
Special Article: Nutrition Diet
Int J Nutr Sci. 2024; 9(1): 1082.
A Novel Synbiotic Blend of Galactooligosaccharide (GOS) and a Two-Strain Probiotic Acts Synergistically to Increase Lactate and Short-Chain Fatty Acid Production in a Short-Term Ex Vivo Colon Fermentation Model
Ghyselinck J1; Teixeira C2; Marzorati M1,3; Önning G2,4; Harthoorn L5*
1ProDigest, Technologiepark 82, 9052 Zwijnaarde, Belgium
2Probi AB, Ideongatan 1A, 223 70 Lund, Sweden
3Center of Microbial Ecology and Technology (CMET), Ghent University, 9000 Ghent, Belgium
4Biomedical Nutrition, Pure and Applied Biochemistry, Lund University, 222 00 Lund, Sweden
5Clasado Biosciences Ltd., Imperium Building, Imperial Way, Worton Grange, Reading RG2 0TD, UK
*Corresponding author: Harthoorn L Clasado Biosciences Ltd., Imperium Building, Imperial Way, Worton Grange, Reading RG2 0TD, UK. Email: lucien.harthoorn@clasado.com
Received: January 30, 2024 Accepted: March 04, 2024 Published: March 11, 2024
Abstract
Synbiotics are mixtures of prebiotics and probiotics that improve health and, when combined, can have superior benefits compared to either component alone. Ex vivo short-term colonic simulations were used to evaluate the synbiotic potential of the prebiotic Bimuno® GOS (galactooligosaccharides) and the probiotic Probi Defendum® (L. plantarum HEAL9 and Lacticaseibacillus paracasei 8700:2). Test conditions included: blank, GOS, the control probiotic Probi Digestis® (Lactiplantibacillus plantarum 299v), Probi Defendum®, Probi Digestis® + GOS, and Probi Defendum® + GOS. Stool samples from five healthy donors were used. GOS supplementation, alone or combined with either probiotic, increased gas pressure, acetate production, propionate production (numeric, non-significant), butyrate production (numeric, non-significant), and lactate production. Additionally, biomass was increased and the microbial community composition shifted, most notably demonstrated by an increase in bifidobacteria. In contrast with Probi Digestis®, Probi Defendum® was able to utilize Bimuno® GOS for growth, which highlights the substrate specificity. Probi Defendum® + GOS resulted in an increased lactogenic effect and a donor dependent increase in butyrate production relative to GOS alone, revealing a synergistic effect in ex vivo short-term colonic simulations.
Keywords: Galactooligosaccharide; Lacticaseibacillus paracasei; Lactiplantibacillus plantarum; Prebiotic; Probiotic; Synbiotic
Abbreviations: AUC: Area Under the Curve; CFU: Colony-Forming Unit; GOS: Galacto-oligosaccharide; LD: Linear Discriminant; LOQ: Limit of Quantification; MRS: Man-Rogosa-Sharpe; OD: Optical Density; PBS: Phosphate-Buffered Saline; RPM: Rotations Per Minute; SCFA: Short-Chain Fatty Acid; LEfSe: Linear Discriminant Analysis Effect Size; LDA: Linear Discriminant Analysis
Introduction
Both prebiotics and probiotics are known for their ability to provide health benefits. Prebiotics are defined as “a substrate that is selectively utilized by host microorganisms conferring a health benefit” [1]. These dietary fibers resist digestion and absorption in the small intestine but are fermented by bacteria that reside in the large intestine. A major byproduct of prebiotic digestion is Short-Chain Fatty Acids (SCFAs), which are known to have several health benefits [2,3]. SCFAs are associated with a reduction in intestinal inflammation and an increase in the integrity of the intestinal epithelial barrier [4] and are reported to be involved in immune system function and the regulation of inflammatory responses [5]. Galactooligosaccharides (GOS) are a well-studied class of prebiotics that are known for their ability to strongly stimulate bifidobacteria expansion, and to a lesser extent, the growth of Bacteroidetes and lactobacilli in the gut [6-12]. Bifidobacteria are considered highly beneficial, largely owing to their ability to produce SCFAs, which, as noted, support intestinal epithelial barrier function and immune regulation [13-16]. The prebiotic supplement, Bimuno®, contains GOS produced from the activity of galactosyltransferases from Bifidobacterium bifidum NCIMB 41170 in the presence of lactose [17]. This GOS has demonstrated a variety of prebiotic effects, including the ability to reduce the incidence and duration of traveler’s diarrhea [18,19], reduce colonization of Salmonella enterica serovar typhimurium [20], increase SCFA and lactic acid production [10,12,21], stimulate the growth of bifidobacteria and lactobacilli [6,7,10,12,21-23], to exert immunomodulatory effects [7,10], to reduce levels of unfavorable metabolites (ammonium and branched SCFAs) [12], and to reduce gastrointestinal symptoms, including flatulence, bloating, and abdominal pain [22,23].
Probiotics are defined as “live microorganisms which, when administered in adequate amounts, confer a health benefit to the host” [24]. They modify the intestinal microbiome composition, have immunomodulatory effects, are able to suppress pathogens, and stimulate the proliferation and differentiation of epithelial cells, which improves the intestinal epithelial barrier [25,26]. Probi Defendum® is a probiotic mixture consisting of Lactiplantibacillus plantarum HEAL9 and Lacticaseibacillus paracasei 8700:2. In studies of L. plantarum HEAL9, supplementation is associated with improved cognition in people who are experiencing moderate stress [27] and a reduction of inflammatory markers associated with acute stress [28]. L. paracasei 8700:2 has been shown to have antagonistic activity against Salmonella enterica subsp. enterica and Helicobacter pylori in vitro [29]. Additionally, a clinical study reported that L. paracasei 8700:2 supplementation was associated with enhanced endothelial function in participants with metabolic syndrome [30]. General health benefits reported for the Lactobacillus casei group, of which L. paracasei is a member, include enhanced brain function, enhanced intestinal barrier function, pathogen resistance, immune modulation, and anti-cancer activity [31]. When supplemented together, these probiotics have been shown to reduce the severity of the common cold in children and adults, to reduce the risk of acquiring the common cold [32-35], and to modulate the peripheral immune response in children with celiac disease [36]. Probi Digestis®, a Lactobacillus-based probiotic that consists of L. plantarum 299v, stands out as the most extensively documented of its species. It was therefore used as a probiotic control in our study. Indeed, clinical evidence on this strain shows its positive impact on gastrointestinal health and iron absorption, and the strain has been reported to affect gut microbiota composition, to inhibit pathogens, and to have immunomodulatory effects [37].
