
Review Article
Int J Nutr Sci. 2023; 8(1): 1070.
Should Rheumatoid Arthritis Patients go on a Gluten-Free Diet?
Lerner A1,2* and Benzvi C1
¹Chaim Sheba Medical Center, The Zabludowicz Research Center for Autoimmune Diseases, Israel
²Ariel University, Ariel, Israel
*Corresponding author: Lerner A Research Department, Chaim Sheba Medical Center, The Zabludowicz Research Center for Autoimmune Diseases, Tel Hashomer, 5262000 Israel
Received: December 07, 2022; Accepted: January 20, 2023; Published: January 27, 2023
Abstract
Rheumatoid arthritis and celiac disease are autoimmune inflammatory diseases that share multiple aspects. The only established therapy for celiac disease is the gluten-free diet. The current therapy for rheumatic arthritis patients is mainly pharmaceutical and physiotherapy. The nutritional therapy is in its first steps, while several diets were suggested. A gluten-free diet was preliminarily assessed with some beneficial effects; however, no guidelines exist in the rheumatic, nutritional, nor in autoimmune literature. The present review expends on rheumatoid arthritis - celiac disease relationship, on the gut-joint axis, on the enteric luminal and mucosal eco events in rheumatoid arthritis patients. Various aspects of Gluten-free diet are reported, guidelines for gluten withdrawal are suggested and the beneficial aspects of Gluten-free-Mediterranean diet are described.
Keywords: Rheumatoid arthritis; Gluten-free diet; Celiac disease; Non-celiac autoimmune diseases; Dietary therapy; Tissue transglutaminase
Abbreviations: Ads: Autoimmune Diseases; RA: Rheumatoid Arthritis; CD: Celiac Disease; GFD: Gluten-Free Diet; MD: Mediterranean Diet; SCFA: Short-Chain Fatty Acids; tTG: Tissue Transglutaminase
Introduction
A wide discrepancy exists between the worldwide increased wheat consumption and its popularity and between the scientific observations on the side effects of gluten, the major protein in wheat [1–4]. The human being first encountered the wheat approximately 15000 years ago in the Fertile Crescent of the Middle East [5]. Due to evolutionary environmental pressures and human breeding, nitrogen fertilizers and pesticides usage, wheat has undergone many phenotypic and genetic changes [6]. Compared to the old wheat, the contemporary one contains 8-folds more gluten and the protein is more immunogenic and more toxic [2,4]. Despite it, wheat is the most popular staple prolamin, representing a major caloric and protein source for most of the world’s population [2,3], while its annual consumption is steadily increasing [1]. Interestingly, efforts are continuously made, aiming to develop low-gluten, non-transgenic wheat variety [7–9].
Alongside to the last decades’ increased incidences of Autoimmune Diseases (ADs) [10,11], the major gluten-dependent autoimmune condition, namely Celiac Disease (CD) is also increasing [11,12]. Interestingly, the list of gluten-dependent diseases is expanding: gluten ataxia, dermatitis herpetiformis, non-celiac gluten/wheat sensitivity and gluten/wheat allergy are on the list [13]. On the other hand, gluten itself has recently been described as a potential contributor to the development of neurodegenerative diseases [14–16]. In addition, the beneficial effects of gluten withdrawal in AD are increasingly reported [1,2,4,17–19], not excluded are various rheumatic conditions [2,4,17–19]. The present narrative review will concentrate on rheumatoid arthritis aiming to answer the question, should rheumatoid arthritis patients go on a Gluten-Free Diet (GFD)?
Rheumatoid Arthritis in a Nutshell
Rheumatoid Arthritis (RA) is a chronic, inflammatory, multi-factorial and progressive AD that primarily affects joints [20,21]. Its estimated prevalence is 1% in Europe and USA. The most frequently affected joints are the hands and wrists, where the joints are swollen, warm and painful, in a symmetrical presentation. It may affect extra-articular organs or tissues, hence present as a multi-organ systemic disease. The disease affects mainly the female gender and presents more frequently above the age of 50 years. The underlining causes are not clear and as with the other ADs, environmental and genetic factors determine RA susceptibility. The systemic and local immune systems attack the involved joints resulting in arthritis and joint’s capsule thickness, bone erosion and cartilage damage. Many other ADs can be associated, including CD [1,2,4,17–19]. Multiple old modes of therapies exist like NSAIDs, steroids, disease-modifying Anti-Rheumatic Drugs and biologicals but new pharmacological agents are upcoming [22]. They can suppress the inflammation, prevent structural damage and improve the patient’s quality of life. However, all those pharma therapies do not lack side effects, which accelerated the attempts to explore various nutritional therapies.
Dietary Therapy for Rheumatoid Arthritis
An increasing number of reports suggested that various nutrients and selected diets might impact induction, maintenance, behavior and progression in RA patients. The increasing knowledge on pro- and anti-inflammatory or antioxidative food components allow designing diets that are protective and fulfill the desire of targeting the inflammatory joints [23–25]. Recently, such beneficial or harmful nutrients were extensively summarized and their mechanism and potential pathways, starting from the enteric lumen to impact peripheral organs, were listed [1,26–28]. Nutrients, food additives, bugs and we can affect the composition and the diversity of the microbiome, switching the balance towards a dysbiome or to a pathobiome [29–31]. Gut eco-events are pivotal for homeostasis, hence, can orchestrate and drive a plethora of pathogenic mechanisms resulting in metabolic as well as autoimmune chronic diseases [26–31], RA is one of those long-term conditions [2,4,17–19,27,28].
Dietary components can affect gut functions. Nutrients can induce dysbiome, change post-translational modification of naïve peptides in the lumen, affect intestinal permeability and induce a leaky gut, impact digestion, absorption and even gut motility. All those events might operate in the gut-joint axes and induce arthritis when dysfunctional or failed [1,2,33–36,4,16,18,19,27,29,30,32]. Zooming on dietary trails on RA patients, several had some beneficial effects. Reports suggested that caloric restriction and fasting produce therapeutic anti-inflammatory effects in RA [37–40]. Plant-based foods were shown to improve gut microbiome in RA patients, resulting in reduced inflammatory arthritis and joint pain [37]. Comparable beneficial effects were reported on low-fat vegan [41–43] and on gluten-free vegan diets [43]. Anti-inflammatory nutraceuticals had good effects on the inflamed joints [44] and finally, the Mediterranean Diet (MD) can lower the risk for RA [45] and protect disease activity and microbiota composition in RA patients [46]. More so, a systemic review concluded that the MD reduces pain and increases physical activity in RA patients. However, there is not sufficient evidence for a widespread recommendation to follow the diet [47]. On the contrary, a recent study concluded that MD does not affect RA indices [48]. So, the jury is not there yet. The complex cross-talks between ADs in general and dietary therapy is “Well Begun, Is Half-Done” [49].
