
Research Article
Int J Nutr Sci. 2023; 8(2): 1075.
Safety and Efficacy of Vitamin D Supplementation in Patients with Allergic Rhinitis: Randomized Double Blind Placebo Control Study
Ortega-Jordá RE; Rivero YD*; López GAI; Ríos LJJ; Papaqui TJS; Caballero LCG; álvarez CJA
Servicio de Alergología e Inmunología Clínica, Hospital Universitario de Puebla, Puebla, México
*Corresponding author: Rivero YD Servicio de Alergología e Inmunología Clínica, Hospital Universitario de Puebla. Calle 25 poniente 1301 Col. Volcanes, C.P.72410, Puebla, México. Tel: 52 222 708 6434 Email: driveroy@hotmail.com; daniela.riveroy@correo.buap.mx
Received: June 10, 2023 Accepted: July 10, 2023 Published: July 17, 2023
Introduction
Allergic rhinitis is an inflammatory disease of the nasal mucosa mediated by immunoglobulin E, caused by exposure to allergens and characterized by the presence of one or more of the following symptoms: nasal itching, rhinorrhea, nasal obstruction, and sneezing. With a great impact on both health and quality of life deterioration, as well as on productivity, involving high costs [1]. Its worldwide prevalence has increased over the last 30 years, currently reported at 15-25% [2].
Vitamin D is produced when the cholesterol precursor, 7-dehydrocholesterol, is exposed to UVB rays in the liver, where it is converted to 25-OH-D. Kidneys, immune cells and keratinocytes express the CYP27B1 enzyme, which mediates the resulting additional hydroxylation to 1, 25-OH-D (calcitriol). In the innate immune system, 1, 25-OH-D increases chemotaxis, autophagy, phagolysosome fusion, defensin and cathelicidin production by macrophages, monocytes, and keratinocytes. In the adaptive immune system, it causes inhibition of the production of proinflammatory cytokines. In monocytes and macrophages, it decreases the expression of major histocompatibility complex class II (MHC II) molecules, CD80/86, decreasing antigen presentation. In dendritic cells, it inhibits the production of IL-12 and IL-23 and stimulates the production of IL-10. It modulates the response of T lymphocytes, decreasing their proliferation, inducing apoptosis of autoreactive lymphocytes, and increasing regulatory T lymphocytes, FoxP3 and cytotoxic T lymphocyte antigen (CTLA 4) [3-6] (Figure 1).
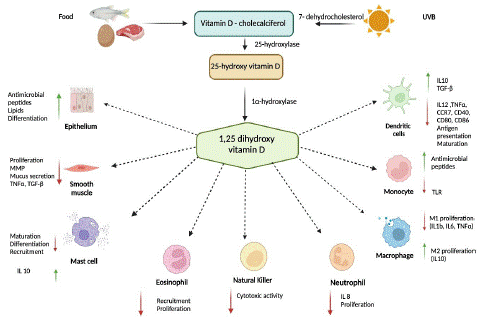
Figure 1: Vitamin D is a fat-soluble vitamin. Few foods naturally contain vitamin D, so dermal synthesis after UVB radiation remains the major route to obtain vitamin D. 7-dehydrocholesterol in skin epidermal cells is converted after UVB radiation into pre-vitamin D. Both vitamin D3 and undergo further enzymatic conversion to its active forms. First, it undergoes 25-hydroxylation in liver to 25(OH)D (calcidiol), then it is converted in kidneys through 1-alpha-hydroxylation to its most active form, 1,25(OH)2D (calcitriol).
