
Research Article (Special Article: Dietetics)
Int J Nutr Sci. 2024; 9(1): 1087.
Is There a Correlation between Endocrine Disorders and the Increasing Severity of Obesity? A Cross-Sectional Study
Mehmet Köklü1; Umut Gök Balci2; Tugce Aytulu3*; Haluk Mergen4
1Family Medicine Specialist Silifke State Hospital, Turkey
2Department of Family Medicine, University of Health Sciences, Izmir Faculty of Medicine, Izmir, Turkey
3Department of Nutrition and Dietetics, American Hospital, Istanbul, 34365 Turkey
4Department of Family Medicine, University of Health Sciences, Izmir Faculty of Medicine, Izmir, Turkey
*Corresponding author: Tugçe Aytulu Department of Nutrition and Dietetics, American Hospital, Istanbul, 34365 Turkey. Email: tugcea@amerikanhastanesi.org
Received: March 04, 2024 Accepted: April 08, 2024 Published: April 15, 2024
Abstract
Introduction: Obesity can lead to various health problems, including endocrine diseases. This study examines endocrine issues in patients with obesity and morbid obesity.
Aim: The aim was to evaluate the changes that occur as the severity of obesity increases.
Methods: The study was single-center and cross-sectional. The study involved 139 patients (age =18 ) randomly selected from the obesity outpatient clinic. Sixty-eight participants with obesity (BMI=30-34,9), and 71 with severe obesity (bmi=35).
Results: The study included 139 participants. The groups of obesity and severe obesity were different regarding age (p=0.005). The groups were similar in terms of gender (p=0.912). There was a significant difference between the groups regarding education level (p=0.027). There was a significant difference in hypertension between two groups in patients aged between 31 and 50 (p=0.003).
Conclusion: Obesity severity increases hypertension in patients aged 31-50, while diabetes increases with obesity severity in those over 50.
Keywords: Obesity; Severe obesity; Nutrition; Endocrine disorders; Dietetics
Introduction
Obesity is associated with serious health risks. Severe obesity further increases the risk of obesity-related complications, such as coronary heart disease and end-stage renal disease [1]. The goal of obesity management is to improve health. Sustained weight loss of more than 10% overall bodyweight improves many of the complications associated with obesity (eg, prevention and control of type 2 diabetes, hypertension, fatty liver disease, and obstructive sleep apnoea) and quality of life [2]. The prevalence of obesity increased in adults <50 years in 2009-2014. This increase was most pronounced for severe obesity [3].
Severe obesity among children and adolescents has emerged as a public health concern in multiple places around the world. The recently published review of severe obesity among primary schoolchildren in 21 European countries is an important resource for both clinicians and investigators. The prevalence of severe obesity varied significantly between countries, ranging from 1.0% in Swedish and Moldovan children to 5.5% in Maltese children [4]. About 650 million adults and 340 million children and adolescents suffer from obesity worldwide. Obesity is more prevalent among women and older persons than among men and young population [5].
Methods
The study was single center, a cross-sectional study and was completed over a period of 6 months, starting from December 2014. Ethics committee approval was obtained before the study. Anthropometric measurements were taken from the patients who were selected for the research study. This was done after they filled out the questionnaires at the obesity polyclinic in a hospital located in the Izmir Region of Turkey. Demographic characteristics were collected, and height and weight were measured to diagnose obesity according to WHO criteria. Laboratory tests were conducted to evaluate endocrine findings in obesity and morbid obesity with the help of statistical methods.
The study involved 139 patients (males/females aged 18 or older) randomly selected from the obesity outpatient clinic. Exclusion criteria included having a Body Mass Index (BMI) less than 30.
Statistical Analysis
The SPSS17 package program was used for all the analyses. Categorical variables were presented with frequencies and percentages. The relationship between variables was assessed using Chi-square and Fisher Exact Test. The research was conducted with a 95% confidence level, and a p-value of less than 0.05 was considered statistically significant.
Results
In the study, a total of 139 people were included. Out of all the participants, 24 people (17.3%) were 30 years old or younger, 61 people (43.8%) were between 31 and 50 years old, and 54 people (38.9%) were over 50 years old. Among the group of participants with obesity, there were 17 people (25%) aged 30 or below, 33 people (48.5%) aged between 31 and 50 years, and 18 people (26.5%) over 50 years old. In the group of severe obesity, there were 7 people (9.9%) aged 30 or below, 28 people (39.4%) aged between 31 and 50 years, and 36 people (50.7%) over 50 years old. Both groups were found to be different in terms of age (p=0.005).