The Detrimental Effect of Gluten
The side effects of gluten were recently summarized [2,4,16,18,32]. The topic is applicable to all gluten-dependent diseases, but also might be of concern to other chronic diseases like non-celiac ADs and even to some parts of the normal population. The reported incidences of the classical gluten-dependent conditions are: CD-1-2%, gluten ataxia- 0-6%, wheat allergy- 0.5-1%, nonceliac wheat/gluten sensitivity- 0.6-13% and dermatitis herpetiformis-0.4-2.6 per 100000 people [50]. It appears that the adverse effects of gluten are present on the systemic, as well as on the local or organ levels. On the systemic levels, gluten is pro-inflammatory, pro-oxidative and impacts epigenetics. On the intestinal level, it breaches tight junction functional integrity thus enhancing gut permeability and inducing dysbiosis. On the cellular level it suppresses viability, it is pro-apoptotic, and decreases cell differentiation and DNA, RNA and glycoprotein synthesis. Gluten affects multiple immune functions. It increases immunogenicity, cytotoxicity, Th-17 activity, neutrophil’s migration, NKG2D expression and TLR4 signalling pathway. Furthermore, it impacts the innate and adaptive immune systems’ functions and Treg phenotype and behavior [2]. It should be stressed that most of the studies were performed on animals and on cell lines and not in vivo on humans. The proof of concept is presented by the numerous non-celiac ADs that might benefit gluten withdrawal, thus curtailing gluten adverse effects [2,4,15,18,19,28,30,50–52]. Intriguingly, even some patients with irritable bowel syndrome, metabolic syndrome, obesity, cardiac conditions and inflammatory bowel diseases might benefit from gluten withdrawal [53–60]. All the above-mentioned dark side of gluten intake might explain the impact of GFD in RA. And now some warnings on the popularity of GFD adaption in unproven, non-gluten-dependent conditions.
The Fashionista of Gluten-Free Diet
Before discussing GFD in RA, a word of caution should be forward due to the fashionista of GFD [3]. Facing the surge of non-infectious human chronic conditions like allergies, ADs, metabolic syndrome and cancer [10] and the surge in popular alternative medicine approaches, GFD has been rising, on a large scale, over the last decades. We are witnessing an uncontrolled, increasingly questioned and criticized by the scientific community contemporary phenomenon [3,61–64]. Despite it, the opponents of gluten consumption reach the center of the popular stage by reinforcing gluten avoidance. “Going gluten-free” became mainstream in the Western world and is an actual fashion trend [3,65–67]. Facing this fashion are the unwanted side effects of gluten avoidance. Indeed, Iron, calcium, sodium, Vitamin D, C, A, E, B12, thiamin, riboflavin and niacin, Folate, trace elements like zinc, magnesium, Selenium, fibers like oligo-fructose, inulin, fructans, HDL, Apo A1, essential amino acids and arachidonic acid abnormalities/deficiencies were described in gluten avoiding patients [3,68–70]. Key inadequacies of currently available GF products are low protein and complex carbohydrate fiber and high fat, simple sugars and salt contents [3,64,69,71]. Furthermore, unfavorable body composition changes might be observed. In celiac patients, after 1-year of GFD, increased fat mass is evident compared to their baseline [72].
An unsupervised GFD is associated with increased consumption of rice- or maize-based products. Those products might contain heavy metals such as copper, arsenic, lead and cadmium or mycotoxins that risk [3]. Maize and its products may contain mycotoxins (fumonisins), which are hepatotoxic, nephrotoxic, hepatocarcinogenic and cytotoxic [43]. However, GF products have also health benefits [73].
Another aspect of the GFD is the adherence difficulties. Applying a GFD is a tough alley and the effort to follow and adhere to gluten withdrawal, represents nowadays also a torrid time [74]. The real-life scenarios of the gluten-dependent affected patients are tough [75] and full of daily challenges [76]. Finally, popular GFD contains several misconceptions that were summarized lately [50]. It is not a healthier option and many will not lose weight.
Rheumatoid Arthritis and Celiac Disease Relationship
Both ADs, despite being separated defined conditions, are related and share many aspects [36,77–81]. Both are autoimmune HLA-dependent diseases that share several non-HLA loci with comparable environmental factors and rising incidences. In both conditions, post-translational modification of naïve peptides is operating [29,36]. Citrullination by the peptidyl arginine deiminase in RA and deamidation and cross-linking by Tissue Transglutaminase (tTG) [36,82]. Clinically, rheumatoid extra-intestinal manifestations exist in CD, while extra-articular gastrointestinal involvement occurs in RA. Notably, enteric inflammation and hepatic damage were reported in rheumatoid patients, even before any joint damage [83,84]. In both conditions, dysbiosis and increased intestinal permeability are major pathophysiological players [29,30,85–88]. Celiac is a typical gluten-induced disease that responds to GFD; hence, parts of RA patients respond to gluten avoidance [2,4,18,19]. Interestingly, Non-celiac Gluten Sensitivity was reported to be associated with fibromyalgia, spondyloarthritis, and refractory RA [89]. Further exploring those shared similarities in the gut-joint axes might improve our knowledge of the mosaic of autoimmunity [90].
GFD in Rheumatoid Arthritis
Many aspects are shared between RA and CD [36,77–81]. GFD will help in gluten-dependent conditions; however, the question of GFD benefit for the RA patients is the topic of the current review. Screening the PubMed for RA and GFD reviles a surge in publications in the last years. Between 1964-2017 the average of publications was much less than 1 per year. It substantially increased to 3.5 per year in the last 4 years. When investigated, GFD alone, or combined with other dietary restrictions, was beneficial in many of them [2,4,94–96,18,19,42,43,82,91–93]. To our knowledge, only one study was negative [97]. Reviewing the literature, some studies explored GFD alone and some others, combined or elimination diets like GF vegan diet [42,43,92], high protein GFD [91] and excluding meat, gluten and lactose [95].
Potential Mechanisms and Pathways for the Beneficial Effect of GFD in RA
Gluten withdrawal might help RA patients in several ways. Some of them are connected to the suggested RA triad: “diet, Microbiota, and Gut Permeability” [32] and are schematically presented in (Figure 1). Following are some of those mechanisms and gut-joint pathways:
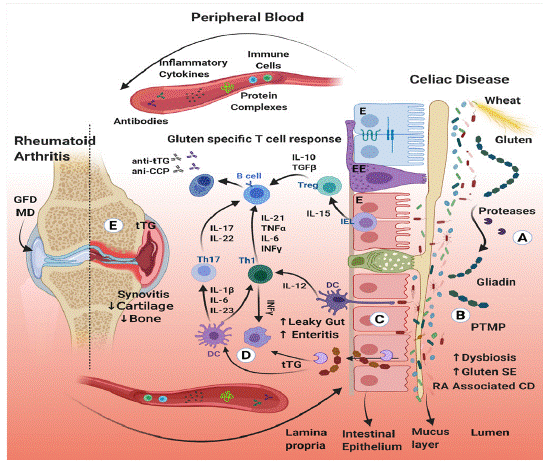
Figure 1: A schematic presentation of the Gut-Joints axis and the relationship between rheumatoid arthritis, gluten-free diet and celiac disease.