The prevalence of vitamin D deficiency in children and adults has increased exponentially in recent years, making it a global epidemic, related to overweight, sedentary lifestyles, decreased intake, gastrointestinal diseases, deficient absorption, renal and hepatic pathologies. Guidelines published by the Endocrine Society on vitamin D defined vitamin D deficiency as 1,25-OH-D <20ng/mL, insufficiency as 21-29ng/mL, and sufficiency as at least 30ng/mL for maximum skeletal muscle health [7]. In Europe, it is estimated that more than 40% of the population has deficiency, 13% being severe. In the U.S. population aged 1 to 21 years, 61% had insufficiency and 9% deficiency [4]. In Mexico, the prevalence of deficiency and insufficiency in children is 16% and 23% respectively, while in the adult population, deficiency was 9.8% and insufficiency 20% [8].
There is currently no international consensus on the supplementation of this vitamin. The dose of 25mcg (1000 IU) increases the serum level of 1,25-OH-D by an average of 15 to 25 nmol/L [9]. According to the Endocrine Society, the daily dose of 10,000 IU of vitamin D should not be exceeded; even with this dose, no adverse reactions occurred in healthy adults [10]. Cholecalciferol supplementation has been shown in several studies to have a positive impact on the clinical outcome of the patient. A study in Australia showed that low vitamin D levels at age 6 years were predictive of increased risk of asthma and sensitization to aeroallergens at age 14 years [11]. Balakrishnan and cols reported that low levels of vitamin D correlated with disease severity, and after supplementation there was a decrease in the TNSS score, an improvement attributed to the immunomodulatory effects of vitamin D [12].
At the University Hospital of Puebla, vitamin D supplementation was evaluated in patients with allergic rhinitis with 6000 IU weekly for adults and 5000 IU weekly for children, with improvement in the severity of symptomatology but without demonstrating an increase in serum 25-OH-D concentrations [13].
Methods
Randomized, double-blind, placebo-controlled, experimental, double-blind, placebo-controlled study involving patients aged 10 to 40 years, who attended the Allergy and Clinical Immunology service of the University Hospital of Puebla, Mexico, during the period from October 2020 to July 2021, with a diagnosis of allergic rhinitis, confirmed by skin tests or specific serum IgE. Patients with pathologies that alter the metabolism, absorption or excretion of cholecalciferol, patients pregnant or breastfeeding, and patients under treatment with corticosteroids, antiepileptic drugs and vitamin D supplements in the previous three months were excluded from the study.
Patients were enrolled and randomized using Excel for Microsoft 365, resulting in an evenly distributed randomized true number with each recalculation, after which they were assigned to each group (active or placebo). Patients in the active group received pharmacological treatment with fluticasone furoate 27.5mcg every 12 hours for two months, subcutaneous specific immunotherapy and cholecalciferol: 5000IU daily for 8 weeks.
On the other hand, patients in the control group received the same treatment, substituting placebo for cholecalciferol.
The severity of allergic rhinitis symptoms was assessed at 0, 30 and 60 days by applying the TNSS, which is the sum of 4 symptom scores evaluated by the patients, for rhinorrhea, nasal congestion, nasal itching, and sneezing. Being mild a total of less than 5 points, moderate from 6 to 10 and severe from 11 to 15 points.
Patients' serum 25(OH)D levels were quantified at baseline and at the end of the study by particle chemiluminescent immunoassay with ARCHITECT i1000SR equipment (Figure 2).
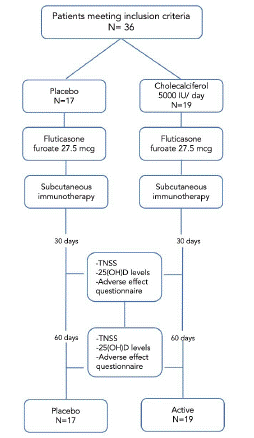
Figure 2: Screening, randomization and follow-up of the patients.
Statistical Analysis
Data were collected and organized in Microsoft Excel. Descriptive summary statistics were used by determining averages, percentages, and frequencies. A comparison of two averages was considered with a bilateral hypothesis, 95% confidence level, and an expected loss ratio of 20%. Statistical analysis of the data was performed with the IBM® SPSS® Statistics 28 program by means of a comparison of means using Student's t-test considering a significance value <0.05.