In this study, 84.2% (n:117) of the participants were women. Both groups with obesity and severe obesity showed a similar gender distribution, with 83.8% and 84.5% women, respectively (p=0.912).
The study found that 70.5% of participants had an education level below high school, while 29.5% had a high school level or above. Among those with obesity, 61.8% had a high school level or above, while 78.9% of those with severe obesity had a high school level or above. The study found a significant difference between the two groups regarding education level (p=0.027). These data are shown in Table 2.
WHO Classification
BMI (kg/m² )
Risk of comorbidities
Underweight
<18.50
Normal Range
18.50 – 24.9
Overweight
25-29.9
Obese
=30
Class 1
30.0-34.9
Moderate
Class 2
35.0 – 39.9
Severe
Class 3
=40
Very severe
Table 1: Criteria of obesity and comorbidities [6].
Descriptive
InformationTOTAL
(n =139)OBESITY
(n =68)SEVERE OBESITY
(n =71)p
n
%
n
%
n
%
Age
=30
24
17.3
17
25.0
7
9.9
0.005
31-50
61
43.8
33
48.5
28
39.4
>50
54
38.9
18
26.5
36
50.7
Gender
Female
117
84.2
57
83.8
60
84.5
0.912
Male
22
15.8
11th
16.2
11th
15.5
Education Status
Under High School
98
70.5
42
61.8
56
78.9
0.027
High School and Above
41
29.5
26
38.2
15
21.1
Operating Status
Working
33
23.7
20
29.4
13
18.3
0.160
Not Working
83
59.7
40
58.8
43
60.6
Retired
23
16.5
8
11.8
15
21.1
Marital Status
Married
111
79.9
54
79.4
57
80.3
0.898
Single
28
20.1
14
20.6
14
19.7
Table 2: Demographic characteristics of individuals.
The study found that as the severity of obesity increased, the diagnosis of hypertension also increased significantly (p=0.003) in the group of age between 31-50. Among the participants with obesity, 12.1% had hypertension, while among those with severe obesity, 46.4% had hypertension (Table 3). However, there However, no statistically significant results were found for diseases such as diabetes, thyroid, urinary tract, heart diseases, hypercolesterolemia or anemia.
Age Between 31-50 (N=61)
With Obesity (N=33)
Severe With Obesity (N=28)
P
Current Diseases
N
%
N
%
Hypertension
Yes
4
12.1
13
46.4
0.003
No
29
87.9
15
53.6
Diabetes
Yes
2
6.1
3
10.7
0.653
No
31
93.9
25
89.3
Thyroid Diseases
Yes
6
18.2
5
17.9
0.974
No
27
81.8
23
82.1
Urinary Tract Diseases
Yes
12
36.4
6
21.4
0.202
No
21
63.6
22
78.6
Heart Diseases
Yes
1
3.0
2
7.1
0.589
No
32
97.0
26
92.9
Hypercholesterolemia
Yes
12
36.4
10
35.7
0.958
No
21
63.6
18
64.3
Anemia
Yes
15
45.5
8
28.6
0.175
No
18
54.5
20
71.4
Table 3: Diseases in participants aged between 31-50.
There were 3 (16.7%) participants with diabetes in the obesity group over the age of 50 and 19 (52.8%) participants with diabetes in the group of participants with severe obesity. Over the age of 50, diabetes increased as the severity of obesity increased (p=0.011) (Shown in Graphic 1).
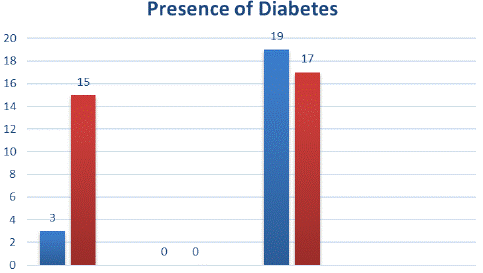
Graphic 1: Comparison of diabetes diagnosis in participants >50 years of age with obesity and severe obesity.
In the obesity group, no patients had pretibial edema (PA) positive. However, in the severe obesity group, 5 (17.9%) patients had PA positive, while 23 (82.1%) patients had no PA. As the participants' obesity severity increased in the 31-50 age group, PA also increased significantly (p=0.017).