(A) Oral consumption of gluten-containing wheat. Gluten is ingested and digested, reaching the gut lumen as gliadin peptides. (B) Gliadins are rich in glutamine and proline, thus are a prime substrate for deamidation and cross-linking by luminal and mucosal transglutaminases, thus, turning those naïve molecules into immunogenic ones. Transglutaminase capacity to deamidate or transamidate results in an increase in post-translation modified proteins (PTMP). Luminal digestive peptidases cannot further break down those bonds, hence, inducing gut inflammation, mucus disruption and intestinal epithelial damage. This affects the microbiome/dysbiome ratio, resulting in the proinflammatory metabolome, pathobionts proliferation, maldigestion, malabsorption and even gut dysmotility. (C) Gluten increases intestinal permeability by binding to epithelial CXCR3 receptors, resulting in zonulin release. Gliadin-transglutaminase transformed peptides can potentially infiltrate through the open junctions or trans-enterocytically into the lamina propria. A breach in the epithelial barrier exposes the highly immunoreactive sub-epithelium to luminal foreign antigens, stimulating the local immune system. Enteritis and leaky gut are major contributors to the local and the articular inflammation in both diseases. (D) In the lamina propria, gliadin-transglutaminase cross-linked complexes induce pro-inflammatory cytokines. Two types of DC are present, sub-epithelial DCs that send protrusions into the lumen and sense the gut microbiota, and the lamina propria DCs that migrate to lymph nodes, where they present antigens to activate T cells. Th1 lymphocytes secrete IFNγ that activates macrophages. Th17 lymphocytes secrete IL-17 and IL-22 which activate B cells. The latter secret autoantibodies against tTG (anti-tTG) or against citrullinated peptides (anti-CCP), both, circulate in the blood. (E) Mucosal immune cells, immunogenic modify peptides, proinflammatory cytokines, autoantibodies and small particles that escaped the immune system enter the blood vessels. They can eventually reach the joints and trigger an autoimmune response, enhancing arthritis, cartilage damage and bone destruction. Local tTG may drive synovitis and bone erosion in RA. Finally, GFD and MD can potentially attenuate RA inflammatory activity in some of the patients.
As gluten have multiple adverse effects [2]. Its avoidance might prevent and curtail those harmful effects, thus lowering the joint inflammation and RA disease activity.
One of the main intestinal contributions to RA evolvement is the leaky gut. Tight junction functional integrity is pivotal for the local and systemic physiological homeostasis. Maintaining tolerance and avoiding autoimmunity are essential for human health [29,30]. A plethora of nutrients enhances or decreases gut permeability [26,32,98]. Since gluten is a major disruptor of the enteric permeability [2], its avoidance might protect the body from unwanted immune reactions on their way to target the joints [1,2,19,27,36,81,99,100].
Gut microbiota, a huge symbiotic prokaryote community inhabiting the human enteric lumen [101–103]. Upon microbiome/dysbiome ratio decrease, chronic diseases ranging from metabolic, cancerous and autoimmune are emerging [29–31,104] and RA is not an exception [2,4,18,19,27,36,85]. Gluten intake perturbates the microbiome balance and changes the composition and the diversity of the normal inhabitants [105–107]. As a result, GFD might partially reverse the abnormality towards a more physiological gut microbial community [108]. Interestingly enough, the effects may be due to the increased consumption of undigested fiber rather than to the gluten withdrawal [109].
Gluten/wheat withdrawal is associated with increased intake of undigested polysaccharides. Higher fiber consumption results in short-chain fatty acid (SCFA,) production and represent the main bacterial fermentation mobilome in the human gut luminal. SCFAs as multiple beneficial effects on the local as well as on the systemic homeostasis [110–112]. Notably, after one year of GFD, SCFA's excretion and the gut microbiome normalized in CD patients [113]. In addition, the MD is rich in fiber and the RA patients that consume it excrete higher SCFA levels [45–47,114,115], thus, exerting anti-inflammatory and immunomodulatory effects for the patient's benefit. It seems logical to suggest a combination of GF MD might help the RA patients [116].
Depression and behavioural problems are known to exist in RA patients [117,118]. Tryptophan deficiency is associated with serotonergic dysfunction, which plays a role in several depressive, affective and behavioural symptoms. In addition, tryptophan metabolism might represent a marker for disease activity and bone destruction in RA patients [119]. It appears that GFD might be beneficial for those behavioural disorders, though, the mechanism is not known [120,121].
Secretory IgA is a major luminal immune protective barrier; however, selective IgA efficiency is associated with ADs, including RA [122]. Its level and functions are microbiome, SCFAs and diet-dependent [123,124]. It is suggested that a GFD that induces a healthier microbiome and higher SCFA production will enhance luminal IgA levels in the gut of the RA patients.
Cross-reactive antibodies between wheat, gluten\gliadin peptides and many human antigens were reported [16,26,27,125–129]. More so, the intestinal fluid of many RA patients contains IgG, IgM and IgA antibodies' activity that react with specific food components, including against gliadin [130]. Of note, an increased serological antibody biomarker positivity exists in RA and wheat-related disorders [131]. Likewise, multiple anti-rheumatic and anti-connective tissue autoantibodies exist in the serum of gluten-depended conditions [36,81,132,133]. Taken together, GFD might suppress those cross-reactive antibodies, thus alleviating the inflammatory activity in their joints.
The tissue transglutaminase is the only proofed autoantigen in CD [82,134]. The enzyme deamidates or cross-link gluten\gliadin peptide, thus, breaking gluten tolerance in CD patients. Tissue transglutaminase exists in normal, as well, in the inflamed joints in RA. This transglutaminase is driving synovitis and bone erosion in RA and osteoarthritis [135–138]. Since gluten/gliadin peptides are the preferred substrate for the enzyme [28,134,139], GFD might deprive tTG of its gluten substrate, thus, preventing or attenuating the articular damage.
In (Table 1), each of these mechanisms is detailed for the beneficial effects of GFD in RA patients.
Mechanisms
Adverse effects of Gluten consumption
GFD effect on RA
Tight junction functional integrity maintains tolerance and avoids autoimmunity [29,30].
A major disruptor of the enteric permeability [2,26,32,98].
Might protect from immune reactions on the joints, lowering inflammation and RA disease activity [1,2,19,27,36,81,99,100].
A healthy gut microbiota composition has implications in preventing chronic conditions ranging from metabolic, cancerous and AD [29–31,101–104].
Perturbates microbiome/dysbiome ratio, change composition and diversity of the normal inhabitants [105–107].
Might partially reverse the abnormality towards a more physiological gut microbial community [108].
Physiologic Microbiome is important for homeostasis [2,4,18,19,27,36,85].
Increased dysbiota and pathobiota [105,106].
The effects may be due to the increased consumption of undigested fiber, rather than to the gluten withdrawal [109].
SCFAs have beneficial effects on local and systemic homeostasis, including anti-inflammatory and immunomodulatory effects [110–112].
Gluten/wheat withdrawal is associated with increased intake of higher fiber consumption which results in a higher SCFA production [113].
RA patients that consume MD excrete higher SCFA levels; GF MD might help RA patients [45–47,114,115].
Tryptophan deficiency is associated with serotonergic dysfunction leading to depression and behavioral problems and is a marker for disease activity and bone destruction in RA patients [117–119].
By its pro-inflammatory activity, gluten might decrease tryptophan absorption [1,2].
Might be beneficial for those behavioral disorders, though, the mechanism is not known [120,121].
IgA is a luminal immune protective barrier; its efficiency depends on microbiome, SCFAs and diet [122–124].
Selective IgA efficiency is associated with several ADs, including RA and CD [81,122].
A healthier microbiome and higher SCFA production might enhance luminal IgA levels of RA patients [123].
Cross-reactive antibodies against specific food components, including gliadin, in RA patients. Positive serological antibody biomarker in RA and wheat-related disorders [36,81,130–133].
Gluten might stimulate cross-reactive antibodies between wheat, gluten\gliadin peptides and human antigens, including joint components reported [16,26,27,125–129].
Might suppress cross-reactive antibodies, alleviating inflammatory activity in RA joints [154].
tTG exists in normal and inflamed joints. It drives synovitis and bone erosion in RA and osteoarthritis [135–138].
tTG is the autoantigen that breaks gluten tolerance in CD patients. Gluten/gliadin peptides are preferred substrate for the enzyme [28,82,134,139].
Might deprive tTG of its gluten substrate, preventing or attenuating the articular damage [82,134].