All procedures during the present study were performed in accordance with the ethical norms, the Regulations of the General Law of Health, on Health Research and the Declaration of Helsinki. All participants expressed their authorization through informed consent and assent.
Results
Thirty-six patients with allergic rhinitis and vitamin D deficiency and insufficiency were included. The experimental group, which received vitamin D3, included 19 patients (52.78%), 10 males and 9 females aged 10-40 years (SD 12.2). The control group included 17 patients (47.25%), 8 men and 9 women between 13 and 40 years of age (SD 9.6). When comparing both groups with respect to gender and age, no statistically significant differences were found between them (p>0.05).
The mean value of serum 25(OH)D levels at baseline in the placebo group was 20.32ng/ml and in the active group 18.10 ng/ml. At 8 weeks of follow-up, the placebo group presented a mean vitamin D level of 21.38 ng/ml even with deficiency and in the active group it was 44.94 ng/ml reaching sufficiency. A statistically significant difference was shown between the initial value and at 8 weeks after treatment, with a mean increase of 26.83. (p=<0.05) (Figure 3). Within the active group, patients between 10 and 20 years old presented a mean serum 25(OH)D elevation of 34.6 ng/ml, while in the 20 to 40 years old age group the mean was 19.7 ng/ml.
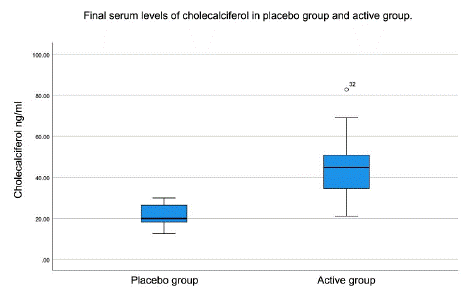
Figure 3: Final serum levels of cholecalciferol in placebo group and active group.
The mean Nasal Symptom Score (TNSS) at baseline was 6.47 in the placebo group and 7.16 in the active group. Consequently, no significant differences were observed between the two groups with respect to this variable (p=0.277). The mean total score at the end of the study was in the placebo group 3.94 and in the active group 2.58, observing a statistically significant decrease in total nasal symptom scores in the active group compared to the placebo group p<0.029 (Table 1 & Figure 4).
Variable
Placebo group Mean ± SD
Active group Mean ± SD
P value
Initial TNSS
6,47±3,002
7,16±3,79
>0.05
Final TNSS
3,94±1,98
2,58±2,16
<0.05
Initial Vitamin D (ng/dl)
20,32±5,24
18,10±7,78
0.277
Final Vitamin D (ng/dl)
21,38±5,58
44,94±16,2
<0.05
Table 1: Comparative data between the active and placebo group pre and post vitamin d supplementation.
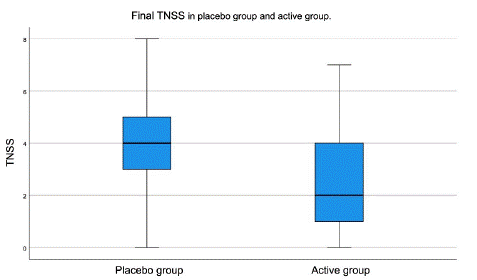
Figure 4: Final TNSS score in placebo group and active group.
Figure 5: Score plot of the sPLS-DA model based on the TNSS score in active group and placebo group.
Discussion
In recent years, multiple investigations have been performed analyzing the relationship between serum vitamin D value and the incidence and severity of allergic diseases.
There are multiple clinical trials evaluating the effects of cholecalciferol supplementation and correction of cholecalciferol levels with doses ranging from 800 IU to 7000 IU daily, showing efficacy and safety at low doses (800-1000 IU). (14-22). However, the safety and incidence of adverse effects associated with it are rarely analyzed.