Synbiotics are mixtures of prebiotics and probiotics that improve health and, when used in combination, can have superior benefits compared to either component alone [38]. When choosing synbiotic pairings, consideration should be given to the positive effects of the prebiotic on the probiotic, ideally pairing a prebiotic that is able to improve the survival of the probiotic and stimulate its proliferation in the gastrointestinal tract. This study was conducted to evaluate the synergistic synbiotic potential of Probi Defendum® + GOS when compared with its individual components, and with another synbiotic (Probi Digestis® + GOS) to demonstrate substrate specificity. Ex vivo short-term colonic simulations were used to evaluate changes in microbial community activity and composition, following supplementation with GOS (Bimuno®), Probi Defendum®, Probi Digestis®, Probi Defendum® + GOS, or Probi Digestis® + GOS.
Materials and Methods
Fecal Samples
Stool samples were collected from five healthy adult donors (no history of antibiotic use within four months prior to stool collection, no history of chronic diseases). The fecal material was processed to 7.5% (w/v) suspensions using PBS under anaerobic conditions and mixed with a cryoprotectant [39], then aliquoted, flash frozen, and stored at –80°C in an anaerobic atmosphere.
GOS Dialysis
To simulate absorption processes during small intestinal passage, Bimuno® GOS (provided by Clasado Biosciences Ltd., Reading, UK) was dialyzed as previously described [12]. Briefly, stock solutions of GOS were prepared in water (35 g/L), added to dialysis membranes (0.5 kDa pore size), and dialyzed in a solution of NaHCO3 (3.75 g/L, pH 7.0) for 24 h to remove monosaccharides and disaccharides.
Short-Term Colonic Incubations
At the start of the experiment, individual colonic reactors were filled with 56 mL nutritional medium (PD01; ProDigest, Gent, Belgium). Next, a single dose (7 mL) of dialyzed GOS and/or probiotic agent was added to respective reactors, resulting in a GOS 3.5 g/L concentration (assuming no absorption), and 1×107 CFU/mL of probiotic agent. Finally, 7 mL of an individual fecal inoculum suspension was added, bringing the total reactor volume to 70 mL. The following six test conditions were used: (a) blank (nutritional medium only), (b) dialyzed GOS (Bimuno® GOS; 3.5 g/L), (c) L. plantarum 299v (Probi Digestis®; 1×107 CFU/mL), (d) L. plantarum 299v (1×107 CFU/mL) + dialyzed GOS (3.5 g/L) (synbiotic), (e) L. plantarum HEAL9 (5×106 CFU/mL) + L. paracasei 8700:2 (5×106 CFU/mL) (Probi Defendum®), and (f) L. plantarum HEAL9 (5×106 CFU/mL) + L. paracasei 8700:2 (5×106 CFU/mL) + dialyzed GOS (3.5 g/L) (synbiotic). Probi Digestis® and Probi Defendum® were provided by Probi AB (Lund, Sweden). Each condition was run in two technical replicates.
Microbial Metabolic Activity Analysis
Change in pH, gas pressure, SCFAs, branched SCFA, lactate, and ammonium were measured at 0 h, 6 h, 24 h, and 48 h. A Senseline F410 pH meter (ProSense, Oosterhout, The Netherlands) was used to measure changes in pH and a hand-held pressure indicator (CPH6200; Wika, Echt, The Netherlands) was used to measure gas pressure at the indicated timepoints. Acetate, propionate, and butyrate, and the branched SCFAs, isobutyrate, isovalerate, and isocaproate, were measured according to the methods of De Weirdt et al. [40]. Lactate levels were assessed according to the manufacturer’s instructions using an enzymatic assay kit from R-Biopharm (Darmstadt, Germany). The method of Tzollas et al. was used to evaluate ammonium levels [41].
Microbial Community Analysis
Samples collected at 24 h were subjected to shallow shotgun sequencing to assess microbial community composition. The Illumina Nextera XT library preparation kit, with protocol modifications, was used to prepare DNA libraries. The library was quantified with Qubit (ThermoFisher, Waltham, MA, USA). An Illumina HiSeq platform 2×150 bp was used for library sequencing. The methods of Ottensen et al. [42], Ponnusamy et al. [43], Hasan et al. [44], and Lax et al. [45] were used to analyze unassembled sequencing reads for multi-kingdom microbiome analysis and quantification of relative abundances. Briefly, we used curated genome databases together with a high-performance data-mining algorithm to rapidly disambiguate hundreds of millions of metagenomic sequence reads into the discrete microorganisms engendering the sequences. A BD FACSVerse Cell Analyzer (BD Biosciences, Franklin Lakes, NJ, USA) was used to determine total bacterial cell counts (high flow rate setting; 200 thresholds on the SYTO channel). The relative abundances of each population in a sample were multiplied with the total cell count obtained with flow cytometry, allowing for the conversion of proportional values obtained using shotgun sequencing to absolute quantities [46].
Growth in GOS
The effect of Bimuno® GOS as a carbohydrate source for the growth of L. plantarum HEALl9 and L. paracasei 8700:2 either individually or in combination, and L. plantarum 299v was assessed. Each probiotic strain was inoculated onto a standard MRS agar plate and incubated anaerobically for 2–3 days at 37°C. Bacteria from 1–2 colonies were then transferred to complete MRS broth with 2% glucose as a carbohydrate source and incubated overnight at 37°C until exponential growth was reached. Next, aliquots were centrifuged (6000 RPM, 3 min) and washed twice using an equal volume of MRS broth without glucose, and then diluted to equivalent OD600. Finally, 2 μL of one individual strain (monocultures) or 1 μL each of both strains (co-cultures, L. plantarum HEAL9 and L. paracasei 8700:2) was added to each well of a sterile 96-well plate containing 198 μL fresh MRS broth (without glucose) with or without 0.5% dialyzed GOS as a carbohydrate source. Plates were incubated at 37°C overnight in a plate reader and periodic OD600 measurements preceded by 5s shaking at 100 rpm were collected to generate growth curves.
Statistical Methods
Between group comparisons of supplementation effects for microbial metabolic activity analysis endpoints across all donors were made using paired two-sided t-tests. Averages of technical replicates per donor were used as input values, with one input value per donor. The following comparisons were made: GOS alone versus blank (prebiotic effect), Probi Digestis® or Probi Defendum® alone versus blank (probiotic effect), Probi Digestis® + GOS or Probi Defendum® + GOS versus GOS alone (synbiotic effect) and versus blank, and Probi Digestis® + GOS versus Probi Defendum® + GOS (differences between two synbiotics).