Table 1: Potential mechanisms for the beneficial effects of GFD in RA patients.
Should Rheumatoid Arthritis Patients go on Gluten-Free Diet?
The answer is not clear-cut and is highly debatable. Many publications are positive for gluten withdrawal [2,4,94–96,18,19,42,43,82,91–93] and only one study found no effect of GFD in RA [9]. We are not endorsing all the RA patients to try GFD. The RA patients should not join the popular GFD "fashionista" in a blind and sweeping way.
It should be remembered that gluten restriction has many limitations [3,43,68–70,72], long-term adherence is difficult and problematic [50,74–76], and should be done under a dietician’s supervision. Based on all of the above, we suggest some guidelines and conditions that will apply to only part of the RA patients to go on a GFD:
A. Gastrointestinal complaints such as abdominal pains, bloating, soft or diarrheal stools, significant burps/belches, etc.
B. Positive CD serology like anti-gluten, anti-endomysial, anti-tTG, anti-deamidated gliadin, anti-neo-epitope tTG antibodies [140–147]
In case the RA patient meets these conditions, they should be referred to a gastroenterologist in order to rule out gluten-dependent conditions, mainly CD. It is suggested that an occasional GFD trail is not sufficient for a long-term gluten withdrawal. Upon applying to the above two clinical and laboratory conditions and after the GI consultation, going on gluten-free Mediterranean diets might represent the most appropriate diet. Since many RA patients, mainly females, are suffering from irritable bowel syndrome and since gluten can cause them GI symptoms, it is suggested that they will follow the above-mentioned work up, before adapting GFD [148].
Finally, it is highly recommended that the rheumatologic/ nutritional/ gastrointestinal communities will explore the GFD in RA, applying a well-designed, double-blind, cross-over study. As recommended, GF-MD should be investigated on RA patients.
Conclusions
Gluten is an autoimmunogenic nutrient [149] and imbeds multiple adverse effects [2,19]. Rheumatoid arthritis and CD share many aspects [36]; both conditions are ADs and are frequent members of the polyautoimmunity syndrome [150] as a part of the mosaic of autoimmunity [90]. The topic of nutritional therapy in RA is expanding towards a more personal approach [23,24,40–49,25,151,32–35,37–39]. There are many reports on the beneficial effects of GFD in RA patients, including amelioration of symptoms, disease activity and quality of life [42,91–96]. However, there are no accepted or established guidelines for GFD application in RA. The present narrative review suggests a few screening conditions, one clinical and the other laboratory. However, those guidelines should be scientifically reassessed for the long-term benefits of RA patients.
Since the compliance to GFD is poor, at least in CD [74–76,152,153] and gluten avoidance has several adverse effects [3,65–72], patients should consult the nutritional teams. It is hoped that targeting the inflamed joints, the enteric barrier functions and the dysbiome by specific dietary means will open new therapeutic strategies to modulate RA evolvement.
Take Home Messages
• GFD might be beneficial in RA patients by ameliorating symptoms, decreasing disease activity and improving quality of life.
• Clinical and serological guidelines for GFD in RA patients should be applied.
Conflict of Interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Author Contributions
AL- screened the literature, designed and wrote the manuscript, CB- screened the literature, edited and revised the manuscript, designed the figure with BioRender.com permission. The two authors agreed to the published version of the manuscript.
References
- Lerner A, Matthias T. Changes in intestinal tight junction permeability associated with industrial food additives explain the rising incidence of autoimmune disease. Autoimmun Rev. 2015; 14: 479–89.
- Lerner A, Shoenfeld Y, Matthias T. Adverse effects of gluten ingestion and advantages of gluten withdrawal in nonceliac autoimmune disease. Nutr Rev. 2017; 75: 1046–58.
- Lerner A, O’Bryan T, Matthias T. Navigating the Gluten-Free Boom: The Dark Side of Gluten Free Diet. Front Pediatr. 2019; 7: 414.
- Lerner A, Ramesh A, Matthias T. Going gluten free in non-celiac autoimmune diseases: the missing ingredient. Expert Rev Clin Immunol. 2018; 14: 873–5.
- Harari YN. Sapiens: A Brief History of Humankind. 2017.
- Mandal S, Verma AK. Wheat Breeding, Fertilizers, and Pesticides: Do They Contribute to the Increasing Immunogenic Properties of Modern Wheat? Gastrointest Disord. 2021; 3: 247–64.
- Verma AK, Mandal S, Tiwari A, Monachesi C, Catassi GN, Srivastava A, et al. Current Status and Perspectives on the Application of CRISPR/Cas9 Gene-Editing System to Develop a Low-Gluten, Non-Transgenic Wheat Variety. Foods. 2021; 10: 2351.
- Smedley MA, Hayta S, Clarke M, Harwood WA. CRISPR-Cas9 Based Genome Editing in Wheat. Curr Protoc. 2021; 1: e65.
- García-Molina MD, Giménez MJ, Sánchez-León S, Barro F. Gluten Free Wheat: Are We There? Nutrients. 2019; 11: 487.
- Bach JF. The hygiene hypothesis in autoimmunity: The role of pathogens and commensals. Nat Rev Immunol. 2018; 18: 105–20.
- Lerner A, Jeremias P, Matthias T. The World Incidence and Prevalence of Autoimmune Diseases is Increasing. Int J Celiac Dis. 2015; 3 151-155.
- Lerner A, Jeremias P, Matthias T. THE WORLD INCIDENCE OF CELIAC DISEASE IS INCREASING: A REVIEW. Int J Recent Sci Res. 2015; 6: 5491–6.
- Taraghikhah N, Ashtari S, Asri N, Shahbazkhani B, Al-Dulaimi D, Rostami-Nejad M, et al. An updated overview of spectrum of gluten-related disorders: Clinical and diagnostic aspects. BMC Gastroenterol. 2020; 20: 1–12.
- Gatta NG, Cammarota G, Gentile V. Possible roles of transglutaminases in molecular mechanisms responsible for human neurodegenerative diseases. AIMS Biophys. 2016; 3: 529–45.
- Lerner A, Matthias T. Don’t forget the exogenous microbial transglutaminases: it is immunogenic and potentially pathogenic. AIMS Biophys. 2016; 3: 546–52.
- Lerner A, Benzvi C. Let Food Be Thy Medicine: Gluten and Potential Role in Neurodegeneration. Cells. 2021; 10: 756.
- Lerner BA, Green PHR, Lebwohl B. Going Against the Grains: Gluten-Free Diets in Patients Without Celiac Disease—Worthwhile or Not? Dig Dis Sci. 2019; 64: 1740–7.
- Lerner A, Ramesh A, Matthias T. Are Non-Celiac Autoimmune Diseases Responsive to Gluten-Free Diet? Int J Celiac Dis. 2017; 5: 164–7.
- Lerner A, Freire de Carvalho J, Kotrova A, Shoenfeld Y. Gluten-free diet can ameliorate the symptoms of non-celiac autoimmune diseases. Nutr Rev. 2020; 80: 525-543.
- Chauhan K, Jandu JS, Goyal A, Bansal P, Al-Dhahir MA. Rheumatoid Arthritis. Rosen and Barkin’s 5-Minute Emergency Medicine Consult: Fifth Edition. StatPearls Publishing; 2021.
- Lerner A, Neidhöfer S, Reuter S, Matthias T. MMP3 is a reliable marker for disease activity, radiological monitoring, disease outcome predictability, and therapeutic response in rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2018; 32: 550–62.