Out of the patients with obesity, 13 of them (39.9%) did not show any signs of insulin resistance, as indicated by HOMA-IR <2.5. Conversely, 20 patients (60.6%) had insulin resistance (HOMA-IR=2.5). In the case of patients with severe obesity, 24 of them (85.7%) had insulin resistance. There was a significant increase in insulin resistance as the severity of obesity increased (p=0.029). While no participants in the obesity group had a TSH level higher than 4.20, 21.4% of patients in the severe obesity group did have elevated TSH levels. This difference was statistically significant (p=0.007). Table 4 shows these data.
Age Range: 31-50 (N=61)
Obesity Group (N=33)
Severe Obesity Group (N=28)
P
N
%
N
%
Blood Pressure Levels (Mmhg)
<120; <80
24
72.7
14
50.0
0.114
120-139; 80-89
5
15.1
5
17.9
=140; =90
4
12.1
9
32.1
Pretibial Edema
Positive
0
0.0
5
17.9
0.017
Negative
33
100.0
23
82.1
Hemoglobin (G/Dl)
<11
1
3.0
2
7.1
0.589
=11
32
97.0
26
92.9
Fasting Plasma Glucose (G/Dl)
<100
23
69.7
16
57.1
0.266
100-125
9
27.3
8
28.6
>125
1
3.0
4
14.3
Postprandial Plasma Glucose (G/Dl)
<140
28
84.8
22
78.6
0.490
140-200
4
12.1
6
21.4
>200
1
3.0
0
0.0
Hba1c (%)
<5.7
21
63.6
12
42.9
0.153
5.7-6.4
12
36.4
15
53.6
>6.4
0
0.0
1
3.6
HOMA-IR
<2.5
13
39.4
4
14.3
0.029
=2.5
20
60.6
24
85.7
Cortisol (μg/Dl)
=19.4
29
87.9
24
85.7
>0.999
>19.4
4
12.1
4
14.3
T4 (Mcg/Dl)
=1.71
33
100.0
28
100.0
-
>1.71
0
0.0
0
0.0
TSH (Mu/Ml)
=4.20
33
100.0
22
78.6
0.007
>4.20
0
0.0
6
21.4
Total Cholesterol (Mg/Dl)
=239
25
75.7
21
75.0
0.945
>239
8
24.2
7
25.0
HDL-Cholesterol (Mg/Dl)
<40
6
18.2
3
10.7
0.412
40-59
19
57.6
21
75.0
>59
8
24.2
4
14.3
LDL-Cholesterol (Mg/Dl)
<100
6
18.2
3
10.7
0.117
100-129
16
48.5
8
28.6
>129
11
33.3
17
60.7
Abbreviations: HbA1c: glycated hemoglobin, HOMA-IR: Homeostatic Model Assessment of Insulin Resistance, T4: Thyroxine, TSH: Thyroid Stimulating Hormone, HDL: High-density lipoprotein, LDL: Low-density lipoprotein
Table 4: Comparison of participants between the ages of 31-50 in terms of some clinical conditions and biochemical values.
Discussion
The World Health Organization has recognised obesity as a disease since 1948, and in 2021, the European Commission categorised obesity as a chronic disease. Accordingly, obesity should be referred to using the language of chronic diseases, with correct and established terminology and definitions. The language of chronic diseases is usually precise, accurate, and respectful. However, despite the international recognition of obesity as a chronic disease, the language used to describe obesity is often inaccurate and sometimes not sufficiently respectful, which can contribute to the misunderstanding of obesity, encourage weight stigma, and lead to systemic bias. This may create barriers and challenges to the treatment and management of people living with obesity [7]. In this article, we chose to use the term "obesity" instead of labeling the participants as "obese."
Obesity is an alarmingly increasing global public health issue. Several countries worldwide have witnessed a double or triple escalation in the prevalence of obesity in the last three decades, probably due to urbanization, sedentary lifestyle, and increase consumption of high-calorie processed food. The alarming increase in childhood obesity foreshows a tremendous burden of chronic disease prevention in the future public healthcare systems worldwide. Obesity prevention is a critical factor in controlling Obesity-Related Non-Communicable Diseases (OR-NCDs), including diabetes, cardiovascular disease, stroke, hypertension, cancer, and psychological problems [8].