Alpha diversity was analyzed using four common indices: observed taxa (species richness), Chao1 (species richness), Shannon (species richness and evenness), and Simpson (species richness and evenness, giving more weight to common or dominant species). Beta diversity was used to determine whether supplementation affected overall community composition. This assessment was made using Discriminant Analysis of Principal Components, which joins two analysis methods to assess effects on population structure. Sequence data were transformed using principal component analysis and clusters were subsequently identified with discriminant analysis, which aims to maximize among-group variation and minimize within-group variation.
Differential abundance analysis was conducted using two statistical methods, treeclimbR and linear discriminant analysis effect size (LEfSe). For both analysis methods, relative abundance data obtained by total sum scaling was used. For treeclimbR analysis [47], bacterial enrichments exceeding a fold change of 4 (corresponding to log2 2) as compared to the reference condition were considered biologically significant; a p-value of <0.05 (corresponding to -log10 0.05=1.3 on the y-axis) was considered statistically significant, i.e., bacterial enrichments with a -log(p-value)>1.3 were considered statistically significant. For LEfSe, the algorithm couples statistical significance with biological consistency and effect size estimation to provide in-depth insight to the biological relevance and magnitude of bacterial enrichments [48]. P-values =0.05 by the Kruskal-Wallis and Wilcoxon tests were considered statistically significant and LDA scores =2.0 or <-2.0 were generally considered biologically relevant.
Results and Discussion
Microbial Metabolic Activity
The pH in the reactors reflected colonic pH in vivo, confirming that the colonic simulations were conducted under optimal conditions to support the growth of a wide diversity of gut microbial community members (Figure 1a). The greatest initial drop in pH was observed with GOS alone, Probi Digestis® + GOS, and Probi Defendum® + GOS. For gas pressure, the blank and probiotic alone conditions demonstrated similar profiles, while the GOS, Probi Digestis® + GOS, and Probi Defendum® + GOS conditions had a significant increase in gas pressure compared with blank (Figure 1b). There was no significant difference in gas production between the two synbiotic conditions (Probi Digestis® + GOS vs Probi Defendum® + GOS).
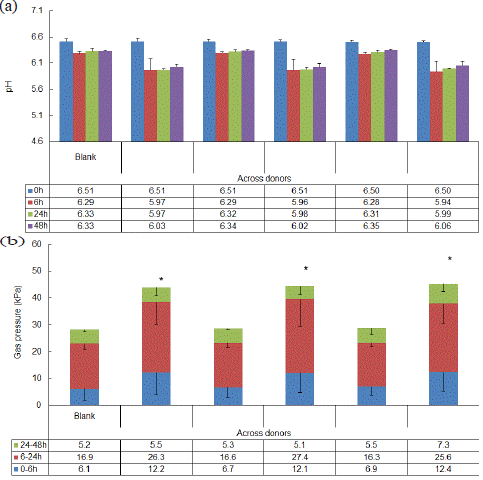
Figure 1: Overall microbial community activity (acidification and gas production) shown as (a) pH and (b) gas pressure. Measurements were collected in triplicate. Data for average values were derived using data from five healthy donors. Error bars represent standard deviation. Two-sided t-tests were used to determine significant differences between each supplemented condition versus blank, between the two synbiotic supplement conditions (Probi Digestis® + GOS and Probi Defendum® + GOS) versus GOS alone, and between Probi Digestis® + GOS versus Probi Defendum® + GOS. * indicates significant differences between the supplemented versus blank test condition when accounting for the entire 48 h period. p<0.05 was considered statistically significant. GOS = Bimuno® galactooligosaccharides.
There was significantly more acetate production with GOS alone and with the two synbiotic test conditions compared with blank, but not with the probiotic alone conditions. Acetate production was also not significantly different between GOS alone and either of the two synbiotic conditions (Figure 2a). Propionate production was also similar between blank and the two probiotics alone, while production was numerically, but not significantly greater with GOS alone compared with blank (Figure 2b). Similar numeric increases in propionate were seen for both synbiotic conditions (compared with GOS alone). As with acetate and propionate, the butyrate profiles for the two probiotics alone were similar to that of blank (Figure 2c). There were numeric, but not significant increases in butyrate production with GOS alone and Probi Digestis® + GOS versus blank, and significantly more butyrate production with Probi Defendum® + GOS compared with blank. GOS stimulated butyrate production in four of five donors, with an average increase of 3.5 mM (+91%) across these four donors. The butyrate production profiles for Probi Digestis® and Probi Defendum® were similar to blank. Co-supplementation of either probiotic + GOS did not significantly alter butyrate levels versus GOS alone, but mild stimulatory effects were observed at the individual level for Probi Defendum® + GOS in two donors, with an average increase in butyrate of +1.4 mM (+26%). GOS alone, Probi Digestis® + GOS, Probi Defendum® alone, and Probi Defendum® + GOS stimulated a significant increase in lactate production versus blank during the first six hours of fermentation (Figure 2d). Production of lactate with Probi Digestis® alone was however, similar to blank during this time. A synergistic synbiotic effect was observed for Probi Defendum® + GOS, as lactate production was significantly higher compared with GOS alone. Lactate production was also significantly higher for the synbiotic Probi Defendum® + GOS compared with Probi Digestis® + GOS.
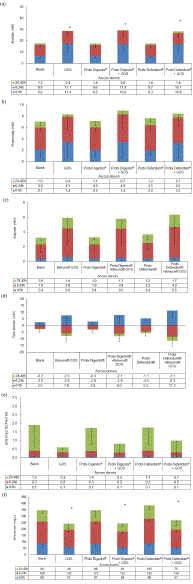
Figure 2: Microbial metabolic activity (a) acetate, (b) propionate, (c) butyrate, (d) lactate, (e) branched SCFA, and (f) ammonium. Data for average values were derived using data from five healthy donors. Error bars represent standard deviation. Paired two-sided t-tests were used to determine significant differences between each supplemented condition versus blank, between the two synbiotic supplement conditions (Probi Digestis® + GOS and Probi Defendum® + GOS) versus GOS alone, and between Probi Digestis® + GOS versus Probi Defendum® + GOS. * indicates significant differences between the supplemented versus blank test condition, § represents significant differences between Probi Digestis® + GOS or Probi Defendum® + GOS compared with GOS alone, and † represents a significant difference between Probi Defendum® + GOS compared with Probi Digestis® + GOS when accounting for the entire 48 h period (acetate, propionate, butyrate, branched SCFA, and ammonium) or the period between 0 and 6 h (lactate). p<0.05 was considered statistically significant. GOS = Bimuno® galactooligosaccharides.