- Mueller AL, Payandeh Z, Mohammadkhani N, Mubarak SMH, Zakeri A, Bahrami AA, et al. Recent Advances in Understanding the Pathogenesis of Rheumatoid Arthritis: New Treatment Strategies. Cells. 2021; 10: 3017.
- Gioia C, Lucchino B, Tarsitano MG, Iannuccelli C, Di Franco M. Dietary Habits and Nutrition in Rheumatoid Arthritis: Can Diet Influence Disease Development and Clinical Manifestations? Nutrients. 2020; 12: 1456.
- Athanassiou P, Athanassiou L, Kostoglou-Athanassiou I. Nutritional Pearls: Diet and Rheumatoid Arthritis. Mediterr J Rheumatol. 2020; 31: 319–24.
- Rondanelli M, Perdoni F, Peroni G, Caporali R, Gasparri C, Riva A, et al. Ideal food pyramid for patients with rheumatoid arthritis: A narrative review. Clin Nutr. 2021; 40: 661–89.
- Lerner A, Neidhöfer S, Matthias T. The Gut Microbiome Feelings of the Brain: A Perspective for Non-Microbiologists. Microorganisms. 2017; 5: 66.
- Lerner A, Matthias T. GUT-the Trojan Horse in Remote Organs’ Autoimmunity. J Clin Cell Immunol. 2016; 7: 1–10.
- Lerner A, Benzvi C. Microbial Transglutaminase Is a Very Frequently Used Food Additive and Is a Potential Inducer of Autoimmune/Neurodegenerative Diseases. Toxics. 2021; 9: 233.
- Lerner A, Aminov R, Matthias T. Dysbiosis May Trigger Autoimmune Diseases via Inappropriate Post-Translational Modification of Host Proteins. Front Microbiol. 2016; 7: 84.
- Lerner A, Aminov R, Matthias T. Transglutaminases in Dysbiosis As Potential Environmental Drivers of Autoimmunity. Front Microbiol. 2017; 8: 66.
- Lerner A, Matthias T, Aminov R. Potential effects of horizontal gene exchange in the human gut. Front Immunol. 2017; 8: 1630.
- Guerreiro CS, Calado Â, Sousa J, Fonseca JE. Diet, microbiota, and gut permeability-the unknown triad in rheumatoid arthritis. Front Med. 2018; 5: 349.
- Winkvist A, Bärebring L, Gjertsson I, Ellegård L, Lindqvist HM. A randomized controlled cross-over trial investigating the effect of anti-inflammatory diet on disease activity and quality of life in rheumatoid arthritis: The Anti-inflammatory Diet in Rheumatoid Arthritis (ADIRA) study protocol. Nutr J. 2018; 17: 1–8.
- Vadell AKE, Bärebring L, Hulander E, Gjertsson I, Lindqvist HM, Winkvist A. Anti-inflammatory Diet In Rheumatoid Arthritis (ADIRA)—a randomized, controlled crossover trial indicating effects on disease activity. Am J Clin Nutr. 2020; 111: 1203–13.
- Chehade L, Jaafar ZA, El Masri D, Zmerly H, Kreidieh D, Tannir H, et al. Lifestyle Modification in Rheumatoid Arthritis: Dietary and Physical Activity Recommendations Based on Evidence. Curr Rheumatol Rev. 2019; 15: 209–14.
- Lerner A, Matthias T. Rheumatoid arthritis-celiac disease relationship: Joints get that gut feeling. Autoimmun Rev. 2015; 14: 1038–47.
- Hartmann AM, Dell’oro M, Kessler CS, Schumann D, Steckhan N, Jeitler M, et al. Efficacy of therapeutic fasting and plant-based diet in patients with rheumatoid arthritis (NutriFast): study protocol for a randomised controlled clinical trial. BMJ Open. 2021; 11: e047758.
- Venetsanopoulou AI, Voulgari P V., Drosos AA. Fasting mimicking diets: A literature review of their impact on inflammatory arthritis. Mediterr J Rheumatol. 2020; 30: 201.
- Ben Nessib D, Maatallah K, Ferjani H, Triki W, Kaffel D, Hamdi W. Sustainable positive effects of Ramadan intermittent fasting in rheumatoid arthritis. Clin Rheumatol. 2022; 41: 399-403.
- Iwashige K, Kouda K, Kouda M, Horiuchi K, Takahashi M, Nagano A, et al. Calorie restricted diet and urinary pentosidine in patients with rheumatoid arthritis. J Physiol Anthropol Appl Human Sci. 2004; 23: 19–24.
- McDougall J, Bruce B, Spiller G, Westerdahl J, McDougall M. Effects of a Very Low-Fat, Vegan Diet in Subjects with Rheumatoid Arthritis. J Altern Complement Med. 2004; 8: 71–5.
- Kjeldsen-Kragh J, Borchgrevink CF, Laerum E, Haugen M, Eek M, F o rre O, et al. Controlled trial of fasting and one-year vegetarian diet in rheumatoid arthritis. Lancet. 1991; 338: 899–902.
- Hafström I, Ringertz B, Spångberg A, Von Zweigbergk L, Brannemark S, Nylander I, et al. A vegan diet free of gluten improves the signs and symptoms of rheumatoid arthritis: The effects on arthritis correlate with a reduction in antibodies to food antigens. Rheumatology. 2001; 40: 1175–9.
- Al-Okbi SY. Nutraceuticals of anti-inflammatory activity as complementary therapy for rheumatoid arthritis. Toxicol Ind Health. 2014; 30: 738–49.
- Johansson K, Askling J, Alfredsson L, Di Giuseppe D. Mediterranean diet and risk of rheumatoid arthritis: a population-based case-control study. Arthritis Res Ther. 2018; 20: 175.
- Diamanti AP, Panebianco C, Salerno G, Di Rosa R, Salemi S, Sorgi ML, et al. Impact of Mediterranean Diet on Disease Activity and Gut Microbiota Composition of Rheumatoid Arthritis Patients. Microorganisms. 2020; 8: 1989.
- Forsyth C, Kouvari M, D’Cunha NM, Georgousopoulou EN, Panagiotakos DB, Mellor DD, et al. The effects of the Mediterranean diet on rheumatoid arthritis prevention and treatment: a systematic review of human prospective studies. Rheumatol Int. 2018; 38: 737–47.
- Vranou P, Gkoutzourelas A, Athanatou D, Zafiriou E, Grammatikopoulou MG, et al. Let Food Be Thy Medicine: The Case of The Mediterranean Diet in Rheumatoid Arthritis. Mediterr J Rheumatol. 2020; 31: 325.
- Mazzucca CB, Raineri D, Cappellano G, Chiocchetti A. How to Tackle the Relationship between Autoimmune Diseases and Diet: Well Begun Is Half-Done. Nutrients. 2021; 13: 3956.
- Sabença C, Ribeiro M, de Sousa T, Poeta P, Bagulho AS, Igrejas G. Wheat/Gluten-Related Disorders and Gluten-Free Diet Misconceptions: A Review. Foods. 2021; 10: 1765.
- Passali M, Josefsen K, Frederiksen JL, Antvorskov JC. Current Evidence on the Efficacy of Gluten-Free Diets in Multiple Sclerosis, Psoriasis, Type 1 Diabetes and Autoimmune Thyroid Diseases. Nutrients. 2020; 12: 2316.
- Gardner JA, Johnson RK, Dong F, Hoffman M, Steck AK, Frohnert BI, et al. Gluten intake and risk of thyroid peroxidase autoantibodies in the Diabetes Autoimmunity Study In the Young (DAISY). Endocrine. 2020; 70: 331–7.