This study, in line with previous research, suggests that the likelihood of obesity rises with age. This could be because the body becomes less capable of expending the energy derived from food as we grow older. The basal metabolic rate decreases with age, and the decrease in physical activity exacerbates this. The study's obesity and severely obesity groups were heterogeneous regarding age (p=0.005). The incidence of obesity begins to increase after the age of 30.Hemmingsson et al. Study in Sweden shows that there were similar increases in obesity across gender and age groups, people with low education (vs high) and rural areas (vs urban) had a higher prevalence increase (both P<0.001) in obesity [9]. A positive correlation was found between age and obesity frequency in the TOHTA study [10]. According to WHO report in May 2022, the state of the obesity pandemic in Europe, stating that 60% of citizens in the area of Europe with impaired BMI and obesity. The age-standardized prevalence of obesity has increased from 4.6% in 1980 to 14.0% in 2019 [11].
The study found that there was a significant difference in the prevalence of hypertension between the groups of obesity and severe obesity in people aged 31-50. As age and BMI increase in individuals with severe obesity, the risk of developing hypertension also increases. Excess body weight is associated with an increased cardiovascular risk and earlier onset of cardiovascular morbidity.1 It is well established that obesity is associated with activation of both the sympathetic nervous system and the renin–angiotensin system contributing to the emergence of hypertension.In Booth et al study, age-standardised hypertension prevalence was twice as high in morbid obesity (men 78.6% women 66.0%) compared with normal weight (men 37.3% women 29.4%). Hypertension treatment was more frequent (AOR 1.75, 1.59–1.92) but hypertension control less frequent (AOR 0.63, 0.59–0.69) in morbid obesity, with similar findings for severe obesity [12].
In the study, the prevalence of obesity was higher in married individuals than in single individuals. This observation is consistent with previous research. The lower prevalence of obesity among single people could be attributed to the fact that they have not undergone pregnancy yet, their average age is lower, and this group is more concerned about maintaining a healthy weight. Similarly, Lee et al. found that married participants showed a higher prevalence of obesity and abdominal obesity than those in other marriage categories, except for widowed women in Korea [13]. Also, Alami et al. showed a high prevalence of overweight/obesity among women, government employees, and married individuals [14].
According to this study, there is a correlation between education level and the severity of obesity. The study found that as education level increases, the severity of obesity decreases (p=0.027). It's possible that as individuals become more educated, they are more likely to have a healthier diet and pay more attention to their physical appearance, which could lead to a decrease in the frequency of obesity. In a study conducted by Chung and Kim in Korea, it was found that gender, education level, and age all had a significant association with the risk of obesity. The association was stronger in women than in men. Additionally, education level was found to have a negative association with obesity risk in middle-aged individuals of both genders. However, the association became positive in old age, particularly among highly educated women [15]. Stival et al. evaluated the prevalence and correlates of overweight and obesity in 12 European countries between 2017 and 2018. Multilevel logistic random-effects analyses showed that the prevalence of obesity was related to higher age and lower level of education and socioeconomic status [16].
This study found a significant difference in hypertension between the obesity and severe obesity groups in patients aged between 31 and 50 (p=0.003). This supports the fact that the risk of developing hypertension increases in people with severe obesity, in advancing age, and increasing BMI.
In this study, 21.4% of patients in the severe obesity group had elevated TSH levels, while no participants in the obesity group had a TSH level higher than 4.20. The difference was statistically significant (p=0.007).In Mele et al.'s study, there was a significant upward trend for TSH levels across incremental BMI classes in females, while the opposite trend was seen for fT4 levels in males (p<0.0001 for both). Expectedly, TSH was associated with %FM and FFM (p<0,0001 for both). TSH and fT4 showed correlations with several metabolic variables, and both declined with aging (TSH, p<0.0001; fT4, p<0.01) [17].
None of the patients with obesity group in the study had PA. However, in the group of patients with severe obesity, 5 out of 28 patients (17.9%) tested positive for PA. As the severity of obesity increased in the age group of 31-50, the prevalence of PA also significantly increased (p=0.017). Obesity can functionally cause iliac vein and Inferior Vena Kava obstruction, resulting in edema with all the cardiovascular disease stigmata without identified infra-inguinal venous disease [18].
Conclusion
The study found that the obesity and severe obesity groups were similar in terms of gender. It also revealed that obesity became more common as age increased. The prevalence of obesity was higher among married individuals compared to single individuals. However, as the level of education increased, the severity of obesity decreased. This study found that as obesity severity increases, there is a significant rise in hypertension in patients aged 31-50.