Supplementation with GOS alone or either of the two synbiotics resulted in a non-significant reduction of branched SCFAs relative to blank (Figure 2e) and a significant reduction in ammonium versus blank (Figure 2f). In contrast, levels of both branched SCFAs and ammonium with either probiotic alone were similar to blank.
Microbial Community Composition
The microbial community composition for each individual donor at the start of the study (prior to supplementation) primarily consisted of members of the Firmicutes, Bacteroidetes, and Actinobacteria phyla (data not shown). Bacterial biomass was significantly increased with GOS supplementation versus blank (Figure 3). There was also an increased biomass with probiotic alone compared with blank, which was significant with Probi Defendum® but not with Probi Digestis®. Biomass was numerically increased with Probi Digestis® + GOS and significantly increased with Probi Defendum® + GOS versus blank. Biomass was similar for GOS alone or GOS + either probiotic (range, 4.26–4.54 × 109 total cells/mL).
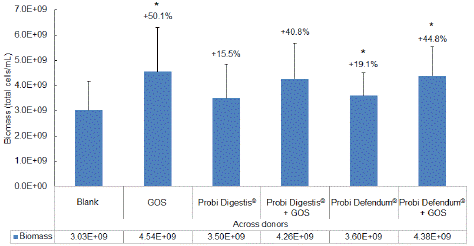
Figure 3: Total bacterial biomass (total cells/mL) at 24 h after the start of incubation. Data for average values were derived using data from five healthy donors. Error bars represent standard deviation. Two-sided t-tests were used to determine significant differences between each supplemented condition versus blank. * indicates significant differences between the supplemented versus blank test condition. p<0.05 was considered statistically significant. GOS = Bimuno® galactooligosaccharides.
Changes in alpha diversity for each of the donors are shown in Figure 4a. The observed and Chao1 indices showed that, on average, there was no major impact of GOS on species richness. Probi Digestis® had an inconsistent effect on species richness across donors, while a moderate decrease was observed for most donors with Probi Defendum®. The overall tendency for synbiotic supplementation was a decrease in species richness compared with blank. The Simpson and Shannon indices revealed that GOS alone and GOS in combination with either probiotic had a tendency to decrease species evenness in most donors, while species evenness was largely unaffected by either probiotic alone. Beta diversity analyses demonstrated that GOS supplementation, with or without probiotics, altered the community composition (Figure 4b). The effect of GOS with or without either probiotic was greater than with probiotic supplementation alone. Supplementation with Probi Defendum® had a stronger impact on the microbial community composition than with Probi Digestis®. This trend was similar when either probiotic was combined with GOS.
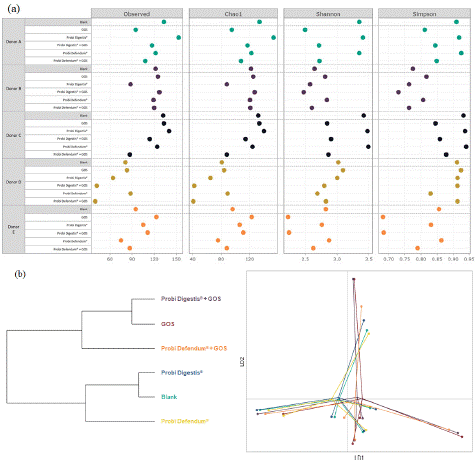
Figure 4: (a) Alpha diversity, represented by the observed, Chao1, Shannon, and Simpson diversity indices, and (b) beta diversity are shown for each donor at 24 h after the start of incubation. Alpha diversity analysis is based on relative abundance data (total sum scaling); each color represents a different donor and each dot represents the average across technical replicates (n=2). Beta diversity is represented by hierarchical clustering showing dissimilarities in community composition (sum of the horizontal lines separating two conditions is a measure for dissimilarity in their community compositions; panel B, left side) and a scatter plot (panel B, right side). Each color represents a different treatment and each dot represents a different donor; calculated from relative abundance data averaged across technical replicates (n=2). GOS = Bimuno® galactooligosaccharides; LD = linear discriminant.
Bacterial enrichments following supplementation with GOS alone versus blank were analyzed using treeclimbR and LEfSe analysis. TreeclimbR analysis across donors revealed a significant enrichment of Bifidobacterium longum with GOS treatment relative to blank (Figure 5), which was also detected with LEfSe analysis (data not shown). TreeclimbR analysis also showed an enrichment of the Anaerobutyricum genus and of several bacterial species, including Bifidobacterium adolescentis, Bifidobacterium ruminantium, Eubacterium ramulus, Slackia isoflavoniconvertens, Streptococcus salivarius, and an uncultured Streptococcus and Roseburia spp (Figure 5). These enrichments reached the threshold for biological relevance, with an average fold change >4, but not statistical significance.
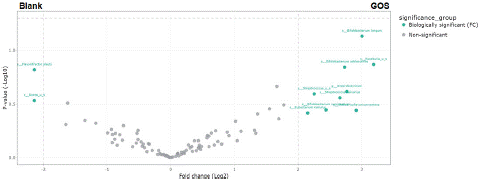
Figure 5: Differential abundance analysis (treeclimbR) to identify differences in community composition at 24 h after the start of incubation with GOS versus blank. The analysis is based on relative abundance data (total sum scaling). The scatter plot classifies taxa into four categories based on abundances in the compared conditions: neither biologically or statistically significant (grey), biologically significant but not statistically significant (teal), statistically significant but not biologically significant (blue), or biologically and statistically significant (red). g= genus; GOS = Bimuno® galactooligosaccharides
The engraftment of the probiotic strains in Probi Digestis® and Probi Defendum® at 24 h was evaluated. The abundance of L. paracasei was elevated with both Probi Defendum® alone and Probi Defendum® + GOS relative to the other conditions (Figure 6a), whereas L. plantarum was not detected with Probi Defendum® supplementation (Figure 6b). The abundances of L. paracasei were higher than the concentration supplemented (5×106 CFU/mL) (Figure 6c). L. plantarum was elevated with Probi Digestis®, both alone and with GOS (Figure 6b); however, the abundance was lower than the concentration supplemented (1×107 CFU/mL) (Figure 6c). To confirm the ability of the probiotic strains to utilize GOS, they were grown in MRS broth media with and without dialyzed GOS as the carbohydrate source. L. plantarum HEAL9 alone, L. paracasei 8700:2 alone, and the combination of L. plantarum HEAL9 and L. paracasei 8700:2 (Probi Defendum® strains) was able to grow in GOS (Figure 7a, 7b, 7c, respectively). However, L. plantarum 299v (Probi Digestis® strain) was not able to utilize GOS for growth (Figure 7d), in agreement with the results of the colonic simulation.