- Verdu EF. Editorial: Can gluten contribute to irritable bowel syndrome. Am J Gastroenterol. 2011; 106: 516–8.
- Reuzé A, Delvert R, Perrin L, Benamouzig R, Sabaté JM, Bouchoucha M, et al. Association between Self-Reported Gluten Avoidance and Irritable Bowel Syndrome: Findings of the Nutri Net-Sant & eacute; Study. Nutrients. 2021; 13: 4147.
- Patel NV. Let Food Be Thy Medicine: Diet and Supplements in Irritable Bowel Syndrome. Clin Exp Gastroenterol. 2021; 14: 377–84.
- Ehteshami M, Shakerhosseini R, Sedaghat F, Hedayati M, Eini-Zinab H, Hekmatdoost A. The Effect of Gluten Free Diet on Components of Metabolic Syndrome: A Randomized Clinical Trial. Asian Pac J Cancer Prev. 2018; 19: 2979–84.
- Emilsson L, Semrad CE. Obesity, Metabolic Syndrome, and Cardiac Risk Factors: Going Gluten-Free, for Better or Worse? Dig Dis Sci. 2017; 62: 2215–6.
- Silva RB, Rodrigues é, Coelho BS, Andrade K, Fonseca L, Fernandes-Braga W, et al. Inconsistent effects of gluten on obesity: is there a role for the haptoglobin isoforms? Clin Nutr ESPEN. 2020; 40: 269–76.
- Weaver KN, Herfarth H. Gluten-Free Diet in IBD: Time for a Recommendation? Mol Nutr Food Res. 2021; 65: 1901274.
- Niland B, Cash BD. Health Benefits and Adverse Effects of a Gluten-Free Diet in Non–Celiac Disease Patients. Gastroenterol Hepatol (NY). 2018; 14: 82.
- Jansson-Knodell CL, Rubio-Tapia A. The fashionable gluten-free diet—wear with caution. Am J Clin Nutr. 2021; 113: 491–2.
- Littlejohns TJ, Chong AY, Allen NE, Arnold M, Bradbury KE, Mentzer AJ, et al. Genetic, lifestyle, and health-related characteristics of adults without celiac disease who follow a gluten-free diet: a population-based study of 124,447 participants. Am J Clin Nutr. 2021; 113: 622–9.
- Jønsson IM, Møller GL, Pærregaard A. Gluten-free diet is for some a necessity, for others a lifestyle. Ugeskr Laeger. 2017; 179: V09160636.
- Melini V, Melini F. Gluten-Free Diet: Gaps and Needs for a Healthier Diet. Nutrients. 2019; 11: 170.
- Newberry C, McKnight L, Sarav M, Pickett-Blakely O. Going Gluten Free: the History and Nutritional Implications of Today’s Most Popular Diet. Curr Gastroenterol Rep. 2017; 19.
- Pearlman M, Casey L. Who Should Be Gluten-Free? A Review for the General Practitioner. Med Clin North Am. 2019; 103: 89–99.
- Buchman AL. Celiac Disease, Gluten-Free, and Today’s Fashionista. Gastroenterol Clin North Am. 2019; 48: xiii–xiv.
- Miranda J, Lasa A, Bustamante MA, Churruca I, Simon E. Nutritional differences between a gluten-free diet and a diet containing equivalent products with gluten. Plant Foods Hum Nutr. 2014; 69: 182–7.
- Sue A, Dehlsen K, Ooi CY. Paediatric Patients with Coeliac Disease on a Gluten-Free Diet: Nutritional Adequacy and Macro- and Micronutrient Imbalances. Curr Gastroenterol Rep. 2018; 20: 2.
- Dennis M, Lee AR, McCarthy T. Nutritional Considerations of the Gluten-Free Diet. Gastroenterol Clin North Am. 2019; 48: 53–72.
- Cardo A, Churruca I, Lasa A, Navarro V, Vázquez-Polo M, Perez-Junkera G, et al. Nutritional Imbalances in Adult Celiac Patients Following a Gluten-Free Diet. Nutrients. 2021; 13: 2877.
- Vereczkei Z, Farkas N, Hegyi P, Imrei M, Földi M, Szakács Z, et al. It Is High Time for Personalized Dietary Counseling in Celiac Disease: A Systematic Review and Meta-Analysis on Body Composition. Nutrients. 2021; 13: 2947.
- Khairuddin MAN, Lasekan O. Gluten-Free Cereal Products and Beverages: A Review of Their Health Benefits in the Last Five Years. Foods. 2021; 10: 2523.
- Lerner A, Matthias T. Gluten-free diet tough alley in torrid time. Int J Celiac Dis. 2017; 5: 50–5.
- Lerner A, Matthias T. The Yin and Yang of dietary gluten transgressions in real-life scenarios of celiac patients. BMC Med. 2020; 18: 70.
- Samasca G, Lerner A, Girbovan A, Sur G, Lupan I, Makovicky P, et al. Challenges in gluten-free diet in coeliac disease: Prague consensus. Eur J Clin Invest. 2017; 47: 394–7.
- Molberg ø, Sollid LM. A gut feeling for joint inflammation - using coeliac disease to understand rheumatoid arthritis. Trends Immunol. 2006; 27: 188–94.
- Elsouri K, Arboleda V, Heiser S, Kesselman MM, Demory Beckler M. Microbiome in Rheumatoid Arthritis and Celiac Disease: A Friend or Foe. Cureus. 2021; 13: e15543.
- Fayyaz B, Gunawan F, Rehman HJ. ‘Preclinical’ rheumatoid arthritis in patients with celiac disease: A cross-sectional study. J Community Hosp Intern Med Perspect. 2019; 9: 86–91.
- Craig E, Cappelli LC. Gastrointestinal and Hepatic Disease in Rheumatoid Arthritis. Rheum Dis Clin North Am. 2018; 44: 89–111.
- lerner A, Wusterhausen P, Ramesh A, Lopez F, Matthias T. The Gut Feeling of the Joints: Celiac Disease and Rheumatoid Arthritis Are Related. Int J Celiac Dis. 2019; 7: 21–5.
- Reif S, Lerner A. Tissue transglutaminase - The key player in celiac disease: A review. Autoimmun Rev. 2004; 3: 40–5.
- De Vos M, Mielants H, Cuvelier C, Elewaut A, Veys E. Long-term evolution of gut inflammation in patients with spondyloarthropathy. Gastroenterology. 1996; 110: 1696–703.
- Ebert EC, Hagspiel KD. Gastrointestinal and hepatic manifestations of rheumatoid arthritis. Dig Dis Sci. 2011; 56: 295–302.
- Horta-Baas G, Romero-Figueroa MDS, Montiel-Jarquín AJ, Pizano-Zárate ML, García-Mena J, Ramírez-Durán N. Intestinal Dysbiosis and Rheumatoid Arthritis: A Link between Gut Microbiota and the Pathogenesis of Rheumatoid Arthritis. J Immunol Res. 2017; 2017: 4835189.
- Hecquet S, Totoson P, Martin H, Prati C, Wendling D, Demougeot C, et al. Intestinal permeability in spondyloarthritis and rheumatoid arthritis: A systematic review of the literature. Semin Arthritis Rheum. 2021; 51: 712–8.
- Lerner A, Matthias T, Wusterhausen P. Autoimmunity in celiac disease: Extra-intestinal manifestations. Autoimmun Rev. 2019; 18: 241–6.
