All procedures followed were in accordance with the ethical standards (institutional and national).
Availability of Data and Materials
The relevant data and materials are available in the present study.
Acknowledgements
Author would like to thank all individuals who provided their efforts for this research especially Pramod Kumbhar and Aaesha Ahmed Alzaabi.
Authors’ Contributions
VB supervised the entire project. Supervision of the laboratory work was performed by VB. VB analysed the data and wrote the manuscript. VB did all experiment work.
References
- APHA (1998). American Public Health Association. Standards methods for the examination of water and wastewater. 20th edition. Washington DC.
- Beysens D. Dew Water; River Publishers: Gistrup, Denmark; 2018. ISBN 9788793609471.
- Binnie C, Kimber M, Smethurst G. Basic water treatment,Cambridge: Royal Society of Chemistry. 2002.
- Griffith JF, Weisberg SB, McGee CD. Evaluation of microbial source tracking methods using mixed fecal sources in aqueous test samples. Journal of water and health. 2003; 1(4): 141-151. doi:10.2166/WH.2003.0017.
- Heo J, Wu B, Abdeen Z, Qasrawi R, Sarnat JA, Sharf G, et al. Source apportionments of ambient fine particulate matter in Israeli, Jordanian, and Palestinian cities. Environmental pollution. 2017; 225: 1-11. doi:10.1016/j. envpol.2017.01.081.
- Li R, Shi Y, Alsaedi M, Wu M, Shi L, Wang P. Hybrid Hydrogel with High Water Vapor Harvesting Capacity for Deployable Solar-Driven Atmospheric Water Generator. Environmental science & technology. 2018; 52(19): 11367- 11377. doi:10.1021/acs.est.8b02852.
- MCL-Maximum Contaminant Level for drinking water from a public water supply system. From “Current Drinking Water Standards”, E.P.A. Office of Water. https://www.freedrinkingwater.com/water_quality/chemical/water
- Mekonnen MM, Hoekstra AY. Four billion people facing severe water scarcity. Science Advances. 2016; 2(2). doi:10.1126/sciadv.1500323.
- Rosborg I, Kozisek F, Precautions S. Drinking Water Minerals and Mineral Balance; Springer International Publishing: Cham, Switzerland; 2019. ISBN 9783030180331.
- Semiat R. Energy issues in desalination processes. Environmental science & technology. 2008; 42(22): 8193-8201. doi:10.1021/ES801330U.
- UNESCO. Leaving No, One Behind; UNSECO: Paris, France. 2019.
- WHO. Calcium and Magnesium in Drinking-Water; WHO: Geneva, Switzerland, ISBN 9789241563550. 2009.
- http://www.who.int/water_sanitation_health/dwq/chemicals/
- https://en.wikipedia.org/wiki/Atmospheric_water_generator