In the present study, the efficacy and safety of vitamin D supplementation in children older than 10 years old and adults with 5,000 units daily for 60 days was analyzed, none of the participants presented adverse events associated with the supplementation, in addition, at the end of the study, all participants within the active group, significantly decreased their symptoms and reached sufficiency levels with the mentioned dose without presenting toxicity levels (>150ng/ml) (Figure 4). These results are in agreement with the proposal of Khan et al., that a daily dose of 4000 to 5000 IU of vitamin D2 or vitamin D3 per day is required to reach levels of 40 to 60 ng/ml 23, and similarly with the safety studies of Bischoff-Ferrari et al. where there were no adverse reactions associated with intakes of up to 50,000IU per day. (24-26). Another study previously conducted at the University Hospital of Puebla, proved the clinical efficacy of supplementation with 5000 IU weekly for children and 6000 IU for adults for 8 weeks, however no significant sufficiency levels were achieved in the experimental group [13].
Bakhshaee et al. demonstrated that supplementation with 50,000 IU cholecalciferol every week for 2 months in patients with AR and vitamin D deficiency resulted in a significant difference between the initial and final nasal symptom severity score measured by TNSS (P=0.007) [27]. Other placebo-controlled studies have used lower doses of cholecalciferol, 1000 IU daily, in shorter periods of time, 21 and 30 days, obtaining the same reduction in the total score of nasal symptoms (P<0.05) [28,29]. Patients aged 10 to 20 years old who received vitamin D supplementation showed an elevation of serum levels 1.75 times higher than patients aged 20 to 40 years old, which is consistent with that described in studies of fixed-dose supplementation in children and adults, where the elevation of serum levels is more significant in children and the elderly [3,30].
Conclusions
This study supports the evidence about the association between vitamin D deficiency and allergic rhinitis. Results showed here, remark the importance of assessing vitamin D levels in allergic rhinitis patients, to provide vitamin D supplementation along with standard treatment in deficient subjects.
References
- Passali D, Cingi C, Staffa P, Passali F, Muluk NB, et al. The International Study of the Allergic rhinitis Survey: outcomes from 4 geographical regions. Asia Pac Allergy. 2018; 8: e7.
- Brozek JL, Bousquet J, Agache I, Agarwal A, Bachert C, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines. J Allergy Clin Immunol. 2016; 140: 950-57.
- Hanel A, Carlberg C. Vitamin D and evolution: pharmacologic implications. Biochem Pharmacol. 2020; 173: 113595.
- Sassi F, Tamone C, D’Amelio P. Vitamin D: nutrient, hormone, and immunomodulator. Nutrients. 2018; 10: 1656-60.
- Martens PJ, Gysemans C, Verstuyf A, Mathieu AC. Vitamin D’s effect on immune function. Nutrients. 2020; 12: 1248-62.
- Charoenngam N, Holick MF. Immunologic effects of vitamin D on human health and disease. Nutrients. 2020; 12: 20-97.
- Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011; 96: 1911-30.
- Khayyatzadeh SS, Bagherniya M, Abdollahi Z, Ferns GA, Ghayour-Mobarhan M. What is the best solution to manage vitamin D deficiency? IUBMB Life. 2019; 71: 1190-1.
- Flores M, Sánchez-Romero LM, Macías-Lozada A, et al. Concentraciones séricas de vitamina D en niños, adolescentes y adultos mexicanos. Resultados de la ENSANUT 2006. Instituto nacional de Salúd pública. 2011; 1: 1-27.
- Amrein K, Scherkl M, Hoffmann M, et al. Vitamin D deficiency 2.0: an update on the current status worldwide. Eur J Clin Nutr. 2020; 85: 1-16.
- Agostoni C, Bresson JL, Fairweather-Tait S, et al. Scientific Opinion on the Tolerable Upper Intake Level of vitamin D. EFSA J. 2012; 10: 1-45.
- Hollams EM. Vitamin D and atopy and asthma phenotypes in children. Curr Opin Allergy Clin Immunol. 2012; 12: 228-34.