The study has certain limitations. Owing to the small sample size and the evaluation criteria for obesity, certain anthropometric assessments such as waist and hip circumference, body fat ratio, and fat-free mass could not be measured. Therefore, there is a requirement for controlled randomized studies that can target larger groups.
Author Statements
Author’s Contribution
Mehmet Köklü did the hypothesis determination and data collection part of the study, Haluk Mergen did the statistical analysis of the data, Tugçe Aytulu did the article writing part, and Umut Gök Balci edited the entire study.
References
- Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017-2018. NCHS Data Brief. 2020: 1-8.
- Perdomo CM, Cohen RV, Sumithran P, Clément K, Frühbeck G. Contemporary medical, device, and surgical therapies for obesity in adults. Lancet. 2023; 401: 1116-1130.
- Patel A, Krishna SG, Patel K, Gray DM, Mumtaz K, Stanich PP, et al. Rising Rates of Severe Obesity in Adults Younger Than 50 Correspond to Rise in Hospitalizations for Non-malignant Gastrointestinal Disease. Dig Dis Sci. 2023; 68: 554-563.
- Pinhas-Hamiel O, Hamiel U, Bendor CD, Bardugo A, Twig G, Cukierman-Yaffe T. The Global Spread of Severe Obesity in Toddlers, Children, and Adolescents: A Systematic Review and Meta-Analysis. Obes Facts. 2022; 15: 118-134.
- Alreshidi FF, Alreshidi FS, Ahmed HG. An insight into the prevalence rates of obesity and its related risk factors among the Saudi community in the Hai’l Region. Eur Rev Med Pharmacol Sci. 2022; 26: 5755-5762.
- Khan A, Raza S, Khan Y, Aksoy T, Kham M, Weinberger Y, et al. Current updates in the medical management of obesity. Recent Pat Endocr Metab Immune Drug Discov. 2012; 6: 117-128.
- Jepsen CH, Bowman-Busato J, Allvin T, Arthurs N, Gossens GH, Govers E, et al. Achieving consensus on the language of obesity: a modified Delphi study. E Clinical Medicine. 2023; 62: 102061.
- Tiwari A, Balasundaram P. Public Health Considerations Regarding Obesity. Stat Pearls. Treasure Island (FL): StatPearls Publishing; 2024.
- Hemmingsson E, Ekblom ö, Kallings LV, et al. Prevalence and time trends of overweight, obesity and severe obesity in 447,925 Swedish adults, 1995-2017. Scand J Public Health. 2021; 49: 377-383.
- Akbay E, Bugdayci R, Tezcan H, Konca K, Yazar A, Pata C. The Prevalence of obesity in adult population in a city on the Mediterranean Coast of Turkey. Turkish Journal of Endocrinology and Metabolism. 2003; 1: 31-35.
- Boutari C, Mantzoros CS. A 2022 update on the epidemiology of obesity and a call to action: as its twin COVID-19 pandemic appears to be receding, the obesity and dysmetabolism pandemic continues to rage on. Metabolism. 2022; 133: 155217.
- Booth HP, Prevost AT, Gulliford MC. Severity of obesity and management of hypertension, hypercholesterolaemia and smoking in primary care: population-based cohort study. J Hum Hypertens. 2016; 30: 40-5.
- Lee J, Shin A, Cho S, Choi JY, Kang D, Lee JK. Marital status and the prevalence of obesity in a Korean population. Obes Res Clin Pract. 2020; 14: 217-224.
- Alami A, Jafari A, Hosseini Z. Differences in overweight/obesity prevalence by demographic characteristics and self-weight misperception status. Clin Nutr ESPEN. 2021; 41: 249-253.
- Chung W, Kim R. A Reversal of the Association between Education Level and Obesity Risk during Ageing: A Gender-Specific Longitudinal Study in South Korea. Int J Environ Res Public Health. 2020; 17: 6755.
- Stival C, Lugo A, Odone A, van den Brandt PA, Fernandez E, Tigova O, et al. Prevalence and Correlates of Overweight and Obesity in 12 European Countries in 2017-2018. Obes Facts. 2022; 15: 655-665.
- Mele C, Mai S, Cena T, Pagano L, Scacchi M, Biondi B, et al. The pattern of TSH and fT4 levels across different BMI ranges in a large cohort of euthyroid patients with obesity. Front Endocrinol (Lausanne). 2022; 13: 1029376.
- Gasparis AP, Kim PS, Dean SM, Khilnani NM, Labropoulos N. Diagnostic approach to lower limb edema. Phlebology. 2020; 35: 650-655.