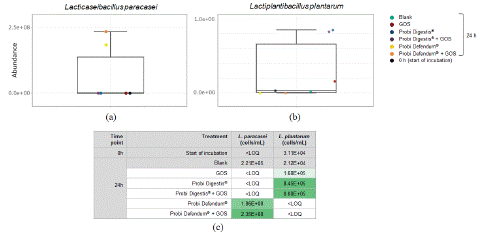
Figure 6: Abundances of (a) Lacticaseibacillus paracasei (b) Lactiplantibacillus plantarum, and (c) an overview of bacterial abundances for all test conditions at 24 h after start of incubation. Data for average values were derived using data from five healthy donors. GOS = Bimuno® galactooligosaccharides; LOQ = limit of quantification.
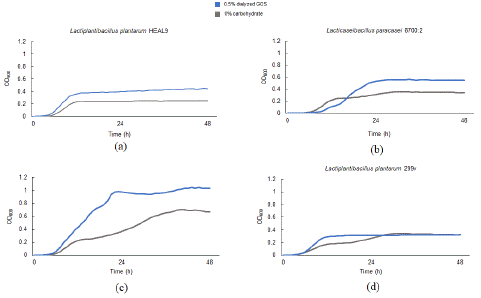
Figure 7: Average growth curves for (a) Lactiplantibacillus plantarum HEAL9, (b) Lacticaseibacillus paracasei 8700:2, (c) Lactiplantibacillus plantarum HEAL9 + Lacticaseibacillus paracasei 8700:2, and (d) Lactiplantibacillus plantarum 299v to determine growth with dialyzed GOS. Measurements were collected in duplicate. Probiotic strains were added to wells of sterile 96 well plates containing MRS broth without a carbohydrate source or with 0.5% dialyzed GOS. Plates were incubated at 37°C overnight on a plate reader and OD600 measurements were taken periodically to generate growth curves. AUC = area under the curve; GOS = Bimuno® galactooligosaccharides
TreeclimbR and LEfSe analysis were also utilized to compare the effects of the two probiotic supplements, Probi Digestis® and Probi Defendum® to blank and each probiotic + GOS to GOS alone. In the treeclimbR analysis, there were no statistically or biologically significant enrichments in the microbial community other than L. plantarum with Probi Digestis® supplementation (Figure 8a). There was a biologically and statistically significant enrichment of L. paracasei and a biologically significant enrichment of Coprococcus catus with Probi Defendum® supplementation compared with blank (Figure 8b). When comparing the effect of Probi Digestis® + GOS with GOS alone, there was a biologically significant enrichment of Phocaeicola vulgatus with GOS alone (Figure 8c). The comparison between Probi Defendum® + GOS and GOS alone showed a biologically and statistically significant enrichment of L. paracasei with Probi Defendum® + GOS supplementation (Figure 8d). LEfSe analysis of the impact of probiotic supplementation only showed differences related to probiotic strains (data not shown).
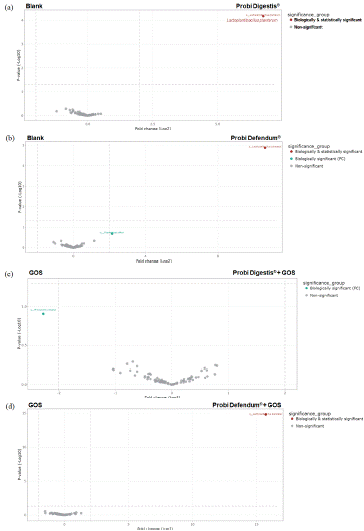
Figure 8: Differential abundance analysis (treeclimbR) to identify differences in community composition at 24 h after the start of incubation with (a) Probi Digestis® versus blank, (b) Probi Defendum® versus blank, (c) Probi Digestis® + GOS versus GOS, and (d) Probi Defendum® + GOS versus GOS. The analysis is based on relative abundance data (total sum scaling). The scatter plot classifies taxa into four categories based on abundances in the compared conditions: neither biologically or statistically significant (grey), biologically significant but not statistically significant (teal), statistically significant but not biologically significant (blue), or biologically and statistically significant (red). GOS = Bimuno® galactooligosaccharides.
Discussion
Using short-term colonic incubations, we evaluated the synbiotic potential of Probi Defendum® with a specific GOS. Evaluations included overall microbial fermentation, microbial metabolic activity, and microbial community composition of colonic bacteria isolated from five healthy adult donors. Overall, the metabolic and metagenomic profile of the Probi Defendum® + GOS synbiotic was comparable with that of GOS alone; however, this synbiotic had a greater lactogenic effect than GOS alone, especially for donors characterized with low lactate production. Butyrate production was also increased, which was likely an effect of the enhanced availability of lactate during the first 6 hours. This enhanced lactogenic effect also differentiated the Probi Defendum® + GOS synbiotic from the Probi Digestis® + GOS synbiotic.
The greatest decreases in pH and increases in gas pressure were observed with GOS, Probi Digestis® + GOS, and Probi Defendum® + GOS, indicating that fermentative activity was higher with these conditions compared with blank or supplementation with either probiotic alone. This activity was driven by GOS, as there was no difference between the two synbiotic conditions and GOS alone. The observation that gas production was similar with GOS supplementation with or without probiotic co-supplementation indicates that administration of the synbiotic formulations to individuals would be tolerable, as GOS intake by humans is tolerable and has been shown to reduce bloating, flatulence, and abdominal pain in participants who suffer these gastrointestinal symptoms [49].
The study findings indicate that GOS was primarily responsible for the increased acetate and butyrate production, given that increases in acetate and butyrate over blank were not observed with either probiotic alone. Although there was also an increase in acetate and butyrate production with both synbiotics relative to blank, the increase was similar to that observed with GOS alone. Butyrate production was significantly greater with the synbiotic Probi Defendum® + GOS compared with blank, and was numerically increased as compared to GOS. Increased SCFA production is a well-known feature of GOS and was confirmed in this study [12,21,50,51]. GOS, and to a lesser extent, Probi Defendum®, stimulated lactate production. Co-supplementation of GOS with Probi Defendum® resulted in significantly higher lactate production than GOS alone, demonstrating an improved lactogenic effect and supporting a synbiotic synergistic effect for this product. This was especially apparent for donors characterized by low lactate production, suggesting that these donors’ inability to produce lactate from GOS was implanted with this probiotic. The greater lactogenic effect can likely be attributed to the probiotic strain L. paracasei, as the L. plantarum probiotics in both the Probi Digestis® and Probi Defendum® formulations were unable to compete with the intestinal microbiome for common nutrients, as evidenced by the metagenomic findings. Given that lactate can be converted to butyrate through cross-feeding, a lactogenic effect is considered beneficial. Indeed, the increase in butyrate production with Probi Defendum® + GOS compared with GOS alone is likely attributable to the strong lactogenic effect of this synbiotic. This is a relevant finding, as butyrate is important for gut membrane health [52-55].