- Balakrishnan M, Charanjeet K, Harsh V, et al. Placebo controlled trial of vitamin D supplementation in Allergic rhinitis. Eur Respir J. 2015; 46: 1-8.
- Payan-Díaz JH, Rivero-Yeverino D, López-García AI, et al. Efecto terapéutico de la suplementación de vitamina D en pacientes con rinitis alérgica: ensayo clínico aleatorizado frente a placebo [tesis] para obtener el Diploma de especialista en Alergia e Inmunología Clínica. Benemérita Universidad Autónoma de Puebla; 2020.
- Gil á, Plaza-Diaz J, Mesa MD. Vitamin D: classic and novel actions. Ann Nutr Metab. 2018; 72: 87-95.
- Hawrylowicz CM, Santos AF. Vitamin D: can the sun stop the atopic epidemic? Curr Opin Allergy Clin Immunol. 2020; 20: 181-7.
- Özdemir O, Karavaizoglu C. Role and importance of vitamin D in asthma and other allergic diseases. JAREM. 2018; 8: 1-8.
- Rosser FJ, Han YY, Forno E, Bacharier LB, Phipatanakul W, et al. Effect of vitamin D supplementation on total and allergen specific IgE in children with asthma and low vitamin D levels. J Allergy Clin Immunol. 2022; 149: 440-444.e2
- Urquiza C, Fernández de Córdova JC, Velasco A, et al. Prevalence of vitamin D deficiency and associated factors in Mexican patients with allergic rhinitis and asthma. Rev Med Hosp Gen Mex. 2020; 83: 113-9.
- Chang SW, Lee HC. Vitamin D and health - the missing vitamin in humans. Pediatr Neonatol. 2019; 60: 237-44.
- Plesa M, Gaudet M, Mogas A, Jalaleddine N, Halayko A, et al. Vitamin D3 attenuates viral-induced inflammation and fibrotic responses in bronchial smooth muscle cells. Front Immunol. 2021; 12: 715848.
- Amorim CLCG, Oliveira JM, Rodrigues A, Furlanetto KC, Pitta F. Vitamin D: association with eosinophil counts and IgE levels in children with asthma. J Bras Pneumol. 2020; 47: e20200279.
- Gaude GS, Kummarganti S, Hattiholi J. Clinical effects of vitamin D on the control of bronchial asthma-is it relevant? J Clin Diagn Res. 2020; 14: 1-4.
- Khan QJ, Fabian CJ. How I treat vitamin D deficiency. J Oncol Pract. 2010; 6: 97-101.
- Rizzoli R. Vitamin D supplementation: upper limit for safety revisited? Aging Clin Exp Res. 2021; 33: 19-24.
- Heaney RP, Davies KM, Chen TC, Holick MF, Barger-Lux MJ. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr. 2003; 77: 204-10.
- Bischoff-Ferrari HA, Shao A, Dawson-Hughes B, Hathcock J, Giovannucci E, Willett WC. Benefit-risk assessment of vitamin D supplementation. Osteoporos Int. 2010; 21: 1121-32.
- Bakhshaee M, Sharifian M, Esmatinia F, Rasoulian B, Mohebbi M. Therapeutic effect of vitamin D supplementation on allergic rhinitis. Eur Arch Otorhinolaryngol. 2019; 276: 2797-801.
- Modh D, Shah P, Thakkar B, Jain A, Joshi K, et al. Role of vitamin D supplementation in allergic rhinitis. Indian J Allergy Asthma Immunol. 2014; 28: 35-9.
- Menon B, Kaur C, Vardhan H, Yusuf Dar M. Placebo controlled trial of vitamin D supplementation in Allergic rhinitis. Research. 2016; 3: 1501-15.
- Holick MF. The vitamin D deficiency pandemic: approaches for diagnosis, treatment and prevention. Rev Endocr Metab Disord. 2017; 18: 153-65.