- Valitutti F, Fasano A. Breaking Down Barriers: How Understanding Celiac Disease Pathogenesis Informed the Development of Novel Treatments. Dig Dis Sci. 2019; 64: 1748–58.
- Isasi C, Tejerina E, Morán LM. Non-celiac Gluten Sensitivity and Rheumatic Diseases. Reumatol Clínica (English Ed. 2016; 12: 4–10.
- Mahroum N, Zoubi M, Lavine N, Ohayon A, Amital H, Shoenfeld Y. The mosaic of autoimmunity - A taste for more. The 12th international congress of autoimmunity 2021 (AUTO12) virtual. Autoimmun Rev. 2021; 20: 102945.
- SHATIN R. PRELIMINARY REPORT OF THE TREATMENT OF RHEUMATOID ARTHRITIS WITH HIGH PROTEIN GLUTEN-FREE DIET AND SUPPLEMENTS. Med J Aust. 1964; 16: 169–72.
- Elkan AC, Sjöberg B, Kolsrud B, Ringertz B, Hafström I, Frostegård J. Gluten-free vegan diet induces decreased LDL and oxidized LDL levels and raised atheroprotective natural antibodies against phosphorylcholine in patients with rheumatoid arthritis: A randomized study. Arthritis Res Ther. 2008; 10: 1–8.
- Palmieri B, Vadalà M, Laurino C. Gluten-free diet in non-celiac patients: beliefs, truths, advantages and disadvantages. Minerva Gastroenterol Dietol. 2019; 65: 153–62.
- Badsha H. Role of Diet in Influencing Rheumatoid Arthritis Disease Activity. Open Rheumatol J. 2018; 12: 19–28.
- Guagnano MT, D’angelo C, Caniglia D, Di Giovanni P, Celletti E, Sabatini E, et al. Improvement of Inflammation and Pain after Three Months’ Exclusion Diet in Rheumatoid Arthritis Patients. Nutrients. 2021; 13: 3535.
- Bruzzese V, Scolieri P, Pepe J. Efficacy of gluten-free diet in patients with rheumatoid arthritis. Reumatismo. 2021; 72: 213–7.
- Binder HJ, O’brien WM, Spiro HM, Hollingsworth JW. Gluten and the small intestine in rheumatoid arthritis. JAMA. 1966; 195: 857–8.
- Bischoff SC, Barbara G, Buurman W, Ockhuizen T, Schulzke JD, Serino M, et al. Intestinal permeability--a new target for disease prevention and therapy. BMC Gastroenterol. 2014; 14: 189.
- Brandl C, Bucci L, Schett G, Zaiss MM. Crossing the barriers: Revisiting the gut feeling in rheumatoid arthritis. Eur J Immunol. 2021; 51: 798–810.
- Kinashi Y, Hase K. Partners in Leaky Gut Syndrome: Intestinal Dysbiosis and Autoimmunity. Front Immunol. 2021; 12: 1390.
- Singh RK, Chang HW, Yan D, Lee KM, Ucmak D, Wong K, et al. Influence of diet on the gut microbiome and implications for human health. J Transl Med. 2017; 15: 1–17.
- Hills RD, Pontefract BA, Mishcon HR, Black CA, Sutton SC, Theberge CR. Gut Microbiome: Profound Implications for Diet and Disease. Nutrients. 2019; 11: 1613.
- Rinninella E, Cintoni M, Raoul P, Lopetuso LR, Scaldaferri F, Pulcini G, et al. Food Components and Dietary Habits: Keys for a Healthy Gut Microbiota Composition. Nutrients. 2019; 11: 2393.
- Potrykus M, Czaja-Stolc S, Stankiewicz M, Kaska L, Malgorzewicz S. Intestinal Microbiota as a Contributor to Chronic Inflammation and Its Potential Modifications. Nutrients. 2021; 13: 3839.
- Sanz Y. Microbiome and Gluten. Ann Nutr Metab. 2015; 67: 28–41.
- Wu X, Qian L, Liu K, Wu J, Shan Z. Gastrointestinal microbiome and gluten in celiac disease. Ann Med. 2021; 53: 1797–805.
- Mohan M, Chow CET, Ryan CN, Chan LS, Dufour J, Aye PP, et al. Dietary Gluten-Induced Gut Dysbiosis Is Accompanied by Selective Upregulation of microRNAs with Intestinal Tight Junction and Bacteria-Binding Motifs in Rhesus Macaque Model of Celiac Disease. Nutrients. 2016; 8: 684.
- Caio G, Lungaro L, Segata N, Guarino M, Zoli G, Volta U, et al. Effect of gluten-free diet on gut microbiota composition in patients with celiac disease and non-celiac gluten/wheat sensitivity. Nutrients. 2020; 12: 1–23.
- Hansen N, Lipp M, Vogelgsang J, Vukovich R, Zindler T, Luedecke D, et al. Autoantibody-associated psychiatric symptoms and syndromes in adults: A narrative review and proposed diagnostic approach. Brain, Behav Immun - Heal. 2020; 9: 100154.
- Lerner A, Patricia J, Matthias T. Nutrients, Bugs and Us: The Short-chain Fatty Acids Story in Celiac Disease. Int J Celiac Dis. 2016; 4: 92–4.
- Cai Y, Folkerts J, Folkerts G, Maurer M, Braber S. Microbiota-dependent and -independent effects of dietary fibre on human health. Br J Pharmacol. 2020; 177: 1363–81.
- Ríos-Covián D, Ruas-Madiedo P, Margolles A, Gueimonde M, De los Reyes-Gavilán CG, Salazar N. Intestinal short chain fatty acids and their link with diet and human health. Front Microbiol. 2016; 7: 185.
- Tjellström B, Högberg L, Stenhammar L, Fälth-Magnusson K, Magnusson KE, Norin E, et al. Faecal short-chain fatty acid pattern in childhood coeliac disease is normalised after more than one year’s gluten-free diet. Microb Ecol Health Dis. 2013; 24.
- Häger J, Bang H, Hagen M, Frech M, Träger P, Sokolova M V., et al. The role of dietary fiber in rheumatoid arthritis patients: A feasibility study. Nutrients. 2019; 11: 2392.
- Dourado E, Ferro M, Guerreiro CS, Fonseca JE. Diet as a modulator of intestinal microbiota in rheumatoid arthritis. Nutrients. 2020; 12: 1–19.
- Casas R, Sacanella E, Estruch R. The immune protective effect of the Mediterranean diet against chronic low-grade inflammatory diseases. Endocr Metab Immune Disord Drug Targets. 2014; 14: 245–54.
- Nerurkar L, Siebert S, McInnes IB, Cavanagh J. Rheumatoid arthritis and depression: an inflammatory perspective. The Lancet Psychiatry. 2019; 6: 164–73.
- Sturgeon JA, Finan PH, Zautra AJ. Affective disturbance in rheumatoid arthritis: Psychological and disease-related pathways. Nat Rev Rheumatol. 2016; 12: 532–42.
- Pongratz G, Lowin T, Sewerin P, Zaucke F, Jenei-Lanzl Z, Pauly T, et al. Tryptophan metabolism in rheumatoid arthritis is associated with rheumatoid factor and predicts joint pathology evaluated by the Rheumatoid Arthritis MRI Score (RAMRIS). Clin Exp Rheumatol. 2019; 37: 450–7.
- Pynnönen PA, Isometsä ET, Verkasalo MA, Kähkönen SA, Sipilä I, Savilahti E, et al. Gluten-free diet may alleviate depressive and behavioural symptoms in adolescents with coeliac disease: a prospective follow-up case-series study. BMC Psychiatry. 2005; 5: 14.