The production of branched SCFAs and ammonium are the result of proteolytic microbial activity. In line with previous reports of GOS fermentation, our study demonstrated a reduction in branched SCFA and ammonium for all GOS-containing test conditions [12,50]. Given that the decreases in branched SCFA and ammonium were similar for GOS alone and both Probi Digestis® + GOS and Probi Defendum® + GOS, the reduction in proteolytic fermentation was driven primarily by GOS. Toxic compounds are produced during proteolytic fermentation; therefore, a reduction in this process, as evidenced by the reduction in branched SCFA and ammonium production, is considered beneficial to human health [56].
Microbial community analysis was conducted 24 h after supplementation since metabolite production largely occurred within 24 h, suggesting that this timepoint was suitable to capture the relevant community shifts. The overall tendency for GOS alone or probiotic + GOS supplementation to decrease species richness versus blank suggests that GOS was primarily fermented by a select group of bacteria, rather than promoting the growth of a diverse array of microbial species. Bacterial biomass increases appeared to be mainly dependent on GOS, as the increase in biomass versus blank was greatest with GOS alone, and reactor biomass following supplementation with GOS alone was similar to that observed with either Probi Digestis® + GOS or Probi Defendum® + GOS. The increase in biomass could be attributed to the enrichment of B. longum, and, to a lesser degree, B. adolescentis and B. ruminantium. The enrichment of Bifidobacterium spp confirms the findings of several clinical trials that have reported the enrichment of bifidobacteria with GOS supplementation [6,7]. The decrease in species richness and/or evenness observed with GOS supplementation alone and in synbiotic combination suggest that GOS favors the growth of a select group of bacteria, shifting the microbial community composition as described above. GOS had the biggest impact on microbial community composition and the impact of Probi Defendum® was stronger than that of Probi Digestis®.
We selected a background medium low in other substrates to observe a specific effect of the added GOS on fermentation by the probiotic strains. In conditions with only probiotics, there were virtually no fermentable substrates available. Therefore, the lack of effect on microbial metabolite production when the probiotics were supplemented without any carbohydrate source (i.e., without GOS) was expected. Further, addition of probiotics alone had no significant impact on the gut microbial community composition. The metagenomic data demonstrated that neither L. plantarum HEAL9 nor L. plantarum 299v, present in Probi Defendum® and Probi Digestis®, respectively, proliferated well in the presence of the donor gut microbiome. In contrast, L. paracasei 8700:2 grew well, correlating with the lactogenic effects observed with Probi Defendum® supplementation.
Both L. plantarum HEAL9 and L. paracasei 8700:2 were able to grow with GOS as a carbohydrate source, but L. plantarum 299v was not. The growth of L. plantarum HEAL9 and L. paracasei 8700:2 appeared to be enhanced when combined compared with either probiotic strain alone, likely due to increase of L. paracasei 8700:2 as observed in the colonic simulations, where only the growth of L. paracasei 8700:2 was enhanced. The growth of these probiotic strains could still be different in vivo, where other carbohydrate substrates are available. An in vitro study demonstrated varied prebiotic carbohydrate utilization by L. plantarum strains, including HEAL9 and 299v [57]. Although L. plantarum 299v was not able to utilize Bimuno® GOS for growth, this probiotic is able to utilize other prebiotic compounds, including another GOS [58,59], and has well established probiotic effects in humans (reviewed in [37]). The inability of L. plantarum 299v to utilize Bimuno® GOS, coupled with the lack of synergistic effect when co-supplemented with Bimuno® GOS highlight the importance of appropriate coupling of prebiotics and probiotics when a synbiotic effect is desired.
Given that the colonic microbiota of five donors were used in this study to address interindividual differences, and that prebiotic effects of easily fermentable fibers (i.e., fibers characterized by low molecular complexity, like GOS) are typically characterized by low interindividual variation, we expect that the effects observed for all donors combined will translate to the general population. In contrast, other substances, such as polyphenols, can be highly donor specific, and would require a larger donor number to be representative for a large population. Although the technology used is validated with in vivo - in vitro correlation [60], this study was limited in that ex vivo findings do not always translate to in vivo effects. Therefore, the synergistic synbiotic effects of co-supplementation of Probi Defendum® and GOS should be validated in clinical trials.
Conclusions
Overall, the known prebiotic effects of GOS, including the stimulation of increased SCFA and lactate production, reduction of proteolytic fermentation, and enrichment of beneficial bifidobacteria were confirmed in this short-term colonic simulation study, and synbiotic effects, most notably an increased lactogenic effect, were identified for the combination of Probi Defendum® + GOS. This effect was most pronounced in donors characterized by an inability to produce lactate, likely because this role was taken up by L. paracasei 8700:2 in the synbiotic formulation.
Author Statements
Acknowledgements
The authors wish to thank Christina Vegge, Probi AB, for her contributions to the study concept and interpretation of the results. The authors also thank Sarah Bubeck, PhD, of Bubeck Scientific Communications for providing medical writing support, which was funded by Clasado Biosciences Ltd. and Probi AB.
References
- Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017; 14: 491-502.
- Roberfroid MB. Health benefits of non-digestible oligosaccharides. Adv Exp Med Biol. 1997; 427: 211-219,.
- Davani-Davari D, Negahdaripour M, Karimzadeh I, Seifan M, Mohkam M, Masoumi SJ, et al. Prebiotics: definition, types, sources, mechanisms, and clinical applications. Foods. 2019; 8: 92.
- Priyadarshini M, Kotlo KU, Dudeja PK, Layden BT. Role of short chain fatty acid receptors in intestinal physiology and pathophysiology. Compr Physiol. 2018; 8: 1091-1115.
- Morrison DJ, Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes. 2016; 7: 189-200.
- Depeint F, Tzortzis G, Vulevic J, I’Anson K, Gibson GR. Prebiotic evaluation of a novel galactooligosaccharide mixture produced by the enzymatic activity of Bifidobacterium bifidum NCIMB 41171, in healthy humans: a randomized, double-blind, crossover, placebo-controlled intervention study. Am J Clin Nutr. 2008; 87: 785-791.
- Vulevic J, Drakoularakou A, Yaqoob P, Tzortzis G, Gibson GR. Modulation of the fecal microflora profile and immune function by a novel trans-galactooligosaccharide mixture (B-GOS) in healthy elderly volunteers. Am J Clin Nutr. 2008; 88: 1438-1446.