- Addolorato G, Capristo E, Ghittoni G, Valeri C, Mascianà R, Ancona C, et al. Anxiety but not depression decreases in coeliac patients after one-year gluten-free diet: a longitudinal study. Scand J Gastroenterol. 2001; 36: 502–6.
- Odineal DD, Gershwin ME. The Epidemiology and Clinical Manifestations of Autoimmunity in Selective IgA Deficiency. Clin Rev Allergy Immunol. 2020; 58: 107–33.
- Tan J, McKenzie C, Vuillermin PJ, Goverse G, Vinuesa CG, Mebius RE, et al. Dietary Fiber and Bacterial SCFA Enhance Oral Tolerance and Protect against Food Allergy through Diverse Cellular Pathways. Cell Rep. 2016; 15: 2809–24.
- Siddiqui MT, Cresci GA. The Immunomodulatory Functions of Butyrate. J Inflamm Res. 2021; 14: 6025–41.
- Vojdani A. Reaction of food-specific antibodies with different tissue antigens. Int J Food Sci Technol. 2020; 55: 1800–15.
- Vojdani A, Tarash I. Cross-Reaction between Gliadin and Different Food and Tissue Antigens. Food Nutr Sci. 2013; 04: 20–32.
- Vojdani A. The Characterization of the Repertoire of Wheat Antigens and Peptides Involved in the Humoral Immune Responses in Patients with Gluten Sensitivity and Crohn’s Disease. ISRN Allergy. 2011; 2011 :1–12.
- Vojdani A, Gushgari LR, Vojdani E. Interaction between food antigens and the immune system: Association with autoimmune disorders. Autoimmun Rev. 2020; 19: 102459.
- Vojdani A, Lerner A, Vojdani E. Cross-Reactivity and Sequence Homology between Al-Pha-Synuclein and Food Products: A Step Further for Parkinson’s Disease Synucleinopathy. Cells. 2021; 10: 1111.
- Hvatum M, Kanerud L, Hällgren R, Brandtzaeg P. The gut-joint axis: Cross reactive food antibodies in rheumatoid arthritis. Gut. 2006; 55: 1240–7.
- Yang Y, Deshpande P, Krishna K, Ranganathan V, Jayaraman V, Wang T, et al. Overlap of characteristic serological antibodies in rheumatoid arthritis and wheat-related disorders. Dis Markers. 2019; 2019: 4089179.
- Lerner A, Blank M, Lahat N, Shoenfeld Y. Increased prevalence of autoantibodies in celiac disease. Dig Dis Sci. 1998; 43: 723–6.
- Shaoul R, Lerner A. Associated autoantibodies in celiac disease. Autoimmun Rev. 2007; 6: 559–65.
- Lerner A, Neidhöfer S, Matthias T. Transglutaminase 2 and Anti Transglutaminase 2 Autoantibodies in Celiac Disease and Beyond: TG2 Double-Edged Sword: Gut and Extraintestinal Involvement. Immunome Res. 2015; 11: 1–4.
- Lauzier A, Charbonneau M, Paquette M, Harper K, Dubois CM. Transglutaminase 2 cross-linking activity is linked to invadopodia formation and cartilage breakdown in arthritis. Arthritis Res Ther. 2012; 14: R159.
- Orlandi A, Oliva F, Taurisano G, Candi E, Di Lascio A, Melino G, et al. Transglutaminase-2 differently regulates cartilage destruction and osteophyte formation in a surgical model of osteoarthritis. Amino Acids. 2009; 36: 755–63.
- Dzhambazov B, Lindh I, Engström å, Holmdahl R. Tissue transglutaminase enhances collagen type II-induced arthritis and modifies the immunodominant T-cell epitope CII260-270. Eur J Immunol. 2009; 39: 2412–23.
- Tarantino U, Ferlosio A, Arcuri G, Spagnoli LG, Orlandi A. Transglutaminase 2 as a biomarker of osteoarthritis: an update. Amino Acids. 2013; 44: 199–207.
- Lerner A, Matthias T. Processed food additive microbial transglutaminase and its cross-linked gliadin complexes are potential public health concerns in celiac disease. Int J Mol Sci. 2020; 21: 1127.
- Tucker NT, Barghuthy FS, Prihoda TJ, Kumar V, Lerner A, Lebenthal E. Antigliadin antibodies detected by enzyme-linked immunosorbent assay as a marker of childhood celiac disease. J Pediatr. 1988; 113: 286–9.
- Lerner A, Lebenthal E. The controversy of the use of anti-gluten antibody (AGA) as a diagnostic tool in celiac disease. J Pediatr Gastroenterol Nutr. 1991; 12: 407–9.
- Kumar V, Lerner A, Jain N, Beutner EH. Are antigliadin antibodies specific for celiac disease? J Pediatr Gastroenterol Nutr. 1984; 3: 815.
- Kumar V, Jain N, Lerner A, Beutner EH, Chorzelski TP, Lebenthal E. Comparative studies of different gliadin preparations in detecting antigliadin antibodies. J Pediatr Gastroenterol Nutr. 1986; 5: 730–4.
- Lerner A, Ramesh A, Matthias T. Serologic Diagnosis of Celiac Disease: New Biomarkers. Gastroenterol Clin North Am. 2019; 48: 307–17.
- Lerner A, Neidhöfer S, Matthias T. Serological Markers and/or Intestinal Biopsies in the Case-finding of Celiac Disease. Int J Celiac Dis. 2015; 3: 53–5.
- Agardh D, Matthias T, Wusterhausen P, Neidhöfer S, Heller A, Lerner A. Antibodies against neo-epitope of microbial and human transglutaminase complexes as biomarkers of childhood celiac disease. Clin Exp Immunol. 2020; 199: 294–302.
- Lerner A, Jeremias P, Neidhöfer S, Matthias T. Comparison of the Reliability of 17 Celiac Disease Associated Bio-Markers to Reflect Intestinal Damage. J Clin Cell Immunol. 2017; 8: 486.
- Biesiekierski JR, Newnham ED, Irving PM, Barrett JS, Haines M, Doecke JD, et al. Gluten Causes gastrointestinal symptoms in subjects without celiac disease: A double-blind randomized placebo-controlled trial. Am J Gastroenterol. 2011; 106: 508–14.
- Lerner A, Matthias T. Gluten and Autoimmunogenesis. Mosaic Autoimmun Nov Factors Autoimmune Dis. 2019; 315–21.
- Samasca G, Ajay R, Sur D, Aldea C, Sur L, Floca E, et al. Polyautoimmunity - The missing ingredient. Autoimmun Rev 2018; 17: 840–1.
- Philippou E, Petersson SD, Rodomar C, Nikiphorou E. Rheumatoid arthritis and dietary interventions: Systematic review of clinical trials. Nutr Rev. 2021; 79: 410–28.
- Freeman HJ. Dietary compliance in celiac disease. World J Gastroenterol. 2017; 23: 2635.
- Cohen IS, Day AS, Shaoul R. Gluten in Celiac Disease-More or Less? Rambam Maimonides Med J. 2019; 10: e0007.
- Natter S, Granditsch G, Reichel GL, Baghestanian M, Valent P, Elfman L, et al. IgA cross-reactivity between a nuclear autoantigen and wheat proteins suggests molecular mimicry as a possible pathomechanism in celiac disease. Eur J Immunol. 2001; 31: 918–28.