- Davis LM, Martinez I, Walter J, Goin C, Hutkins RW. Barcoded pyrosequencing reveals that consumption of galactooligosaccharides results in a highly specific bifidogenic response in humans. PLoS One. 2011; 6: 25200.
- Scalabrin DM, Mitmesser SH, Welling GW, Harris CL, Marunycz JD, Walker DC, et al. New prebiotic blend of polydextrose and galacto-oligosaccharides has a bifidogenic effect in young infants. J Pediatr Gastroenterol Nutr. 2012; 54: 343-352.
- Vulevic J, Juric A, Walton GE, Claus SP, Tzortzis G, Toward RE, et al. Influence of galacto-oligosaccharide mixture (B-GOS) on gut microbiota, immune parameters and metabonomics in elderly persons. Br J Nutr. 2015; 114: 586-595.
- Ladirat SE, Schuren FH, Schoterman MH, Nauta A, Gruppen H, Schols HA. Impact of galacto-oligosaccharides on the gut microbiota composition and metabolic activity upon antibiotic treatment during in vitro fermentation. FEMS Microbiol Ecol. 2014; 87: 41-51.
- Marzorati M, Ghyselinck J, Abbeele P, Maruszak A, Harthoorn L. Galactooligosaccharide (GOS) reduces branched short-chain fatty acids, ammonium, and pH in a short-term colonic fermentation model. Appl Microbiol. 2023; 3: 90–103.
- Griffiths EA, Duffy LC, Schanbacher FL, Qiao H, Dryja D, Leavens A, et al. In vivo effects of bifidobacteria and lactoferrin on gut endotoxin concentration and mucosal immunity in Balb/c mice. Dig Dis Sci. 2004; 49: 579-589.
- Wang ZT, Yao YM, Xiao GX, Sheng ZY. Risk factors of development of gut-derived bacterial translocation in thermally injured rats. World J Gastroenterol. 2004; 10: 1619-1624.
- Wang Z, Xiao G, Yao Y, Guo S, Lu K, Sheng Z. The role of bifidobacteria in gut barrier function after thermal injury in rats. J Trauma. 2006; 61: 650-657.
- Ruiz L, Delgado S, Ruas-Madiedo P, Sanchez B, Margolles A. Bifidobacteria and their molecular communication with the immune system. Front Microbiol. 2017; 8: 2345.
- Tzortzis G, Goulas AK, Gibson GR. Synthesis of prebiotic galactooligosaccharides using whole cells of a novel strain, Bifidobacterium bifidum NCIMB 41171. Appl Microbiol Biotechnol. 2005; 68: 412-416.
- Drakoularakou A, Tzortzis G, Rastall RA, Gibson GR. A double-blind, placebo-controlled, randomized human study assessing the capacity of a novel galacto-oligosaccharide mixture in reducing travellers’ diarrhoea. Eur J Clin Nutr. 2010; 64: 146-152.
- Hasle G, Raastad R, Bjune G, Jenum PA, Heier L. Can a galacto-oligosaccharide reduce the risk of traveller’s diarrhoea? A placebo-controlled, randomized, double-blind study. J Travel Med. 2017; 24.
- Searle LEJ, Cooley WA, Jones G, Nunez A, Crudgington B, Weyer U, et al. Purified galactooligosaccharide, derived from a mixture produced by the enzymic activity of Bifidobacterium bifidum, reduces Salmonella enterica serovar Typhimurium adhesion and invasion in vitro and in vivo. J Med Microbiol. 2010; 59: 1428-1439.
- Grimaldi R, Swann JR, Vulevic J, Gibson GR, Costabile A. Fermentation properties and potential prebiotic activity of Bimuno(R) galacto-oligosaccharide (65% galacto-oligosaccharide content) on in vitro gut microbiota parameters. Br J Nutr. 2016; 116: 480-486.
- Silk DB, Davis A, Vulevic J, Tzortzis G, Gibson GR. Clinical trial: the effects of a trans-galactooligosaccharide prebiotic on faecal microbiota and symptoms in irritable bowel syndrome. Aliment Pharmacol Ther. 2009; 29: 508-518.
- Huaman JW, Mego M, Manichanh C, Canellas N, Canueto D, Segurola H, et al. Effects of prebiotics vs a diet low in FODMAPs in patients with functional gut disorders. Gastroenterology. 2018; 155: 1004-1007.
- Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014; 11: 506-514.
- Thomas CM, Versalovic J. Probiotics-host communication: Modulation of signaling pathways in the intestine. Gut Microbes. 2010; 1: 148-163.
- Pyne DB, West NP, Cox AJ, Cripps AW. Probiotics supplementation for athletes - clinical and physiological effects. Eur J Sport Sci. 2015; 15: 63-72.
- Onning G, Montelius C, Hillman M, Larsson N. Intake of Lactiplantibacillus plantarum HEAL9 improves cognition in moderately stressed subjects: a randomized controlled study. Nutrients. 2023; 15: 3466.
- Onning G, Hillman M, Hedin M, Montelius C, Eriksson J, Ahrne S, et al. Intake of Lactiplantibacillus plantarum HEAL9 reduces the inflammatory markers soluble fractalkine and CD163 during acute stress: A randomized, double blind, placebo-controlled study. Physiol Behav. 2020; 225: 113083.
- Hutt P, Shchepetova J, Loivukene K, Kullisaar T, Mikelsaar M. Antagonistic activity of probiotic lactobacilli and bifidobacteria against entero- and uropathogens. J Appl Microbiol. 2006; 100: 1324-1332.
- Yang J, Huang J, Huang Z, Xu Y, Li W, Zhu S, et al. Cardiometabolic benefits of Lacticaseibacillus paracasei 8700:2: A randomized double-blind placebo-controlled trial. Clin Nutr. 2023; 42: 1637-1646.
- Hill D, Sugrue I, Tobin C, Hill C, Stanton C, Ross RP. The Lactobacillus casei group: history and health related applications. Front Microbiol. 2018; 9: 2107.
- Lazou Ahren I, Berggren A, Teixeira C, Martinsson Niskanen T, Larsson N. Evaluation of the efficacy of Lactobacillus plantarum HEAL9 and Lactobacillus paracasei 8700: 2 on aspects of common cold infections in children attending day care: a randomised, double-blind, placebo-controlled clinical study. Eur J Nutr. 2020; 59: 409-417.
- Ahren IL, Hillman M, Nordstrom EA, Larsson N, Niskanen TM. Fewer community-acquired colds with daily consumption of Lactiplantibacillus plantarum HEAL9 and Lacticaseibacillus paracasei 8700:2. A randomized, placebo-controlled clinical trial. J Nutr. 2021; 151: 214-222.
- Busch R, Gruenwald J, Dudek S. Randomized, double blind and placebo-controlled study using a combination of two probiotic lactobacilli to alleviate symptoms and frequency of common cold. Food Nutr Sci. 2013; 4: 13–20.
- Berggren A, Lazou Ahren I, Larsson N, Onning G. Randomised, double-blind and placebo-controlled study using new probiotic lactobacilli for strengthening the body immune defence against viral infections. Eur J Nutr. 2011; 50: 203-210.
- Hakansson A, Andren Aronsson C, Brundin C, Oscarsson E, Molin G, Agardh D. Effects of Lactobacillus plantarum and Lactobacillus paracasei on the peripheral immune response in children with celiac disease autoimmunity: a randomized, double-blind, placebo-controlled clinical trial. Nutrients. 2019; 11: 1925.
- Nordstrom EA, Teixeira C, Montelius C, Jeppsson B, Larsson N. Lactiplantibacillus plantarum 299v (LP299V((R))): three decades of research. Benef Microbes. 2021; 12: 441-465.
- Markowiak P, Slizewska K. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients. 2017; 9: 1021.
- Hoefman S, Pommerening-Roser A, Samyn E, Vos P, Heylen K. Efficient cryopreservation protocol enables accessibility of a broad range of ammonia-oxidizing bacteria for the scientific community. Res Microbiol. 2013; 164: 288-292.
- Weirdt R, Possemiers S, Vermeulen G, Moerdijk-Poortvliet TC, Boschker HT, Verstraete W, et al. Human faecal microbiota display variable patterns of glycerol metabolism. FEMS Microbiol Ecol. 2010; 74: 601-611.
- Tzollas NM, Zachariadis GA, Anthemidis AN, Statis JA. A new approach to indophenol blue method for determination of ammonium in geothermal waters with high mineral content. Int J Environ Anal Chem. 2010; 90: 115-126.
- Ottesen A, Ramachandran P, Reed E, White JR, Hasan N, Subramanian P, et al. Enrichment dynamics of Listeria monocytogenes and the associated microbiome from naturally contaminated ice cream linked to a listeriosis outbreak. BMC Microbiol. 2016; 16: 275.
- Ponnusamy D, Kozlova EV, Sha J, Erova TE, Azar SR, Fitts EC, et al. Cross-talk among flesh-eating Aeromonas hydrophila strains in mixed infection leading to necrotizing fasciitis. Proc Natl Acad Sci USA. 2016; 113: 722-727.
- Hasan NA, Young BA, Minard-Smith AT, Saeed K, Li H, Heizer EM, et al. Microbial community profiling of human saliva using shotgun metagenomic sequencing. PLoS One. 2014; 9: 97699.
- Lax S, Smith DP, Hampton-Marcell J, Owens SM, Handley KM, Scott NM, et al. Longitudinal analysis of microbial interaction between humans and the indoor environment. Science. 2014; 345: 1048-1052.
- Vandeputte D, Kathagen G, D’Hoe K, Vieira-Silva S, Valles-Colomer M, Sabino J, et al. Quantitative microbiome profiling links gut community variation to microbial load. Nature. 2017; 551: 507-511.
- Huang R, Soneson C, Germain PL, Schmidt TSB, Mering CV, Robinson MD. treeclimbR pinpoints the data-dependent resolution of hierarchical hypotheses. Genome Biol. 2021; 22: 157.
- Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, et al. Metagenomic biomarker discovery and explanation. Genome Biol. 2011; 12: R60.
- Vulevic J, Tzortzis G, Juric A, Gibson GR. Effect of a prebiotic galactooligosaccharide mixture (B-GOS(R)) on gastrointestinal symptoms in adults selected from a general population who suffer with bloating, abdominal pain, or flatulence. Neurogastroenterol Motil. 2018; 30: 13440.
- Maathuis AJ, Heuvel EG, Schoterman MH, Venema K. Galacto-oligosaccharides have prebiotic activity in a dynamic in vitro colon model using a (13)C-labeling technique. J Nutr. 2012; 142: 1205-1212.
- Perdijk O, Baarlen P, Fernandez-Gutierrez MM, Brink E, Schuren FHJ, Brugman S, et al. Sialyllactose and galactooligosaccharides promote epithelial barrier functioning and distinctly modulate microbiota composition and short chain fatty acid production in vitro. Front Immunol. 2019; 10: 94.
- Clausen MR, Mortensen PB. Kinetic studies on colonocyte metabolism of short chain fatty acids and glucose in ulcerative colitis. Gut. 1995; 37: 684-689.
- Geirnaert A, Calatayud M, Grootaert C, Laukens D, Devriese S, Smagghe G, et al. Butyrate-producing bacteria supplemented in vitro to Crohn’s disease patient microbiota increased butyrate production and enhanced intestinal epithelial barrier integrity. Sci Rep. 2017; 7: 11450.
- Peng L, Li ZR, Green RS, Holzman IR, Lin J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J Nutr. 2009; 139: 1619-1625.
- Chelakkot C, Ghim J, Ryu SH. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp Mol Med. 2018; 50: 1-9.
- Diether NE, Willing BP. Microbial Fermentation of Dietary Protein: An Important Factor in Diet(-)Microbe(-)Host Interaction. Microorganisms. 2019; 7.
- Fuhren J, Rosch C, Ten Napel M, Schols HA, Kleerebezem M. Synbiotic matchmaking in Lactobacillus plantarum: substrate screening and gene-trait matching to characterize strain-specific carbohydrate utilization. Appl Environ Microbiol. 2020; 86: 18.
- Barnett AM, Roy NC, Cookson AL, McNabb W.C. Metabolism of caprine milk carbohydrates by probiotic bacteria and Caco-2:HT29(-)MTX epithelial co-cultures and their impact on intestinal barrier integrity. Nutrients 2018; 10: 949.
- Probi AB. GRAS notification for Lactobacillus plantarum strain 299v. Nutrients. 2018; 10.
- Perreau C, Thabuis C, Verstrepen L, Ghyselinck J, Marzorati M. Ex vivo colonic rermentation of NUTRIOSE((R)) exerts immuno-modulatory properties and strong anti-inflammatory effects. Nutrients. 2023; 15: 4229.
Citation: Ghyselinck J, Teixeira C, Marzorati M, Önning G, Harthoorn L. A Novel Synbiotic Blend of Galactooligosaccharide (GOS) and a Two-Strain Probiotic Acts Synergistically to Increase Lactate and Short-Chain Fatty Acid Production in a Short-Term Ex Vivo Colon Fermentation Model. Int J Nutr Sci. 2024; 9(1): 1082.